Abstract
Ethyl pyruvate (EP), as a pyruvate derivative, has the protective effect on oxidative-stress-mediated cellular injury. In previous studies, it has been reported that EP provides protection against neuronal injury in brain, for example transient cerebral ischemia. However, the inhibition effect of EP on peripheral nerve injury is still unknown. Here, we investigated the inhibition effect of EP in Schwann cell de-differentiation and proliferation during Wallerian degeneration using ex vivo sciatic nerve explant system. We demonstrated that EP inhibits p-ERK1/2, p75NGFR, and lysosomal associated membrane protein 1 as factors implicated in Schwann cell de-differentiation during Wallerian degeneration. In addition, EP is sufficient to inhibit Schwann cell proliferation during Wallerian degeneration. Together, these findings suggest that EP has a strong protective effect on Wallerain degeneration.
Introduction
Pyruvate, the product of glycolysis, is a scavenger of reactive oxygen species (ROS), and thus has a strong neuroprotective effect in various models of oxidative stress. In vitro, pyruvate is neuroprotective against hydrogen peroxide (H2O2) (Desagher et al. Citation1997; Wang et al. Citation2007), β-amyloid (Alvarez et al. Citation2003), and zinc (Kawahara et al. Citation2002; Chen & Liao Citation2003); in in vivo models, pyruvate protects against cerebral ischemic injury (Lee et al. Citation2001; Yu et al. Citation2005) and zinc toxicity (Lee et al. Citation2001). However, in the peripheral nerve, previous studies have only reported pyruvate's involvement in energy metabolism (Miller et al. Citation2002; Pfeiffer-Guglielmi et al. Citation2007), but not in nerve injury.
After injury, the peripheral nerve shows a vigorous response, especially in the distal stump. During this morphologically and molecularly degenerative process, called Wallerian degeneration, Schwann cells, the glial cells of the peripheral nervous system, de-differentiate to an immature state, and no longer express myelin-related genes (Jessen & Mirsky Citation2008). These de-differentiated Schwann cells proliferate to facilitate axonal regeneration. Thus, because Schwann cell de-differentiation and proliferation are phenomena characteristic of Wallerian degeneration, these processes may be essential to treating peripheral degenerative or demyelinating diseases.
Peripheral nerve injury increases the expression of inducible nitrogen oxide synthase, which is significantly involved in Wallerian degeneration (Levy et al. Citation2001; Lee et al. Citation2007a; Campuzano et al. Citation2008). The present study tested the effects of pyruvate against oxidative stress in peripheral nerve injury, and defined its role in mediating Schwann cell de-differentiation and proliferation during Wallerian degeneration. Towards the latter aim, we measured its effects on signaling proteins involved in Schwann cell de-differentiation (i.e. a lysosomal protein, a neurotrophic factor, and mitogen-activated protein (MAP) kinase). However, despite pyruvate's effectiveness as an ROS scavenger, its potential as a therapeutic agent is limited due to poor stability in solution (Vonkorff Citation1964). Thus, we used ethyl pyruvate (EP), a stable derivative (Sims et al. Citation2001), and demonstrated that its direct antioxidant effect remarkably inhibited Schwann cell de-differentiation and proliferation during Wallerian degeneration in an ex vivo sciatic nerve explant model.
Materials and methods
Animals
All the animal experiments were conducted using male C57BL/six mice (five-weeks-old, Samtako, Osan, Korea). Mice were housed with food and water available ad libitum in a temperature- (23 ± 1°C) and humidity-controlled (50%) environment on a 12 h light/dark cycle.
Materials
The primary antibodies used for immunostaining or Western blotting were commercially purchased. Antibodies against p75 nerve growth factor receptor (p75NGFR) and lysosomal associated membrane protein 1 (LAMP1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), whereas those against phospho-p44/42 MAPK (p-ERK1/2) and Ki67 were obtained from Cell Signaling (Beverly, MA, USA) and Abcam (Cambridge, UK), respectively. Alexa Fluor 488- and 594-conjugated secondary antibodies were purchased from Life Technologies (Grand Island, NY, USA). All of the other antibodies and reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Explant culture
Sciatic nerve explants were cultured as described (Park et al. Citation2015). Briefly, connective tissues surrounding the sciatic nerves removed from mice were detached under a stereomicroscope. Sciatic nerves were cut into small explants of 3–4 mm in length. Several sciatic explants which were fixed in 4% paraformaldehyde (PFA) directly after removed from mice were used as control. Sciatic nerve explants for a model of Wallerian degeneration were cultured at 37°C, 5% CO2 in Dulbecco's Modified Eagle's Medium containing 100 units/mL penicillin, 100 μg/mL streptomycin, 10% (vol/vol) heat-inactivated fetal bovine serum, and 2 mM L-glutamine with or without EP treatment. In all the experiments, nerve explants were maintained in culture for three days, after which they were fixed in 4% PFA for 6–12 h for immunostaining analysis or homogenized to obtain protein extracts.
Western blot analysis
Western blot analysis was carried out as described elsewhere (Lee et al. Citation2007b). Briefly, total protein (10 μg) from cultured sciatic explants was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, electrophoretically transferred onto a nitrocellulose membrane (Amersham Bioscience, Piscataway, NJ, USA), blocked in 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) at 4°C overnight, and hybridized for 1 h with primary antibodies diluted in the same blocking buffer. Protein loading was detected by Ponceau S staining. For quantitative analysis, X-ray films were scanned using a Hewlett-Packard company scanner and analyzed with the luminescence image analysis system (Fujifilm, Tokyo, Japan).
Immunostaining
Sciatic explant slides were post-fixed in 4% PFA for 20 min, washed three times with phosphate-buffered saline (PBS) for 10 min, and blocked at room temperature (RT) for 1 h in PBS containing 0.3% Triton X-100 (PBST) and 10% bovine serum albumin. Slides were incubated overnight at 4°C with the appropriate primary antibodies in blocking solution. After washing three times, slides were incubated in PBST containing Alexa 488- or 594-conjugated secondary antibodies for 2 h at RT. After further washing three times with PBS, coverslips were adhered to the slides with Gelmount (Biomeda, Foster City, CA, USA) and analyzed using a laser confocal microscope (LSM700, Carl Zeiss, Oberkochen, Germany). For morphometric analysis for LAMP1 immunofluorescence, we used a software accompanied by the LSM 700 microscopy. The intensity was calculated in seven randomly selected 300 × 300 μm2 areas from each sample from tree mice. For quantification of the relative intensity, we conducted to evaluate the relative number of the pixels that showed each intensity unit (Lee et al. Citation2009).
Statistical analysis
All the experiments were conducted at least three times, and values are expressed as means ± SEM. Statistically significant differences between groups were determined using the one-way analysis of variance; all of the analyses were carried out using SPSS 21.0 software (IBM, USA).
Ethics statement
Animals were handled in accordance with the guidelines of the Korean Academy of Medical Science, and approved by the Kyung Hee University Committee on Animal Research [KHUASP(SE)-13-027]. All of the procedures were designed to minimize animal suffering and the number of animals used.
Results
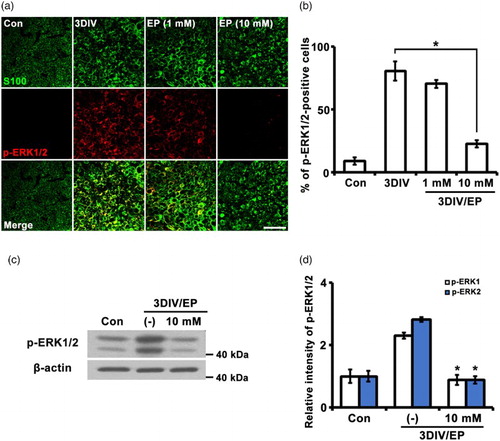
The de-differentiation of Schwann cells involves activation of MAP kinases, including JNK, ERK, and p38 MAP kinase, after peripheral nerve injury (Harrisingh et al. Citation2004; Monje et al. Citation2010; Yang et al. Citation2012). ERK1/2 is highly phosphorylated (p-ERK1/2) in the distal stump after nerve injury (Harrisingh et al. Citation2004). To determine whether Schwann cell de-differentiation is inhibited by EP during Wallerian degeneration, we examined the effect of three days in vitro (3DIV) treatment with EP (1 or 10 mM) on p-ERK1/2 by immunostaining. Levels of p-ERK1/2 were significantly increased 3DIV compared to non-degenerated nerves, and the increased p-ERK1/2 signals co-localized with S100 (a Schwann cell marker) ((a) and (b)). In contrast, sciatic explants treated with EP (10 mM) showed no change in p-ERK1/2, with levels similar to control in Schwann cells, but EP (1 mM) did not show any effect on the inhibition of p-ERK1/2 expression in the denervated Schwann cells ((a) and (b)). Thus, we found that 10 mM EP is suitable to inhibit Schwann cell de-differentiation. Quantitative Western blot analyses also showed that 3DIV EP treatment suppressed induction of p-ERK1/2 ((c) and (d)). Thus, exposure of sciatic nerve to EP appears to significantly inhibit p-ERK1/2 expression in Schwann cells during Wallerian degeneration.
Figure 1. EP (10 mM) inhibits p-ERK1/2 expression. (a) Sciatic explants cross sections were double-immunostained with antibodies against p-ERK1/2 (red) and S100 (green, Schwann cell marker). Sciatic nerve explants were cultured for 3DIV in the presence or absence of EP (1 or 10 mM). Scale bar = 50 μm. (b) Percentage of p-ERK1/2-positive cells among 200 S100-positive cells. n = 3. (c) Protein lysates (10 μg) from sciatic explants were analyzed by Western blotting. EP, 10 mM. (d) Quantification of relative intensity of p-ERK1 and p-ERK2 expression levels by Western blot. n = 4. *P < .001 compared to non-treated explants at 3DIV.
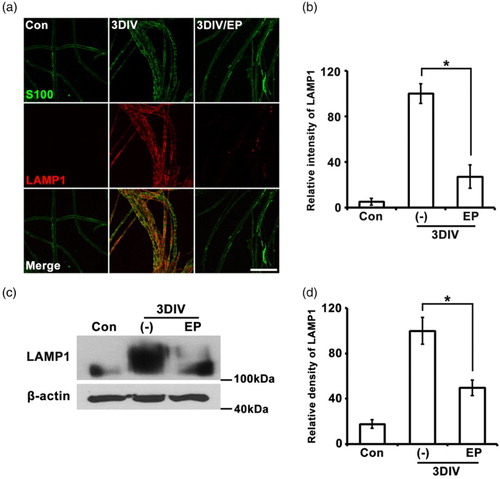
In de-differentiated Schwann cells, lysosomal and proteasomal protein degradation are activated to remove myelin sheath debris. Previous studies have reported that expression of LAMP1, a marker of lysosomal vesicles, is dramatically increased in sciatic nerves after nerve injury (Jung et al. Citation2011; Shin et al. Citation2013). To assess the effect of EP on lysosomal activation during Wallerian degeneration, we immunostained for LAMP1 and S100. Three days of EP treatment significantly decreased LAMP1 immunoreactivity compared to non-treated explants at 3DIV ( (a) and (b)). This decrease was quantitatively confirmed by analysis of relative densities on Western blots using Image J software (Khan et al. Citation2012; Lee et al. Citation2014) ( (c) and (d)). These results indicate that EP is sufficient to inhibit lysosomal protein degradation during Wallerian degeneration.
Figure 2. Lysosomal activation is suppressed by EP (10 mM). (a) Teased sciatic nerve fibers were double-immunostained with LAMP1 (red, lysosomal vesicle marker) and S100 (green). Scale bar = 100 μm. (b) Quantitation of the fluorescent intensity of LAMP1 immunoreactivity from teased nerve fiber samples. n = 3. (c) Western blots for LAMP1; β-actin, loading control. (d) Densitometric quantification of LAMP1 expression. n = 4.*P < .001 compared to non-treated explants at 3DIV.
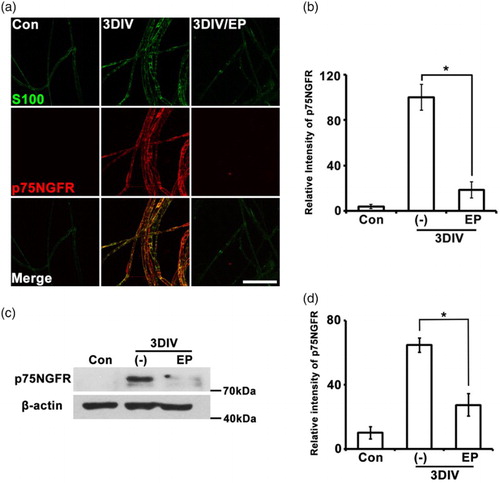
During Wallerian degeneration, de-differentiated Schwann cells induce expression of several neurotrophic factors involved in neuronal survival and axonal regeneration (Chen et al. Citation2007). The p75 NGFR, the low-affinity NGFR, is highly expressed in de-differentiating Schwann cells during Wallerian degeneration (Jung et al. Citation2011; Shin et al. Citation2013). To assess whether EP inhibits Schwann cell de-differentiation, we immunostained teased sciatic nerve fibers with p75NGFR antibody at 3DIV in the presence or absence of EP. Three days of EP treatment dramatically reduced p75NGFR expression compared to non-treated nerves at 3DIV ( (a) and (b)), and this effect was confirmed in Western blot analyses ( (c) and (d)). We conclude that EP downregulates p75NGFR expression in de-differentiated Schwann cells during Wallerian degeneration.
Figure 3. p75NGFR expression is regulated by EP (10 mM). (a) Teased sciatic explants immunostained for p75NGFR (red) and S100 (green). Scale bar = 100 μm. (b) Quantification of fluorescent intensity of p75NGFR expression. n = 3. (c) Western blot analyses show the decrease of p75NGFR expression in EP-treated explants. (d) Quantification of Western blot bands. n = 4. *P < .001 compared to non-treated explants at 3DIV.
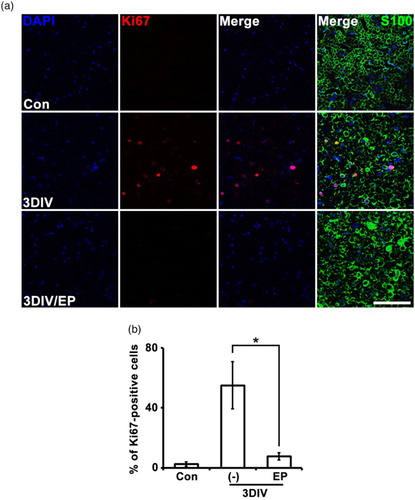
Because Schwann cell proliferation is a distinctive characteristic of Wallerian degeneration, we examined the effect of EP on this process by immunostaining for the cell proliferation marker Ki67. Ki67 signal was increased in explant cross sections of non-treated explants at 3DIV ( (a) and (b) relative to non-denervated controls. Ki67 signal localized to S100-positive, 4′,6-diamidino-2-phenylindole (DAPI)-positive cells ( (a) and (b)). However, EP treatment decreased the number of Ki67-positive cells at 3DIV compared to non-treated explants ( (a) and (b)). This result suggests that EP inhibits Schwann cell proliferation during Wallerian degeneration.
Figure 4. EP (10 mM) suppresses Schwann cell proliferation during Wallerian degeneration. (a) Cross-sections of mouse sciatic explants were double-immunostained for Ki67 (red, cell proliferation marker) and S100. Scale bar = 50 μm. (b) Mean percentage of Ki67-positive cells shown in (a) among 200 DAPI-positive cells. *P < .001 compared the non-treated explants at 3DIV, n = 4.
Discussion
Schwann cell de-differentiation and proliferation, characteristic of Wallerian degeneration, are also essential for axonal regeneration and effective reinnervation during peripheral nerve regeneration. However, in demyelinating diseases such as Guillain–Barré syndrome, Schwann cell de-differentiation can be lethal if patients do not receive treatment. In addition, in inherited peripheral neuropathies, including Charcot–Marie–Tooth disease, de-differentiated Schwann cells continuously promote demyelination during Wallerian-like degeneration, suppressing entry into the nerve regeneration cycle. Thus, development of methods of inhibiting Schwann cell de-differentiation during Wallerian or Wallerian-like degeneration (Coleman & Freeman Citation2010) is essential for the treatment of peripheral degenerative or demyelinating disease.
In this study, we showed that EP (a simple, stable derivative of the endogenous metabolite pyruvate) inhibits Schwann cell de-differentiation during Wallerian degeneration. In previous studies, EP was reported to be effective against oxidative stress-related cellular injury (Varma et al. Citation1998), ischemia/reperfusion-induced epithelial injury (Sims et al. Citation2001), and systemic inflammation (Ulloa et al. Citation2002). Thus, because oxidative stress is related to Wallerian degeneration (Levy et al. Citation2001; Lee et al. Citation2007a; Campuzano et al. Citation2008) and EP protects against ROS and reduces inflammation, we hypothesized that EP might be effective for inhibiting Schwann cell de-differentiation.
During Wallerian degeneration, the MAP kinase pathway is activated in de-differentiated Schwann cells (Harrisingh et al. Citation2004; Monje et al. Citation2010; Yang et al. Citation2012), and EP suppresses activation of p38MAPK in RAW 264.7 cells (Song et al. Citation2004). In the present study, EP (10 mM) directly inhibited p-ERK1/2 in sciatic nerve explants (). Thus, inhibition of Schwann cell de-differentiation by EP may be a result of its suppression of MAP kinase. We also examined EP's effects on p75NGFR expression because this receptor has previously been implicated in oxidative stress; ROS activates p75NGFR, leading to neurodegeneration (Shin et al. Citation2011; Kraemer et al. Citation2014). Based on our data, we infer that EP's inhibition of p75NGFR expression in Schwann cells during Wallerian degeneration may result from its function as a ROS scavenger. Finally, oxidative stress increases lysosomal function, which supports antimicrobial activity and apoptotic cell death (Kurz et al. Citation2008; Yoon et al. Citation2011). Thus, inhibition of oxidative stress by EP may alter lysosomal function. In the present study, we found that EP, an inhibitor of oxidative stress, effectively suppresses Schwann cell de-differentiation during Wallerian degeneration. In addition, EP's inhibition of Schwann cell de-differentiation could be related to its prevention of Schwann cell entry into the nerve regeneration process. This effect is likely upstream of EP's suppression of Schwann cell proliferation in this ex vivo model.
In conclusion, we demonstrated that EP inhibits Schwann cell de-differentiation and proliferation in sciatic explants during Wallerian degeneration. EP-induced inhibition of Schwann cell de-differentiation and proliferation is likely a result of its reduction of oxidative stress. Thus, new therapeutic strategies related to EP may be helpful to treat peripheral degenerative or demyelinating diseases.
Disclosure
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alvarez G, Ramos M, Ruiz F, Satrústegui J, Bogónez E. 2003. Pyruvate protection against beta-amyloid induced neuronal death: role of mitochondrial redox state. J Neurosci Res. 73:260–269. doi: 10.1002/jnr.10648
- Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B. 2008. Distinct pattern of microglial response, cyclooxygenase-2, and inducible nitric oxide synthase expression in the aged rat brain after excitotoxic damage. J Neurosci Res. 86:3170–3183. doi: 10.1002/jnr.21751
- Chen CJ, Liao SL. 2003. Zinc toxicity on neonatal cortical neurons: involvement of glutathione chelation. J Neurochem. 85:443–453. doi: 10.1046/j.1471-4159.2003.01691.x
- Chen ZL, Yu WM, Strickland S. 2007. Peripheral regeneration. Annu Rev Neurosci. 30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337
- Coleman MP, Freeman MR. 2010. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 33:245–267. doi: 10.1146/annurev-neuro-060909-153248
- Desagher S, Glowinski J, Prémont J. 1997. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 17:9060–9067.
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. 2004. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 23:3061–3071. doi: 10.1038/sj.emboj.7600309
- Jessen KR, Mirsky R. 2008. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 56:1552–1565. doi: 10.1002/glia.20761
- Jung J, Cai W, Jang SY, Shin YK, Suh DJ, Kim JK, Park HT. 2011. Transient lysosomal activation is essential for p75 nerve growth factor receptor expression in myelinated Schwann cells during Wallerian degeneration. Anat Cell Biol. 44:41–49. doi: 10.5115/acb.2011.44.1.41
- Kawahara M, Kato-Negishi M, Kuroda Y. 2002. Pyruvate blocks zinc-induced neurotoxicity in immortalized hypothalamic neurons. Cell Mol Neurobiol. 22:87–93. doi: 10.1023/A:1015345813075
- Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, Ashok A, Mirshahidi S, Mercola D, Lilly MB, Wall NR. 2012. Plasma-derived exosomal surviv in, a plausible biomarker for early detection of prostate cancer. PLoS One. 7:e46737. doi:10.1371/journal.pone.0046737
- Kraemer BR, Snow JP, Vollbrecht P, Pathak A, Valentine WM, Deutch AY, Carter BD. 2014. A role for the p75 neurotrophin receptor in axonal degeneration and apoptosis induced by oxidative stress. J Biol Chem. 289:21205–21216. doi: 10.1074/jbc.M114.563403
- Kurz T, Terman A, Gustafsson B, Brunk UT. 2008. Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta. 1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009
- Lee H, Park C, Cho IH, Kim HY, Jo EK, Lee S, Kho HS, Choi SY, Oh SB, Park K, et al. 2007a. Double-stranded RNA induces iNOS gene expression in Schwann cells, sensory neuronal death, and peripheral nerve demyelination. Glia. 55:712–722. doi: 10.1002/glia.20493
- Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. 2007b. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 102:686–698. doi: 10.1111/j.1471-4159.2007.04580.x
- Lee HK, Seo IA, Suh DJ, Hong JI, Yoo YH, Park HT. 2009. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 108:776–786. doi: 10.1111/j.1471-4159.2008.05826.x
- Lee JK, Jin HK, Park MH, Kim BR, Lee PH, Nakauchi H, Carter JE, He X, Schuchman EH, Bae JS. 2014. Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer's disease. J Exp Med. 211:1551–1570. doi: 10.1084/jem.20132451
- Lee JY, Kim YH, Koh JY. 2001. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 21:RC171.http://www.jneurosci.org/content/21/20/RC171.full.pdf
- Levy D, Kubes P, Zochodne DW. 2001. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J Neuropathol Exp Neurol. 60:411–421.
- Miller KE, Richards BA, Kriebel RM. 2002. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 945:202–211. doi: 10.1016/S0006-8993(02)02802-0
- Monje PV, Soto J, Bacallao K, Wood PM. 2010. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J Biol Chem. 285:31024–31036. doi: 10.1074/jbc.M110.116970
- Park BS, Kim HW, Rhyu IJ, Park C, Yeo SG, Huh Y, Jeong NY, Jung J. 2015. Hydrogen sulfide is essential for Schwann cell responses to peripheral nerve injury. J Neurochem. 132:230–242. doi: 10.1111/jnc.12932
- Pfeiffer-Guglielmi B, Francke M, Reichenbach A, Hamprecht B. 2007. Glycogen phosphorylase isozymes and energy metabolism in the rat peripheral nervous system--an immunocytochemical study. Brain Res. 1136:20–27. doi: 10.1016/j.brainres.2006.12.037
- Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, Hong JS, Yoneda Y, Kim HC. 2011. Role of oxidative stress in epileptic seizures. Neurochem Int. 59:122–137. doi: 10.1016/j.neuint.2011.03.025
- Shin YH, Lee SJ, Jung J. 2013. Extracellular ATP inhibits Schwann cell dedifferentiation and proliferation in an ex vivo model of Wallerian degeneration. Biochem Biophys Res Commun. 430:852–857. doi: 10.1016/j.bbrc.2012.11.057
- Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. 2001. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 29:1513–1518. doi: 10.1097/00003246-200108000-00003
- Song M, Kellum JA, Kaldas H, Fink MP. 2004. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J Pharmacol Exp Ther. 308:307–316. doi: 10.1124/jpet.103.056622
- Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. 2002. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 99:12351–12356. doi: 10.1073/pnas.192222999
- Varma SD, Devamanoharan PS, Ali AH. 1998. Prevention of intracellular oxidative stress to lens by pyruvate and its ester. Free Radic Res. 28:131–135. doi: 10.3109/10715769809065799
- Vonkorff RW. 1964. Pyruvate-C14, purity and stability. Anal Biochem. 8:171–178. doi: 10.1016/0003-2697(64)90043-0
- Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH. 2007. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 1132:1–9. doi: 10.1016/j.brainres.2006.11.032
- Yang DP, Kim J, Syed N, Tung YJ, Bhaskaran A, Mindos T, Mirsky R, Jessen KR, Maurel P, Parkinson DB, Kim HA. 2012. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 32:7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012
- Yoon J, Bang SH, Park JS, Chang ST, Kim YH, Min J. 2011. Increased in vitro lysosomal function in oxidative stress-induced cell lines. Appl Biochem Biotechnol. 163:1002–1011. doi: 10.1007/s12010-010-9104-z
- Yu YM, Kim JB, Lee KW, Kim SY, Han PL, Lee JK. 2005. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 36:2238–2243. doi: 10.1161/01.STR.0000181779.83472.35
