Abstract
Effects of histamine receptors agonists or antagonists administered centrally (supraspinally or spinally) on immobilization stress-induced blood glucose levels were studied in Institute for Cancer Research mice. Mice were pretreated intracerebroventricularly (i.c.v.) or intrathecally (i.t.) with histamine (H1, H2, H3 and H4) receptor agonists or antagonists 10 min prior to immobilization stress (IMO) stimulation for 30 min. We found that the increased blood glucose level induced by IMO was attenuated by the i.c.v. pretreatment with α-methylhistamine (H3 agonist). However, H3 antagonist, H1, H2 and H4 receptor agonists and antagonists did not affect the blood glucose level. Also, i.t. pretreatment with α-methylhistamine or VUF 8430 attenuated the increased blood glucose level induced by IMO. In addition, cetirizine and carcinine enhanced the increased blood glucose level induced by IMO. However, the blood glucose level was not affected by H1 and H2 receptor agonists, H2 and H4 receptor antagonists. Corticosterone and insulin levels were also measured in α-methylhistamine (i.c.v or i.t) and with VUF 8430, carcinine and cetirizine (i.t.) treated group. We found that the corticosterone level was increased only by i.t treatment with α-methylhistamine, whereas the insulin level was attenuated by i.c.v or i.t. treatment with α-methylhistamine and i.t treatment with VUF 8430, carcinine and cetirizine. Our results suggest the active involvement of histamine receptor in blood glucose regulation in IMO.
Introduction
The stressful stimulus is unavoidable in modern life and has a negative influence on the cognitive and emotional functions (Chen et al. Citation2013). It has been demonstrated that stress influences the brain activity and induces neuroplastic changes in different neural systems (Seo et al. Citation2006). For example, stress can induce the release of various hormones and blood glucose levels (Surwit et al. Citation1992). In addition, stress results in the activation of the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) responses (Gadek-Michalska et al. Citation2011). It has been well known that the increased activity of the SNS is involved in the regulation of glucose metabolism. The alterations in glucose and lipid metabolism induced by stress can cause the development of diabetes mellitus (Uresin et al. Citation2004). The main regulator of the HPA axis is a corticotrophin-releasing hormone, finally causing a glucocorticoid secretion by the adrenal cortex (Kalantaridou et al. Citation2010).
Several studies have demonstrated that the various regions of the brain play pivotal roles to keep the homeostatic status of the blood glucose level (Benzo Citation1983; Sandoval et al. Citation2009). Supraspinal injection of atropine methyl bromide (methylatropine) exerts the modulatory action for the regulation of the blood glucose and insulin levels (Ohnuma et al. Citation1996). Lux et al. reported that the effect of intrathecally (i.t.) morphine may be facilitated by the production of a profound hypoglycemia, which involves a spinal, rather than supraspinal or systemic, action of morphine (Citation1988). Furthermore, in our recent study, we reported that pro-inflammatory cytokines such as IL–1β spinally administered leads to an elevation of the blood glucose level (Sim et al. Citation2012).
Several lines of evidence suggest that the regulation of blood glucose level is one of the important function of histamine system. Histamine H1 receptors stimulate adrenaline and noradrenaline secretions (Kuzmin et al. Citation1999). Histamine H1 receptors stimulate glycogenolysis as inducing a rapid increase in inositol 1,4,5-trisphosphate levels and intracellular Ca2+ concentration (Mine et al. Citation1991; Cheng et al. Citation2000). These reactions represent hyperglycemic effect through an increase in the sympathetic outflow and catecholamine secretion (Nishibori et al. Citation1987). However, histamine H2 receptors show widespread expression in the brain and spinal cord. Histamine H2 receptors stimulate noradrenaline release, but not adrenaline (Kuzmin et al. Citation1999). Histamine H3 receptors located on various central and peripheral nerves negatively regulates the release of norepinephrine and acetylcholine in the central nervous system (CNS) (Molderings et al. Citation1992; Tokita et al. Citation2006). Strakhova et al. reported that the transcripts of H4 receptor are present in all analyzed regions of the human CNS, including spinal cord, hippocampus, cortex, thalamus and amygdala, with the highest levels of H4 messenger ribonucleic acid (mRNA) detected in the spinal cord. Moreover, in rat, H4 mRNA was detected in cortex, cerebellum, brainstem, amygdala, thalamus and striatum. And also observed that H4 mRNA was highly expressed in rat dorsal root ganglia and spinal cord (Strakhova et al. Citation2009). Another study reported that H3 and H4 receptors are expressed in rat brain endothelial cells, and activation of the histamine H4 receptor activates the Erk (extracellular signal-regulated kinases) 1/2 pathway. H3 and H4 receptors in endothelial cells are important for regulation of blood–brain barrier permeability, including trafficking of immunocompetent cells (Karlstedt et al. Citation2013). However, the role of H4 in blood glucose regulation is not clearly known.
The peripheral and central actions of histamine receptors have been well studied and characterized. However, the central action of histamine receptors on the blood glucose level regulation in stress model has not been well known yet. Thus, the aim of the present study was to examine the effects of histamine receptors activator and inactivator spinally or supraspinally administered on the regulation of the blood glucose level in the immobilization stress (IMO) model.
Materials and methods
These experiments were approved by the Hallym University Animal Care and Use Committee (Registration Number: Hallym 2009-05-01). All procedures were conducted in accordance with the ‘Guide for Care and Use of Laboratory Animals' published by the National Institutes of Health and the ethical guidelines of the International Association for the Study.
Experimental animals
Male Institute for Cancer Research mice (MJ Co., Seoul, Korea) weighing 20–25 g were used for all the experiments. Five animals were housed per cage in a room maintained at 22 ± 0.5°C with an alternating 12 h light–dark cycle. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 h before testing and were only used once. Experiments were performed during the light phase of the cycle (10:00–17:00). The animals were fasted for 16 h.
Intracerebroventricular and intrathecal injections
The i.t. injections were made as stated by Hylden and Wilcox (Citation1981) using a 25 µl Hamilton syringe with a 30-gage needle. The intracerebroventricular (i.c.v.) administration was performed according to Haley and McCormick (Citation1957). The i.c.v. and i.t. injection volumes were 5 µl and the injection sites were verified by injecting a similar volume of 1% methylene blue solution and determining the distribution of the injected dye in the ventricular space or in the spinal cord. The dye injected i.c.v. was observed to be dispersed through the ventricular spaces and reached the ventral surface of the brain and the upper cervical portion of the spinal cord. The dye injected i.t. was distributed both rostrally and caudally but within a short distance (about 0.5 cm) and no dye was found visually in the brain. The success rate for prior injections with this technique was over 95%.
Drugs
2-Pyridylethylamine, cetirizine, dimaprit, ranitidine, α-methylhistamine, carcinine, VUF 8430 and JNJ 7777120 were purchased from Tocris Bioscience Co. (Minneapolis, MN, USA). 2-Pyridylethylamine, cetirizine, dimaprit, ranitidine, α-methylhistamine, carcinine and VUF 8430 were dissolved in saline. JNJ 7777120 was prepared following steps: (a) 1 g of JNJ 7777120 was dissolved in 0.5 ml of ethanol plus 0.5 ml of polyethylene glycol 400. (b) Separately, 100 mg of sodium carboxymethylcellulose was dissolved in 9 ml of distilled water. (c) Finally, Solution (a) and Solution (b) were vigorously mixed. This solution (polyethylene glycol 400 Ethanol Carboxymethyl Cellulose) excluding JNJ 7777120 was used as vehicle control. All drugs were prepared just before use. Blood glucose meter, lancing device and strips were purchased from Roche Diagnostics (Sandhofer Strasse, Mannheim, Germany).
IMO procedure
The mice were subjected to restraint stress as mentioned in a study by Sim et al. (Suh et al. Citation2000). In brief, mouse was placed in a 50 ml corning tube, and adjusting it with an iron nail on the outside, which crossed in the caudal part of the animal. Adequate ventilation was provided by means of holes at the sides of the tubes. The mice were stressed by restraint for 30 min. In the stress model, there was a single exposure.
Measurement of the blood glucose level
The blood glucose level was measured at 30, 60 and 120 min after the IMO initiated. The blood was collected shortly as much as possible with a minimum volume (1 µl) from the tail-vein. The glucose level was quantified by using Accu-Chek Performa blood glucose monitoring system (glucometer) (Mannheim, Baden-Württemberg, Germany).
Corticosterone assay and blood sampling
The plasma corticosterone level was measured by the fluorometric determination method (Glick et al. Citation1964). Four hundred microliters of blood were collected by puncturing the retro-orbital venous plexus. Plasma was separated by centrifugation and stored at ‒80°C until assayed.
Insulin ELISA assay
In Mouse Insulin enzyme-linked immunosorbent Assay (ELISA), biotin-conjugated anti-insulin and standard or samples are incubated in monoclonal anti-insulin-coated wells to capture insulin bound with biotin-conjugated anti-insulin. After 2 h incubation and washing, horseradish peroxidase (HRP)-conjugated streptavidin is added, and incubated for 30 min. After washing, HRP-conjugated streptavidin remaining in wells are reacted with a substrate chromogen reagent (3,3', 5,5'-tetramethylbenzidine) for 20 min, and reaction is stopped by the addition of acidic solution, and absorbance of yellow product is measured spectrophotometrically at 450 nm. The absorbance is proportional to insulin concentration. The standard curve is prepared by plotting absorbance against standard insulin concentrations. Insulin concentrations in unknown samples are determined using this standard curve.
Statistical analysis
All values were expressed as mean ± SEM. The statistical significance of differences between groups was assessed with one-way ANOVA with Bonferroni's post-hoc test using GraphPad Prism Version 4.0 for Windows XP (GraphPad Software, San Diego, CA, USA). P-value less than .05 were considered to be statistically significant. In our study, the mean blood glucose value of the control group was established through several experiments under matching conditions. Selected mice of established blood glucose level were then used in replication experiments.
Results
Effects of histamine receptor agonists or antagonists administered i.c.v on the blood glucose level in IMO model
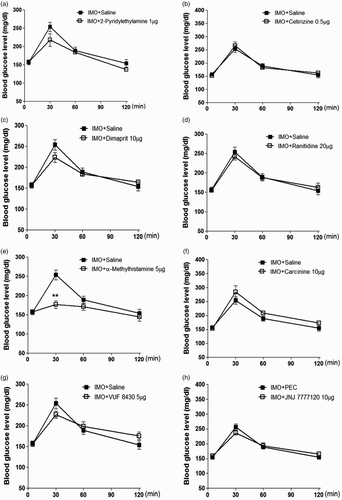
Doses of histamine agonist and antagonist were selected according to our previous study (Sim et al. Citation2014). Mice were pretreated i.c.v. with 2-pyridylethylamine (1 µg), cetirizine (0.5 µg), dimaprit (10 µg), ranitidine (20 µg), α-methylhistamine (5 µg), carcinine (10 µg), VUF 8430 (5 µg) or JNJ 7777120 (10 µg) for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. The blood glucose level was measured at 30, 60 and 120 min after IMO initiated. As shown in (e), α-methylhistamine pretreated i.c.v. attenuated the elevation of the blood glucose level induced by IMO. However, the elevation of the blood glucose level induced by IMO was not affected by 2-pyridylethylamine, cetirizine, dimaprit, ranitidine, carcinine, VUF 8430 and JNJ 7777120 ((a), 1(b), 1(c), 1(d), 1(f), 1(g) and 1(h), respectively). Our results indicated that histamine H3 receptor supraspinally located may be involved in blood glucose regulation during IMO.
Figure 1. Mice were pretreated i.c.v. with of 2-pyridylethylamine (1 µg), cetirizine (0.5 µg), dimaprit (10 µg), ranitidine (20 µg), methylhistamine (5 µg), carcinine (10 µg), VUF 8430 (5µg) and JNJ7777120 (10 µg) for 10 min. Then, mice were enforced into IMO for 30 min and returned to the cage. The blood glucose level was measured at 30, 60 and 120 min after the IMO, (a)–(h), respectively. The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean. **P < .01; compared to IMO + saline group. The number of animals used for each group was 8.
Effects of histamine receptor agonist or antagonist administered i.t. on the blood glucose level in the IMO model
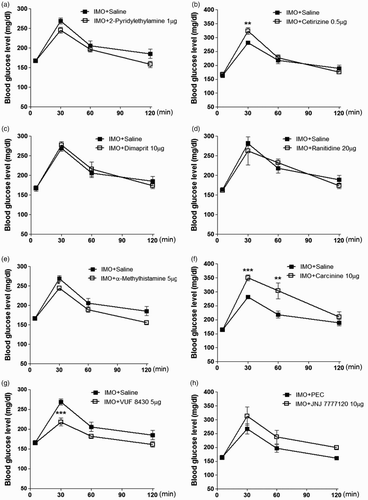
Doses of histamine agonist and antagonist were selected according to our previous study by Sim et al. (Citation2014). Mice were pretreated i.t. with 2-pyridylethylamine (1 µg), cetirizine (0.5 µg), dimaprit (10 µg), ranitidine (20 µg), α-methylhistamine (5 µg), carcinine (10 µg), VUF 8430 (5 µg) or JNJ 7777120 (10 µg) for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. The blood glucose level was quantified at 30, 60 and 120 min after IMO initiated. As shown in (b) and 2(f), cetirizine or carcinine pretreated i.t. enhanced the elevation of the blood glucose level induced by IMO. However, α-methylhistamine and VUF 8430 attenuated the elevation of the blood glucose level induced by IMO ((e) and 2(g)). In addition, 2-pyridylethylamine, dimaprit, ranitidine or JNJ 7777120 did not affect the elevation of the blood glucose level induced by IMO ((a), 2(c), 2(d) and 2(h)), respectively. These results suggest that H1, H3 and H4 receptors spinally located are involved in blood glucose regulation during IMO.
Figure 2. Effect of 2-pyridylethylamine (1 µg), cetirizine (0.5 µg), dimaprit (10 µg), ranitidine (20 µg), methylhistamine (5 µg), carcinine (10 µg), VUF 8430 (5 µg) and JNJ7777120 (10 µg) administered i.t.. Then, the mice were enforced into IMO for 30 min and returned to the cage. The blood glucose level was measured at 30, 60 and 120 min after the IMO, (a)–(h). The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean. *P < .05; compared to saline group, **P < .01, ***P < .005; compared to IMO + saline group. The number of animals used for each group was 8.
Effects of histamine receptor (H3) agonist α-methylhistamine administered i.c.v. or i.t. on plasma corticosterone and insulin levels induced by IMO
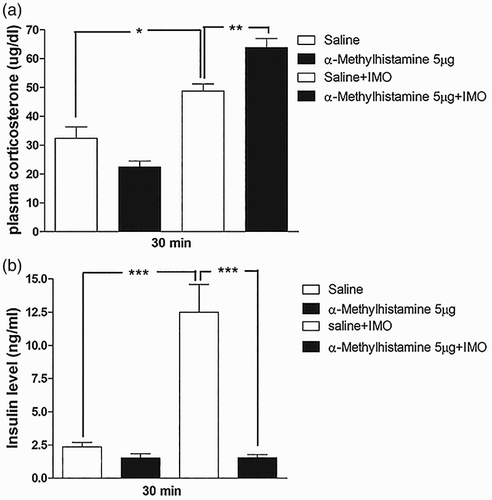
As shown in (e) and (e), α-methylhistamine administered i.c.v. or i.t. attenuated the blood glucose level induced by IMO. Thus, further, we evaluated the plasma corticosterone and insulin levels at 30 min after IMO. We found that the plasma corticosterone level was increased after 30 min of IMO, but not affected by i.c.v pretreatment with α-methylhistamine (5 µg; (a)). However, i.t. pretreated with α-methylhistamine (5 µg) significantly increases the plasma corticosterone level after 30 min of IMO ((a)). In addition, we observed that insulin level was significantly reduced by i.c.v or i.t. pretreatment with α-methylhistamine (5 µg) after 30 min of IMO ((b) and (b), respectively). These findings indicate that the corticosterone level increased by IMO is just to supply the increased demand of energy to the organs during stress. These results represent that insulin and corticosterone may not be involved in the attenuation of blood glucose level after 30 min of IMO. However, corticosterone and insulin may help in blood glucose homeostasis against lowering effect of α-methylhistamine.
Figure 3. Effect of α-methylhistamine administered i.c.v. on plasma corticosterone and plasma insulin levels in IMO model. Mice were pretreated i.c.v. with 5 µg of α-methylhistamine for 10 min. Then, mice were enforced into IMO for 30 min and returned to the cage. Plasma corticosterone and insulin levels were also measured at 30 min after IMO ((a) and 3(b), respectively). The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean (*P < .05, **P < .01, ***P < .005; compared to saline + IMO group), compared to IMO + saline group). The number of animals used for each group was 8.
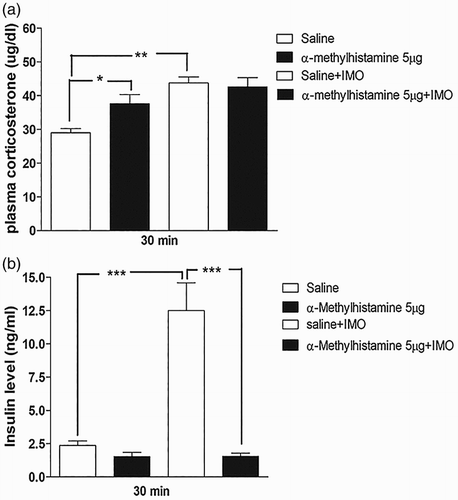
Figure 4. Effect of α-methylhistamine administered i.t. on plasma corticosterone and plasma insulin levels in the IMO model. Mice were pretreated i.t. with 5 µg of α-methylhistamine for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. Plasma corticosterone and insulin levels were also measured at 30 min after IMO ((a) and 4(b), respectively). The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean (*P < .05, **P < .01, ***P < .005; compared to saline + IMO group). The number of animals used for each group was 8.
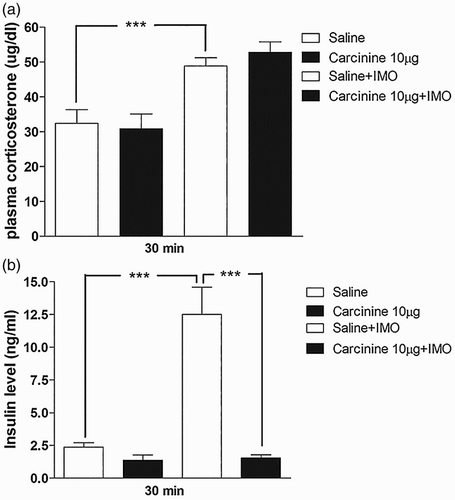
Effects of histamine receptor (H3) antagonist carcinine administered i.t. on plasma corticosterone and insulin levels induced by IMO
As described above, i.t. pretreated with carcinine further enhanced the blood glucose level ((f)). Therefore, in further investigation, mice were only pretreated i.t. with carcinine (10 µg) for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. The plasma corticosterone and insulin levels were measured at 30 min after IMO initiated. (a) and 5(b), respectively, shows that corticosterone and insulin levels were significantly increased after IMO as compared to the control group. Carcinine pretreated i.t. has no effect on the corticosterone level, whereas it significantly reduced the insulin level. It indicates that H3 antagonist increases the plasma glucose level after 30 min of IMO via an insulin-mediated pathway.
Figure 5. Effect of carcinine administered i.t. on plasma corticosterone and plasma insulin levels in the IMO model. Mice were pretreated i.t. with 10 µg of cetirizine for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. Plasma corticosterone and insulin levels were also measured at 30 min after IMO in carcinine i.t. pretreated mice ((a) and 5(b), respectively). The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean (***P < .005; compared to saline + IMO group). The number of animal used for each group was 8.
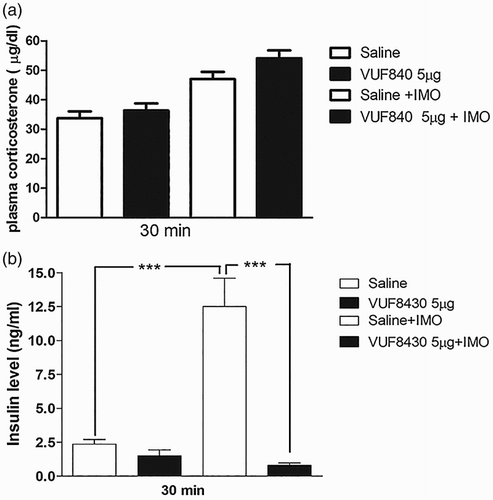
Effects of histamine receptor (H4) agonist VUF 8430 administered i.t. on plasma corticosterone and insulin levels induced by IMO
VUF 8430 (5 µg) administered i.t. attenuated the blood glucose level, whereas VUF 8430 administered i.c.v did not affect the blood glucose level after 30 min of IMO. Furthermore, the plasma corticosterone and insulin levels were investigated at 30 min after IMO initiated, in i.t. pretreated with VUF 8430. (a) shows that the corticosterone level was not altered after i.t. pretreatment with VUF 8430. In addition, the plasma insulin level was significantly downregulated by the i.t. pretreatment with VUF 8430 after IMO ((b)).
Figure 6. Effect of VUF 8430 administered i.t. on the plasma corticosterone and insulin levels in the IMO model. Mice were pretreated i.t. with 5 µg of VUF 8430 for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. Plasma corticosterone and insulin levels were also measured at 30 min after IMO in VUF 8430 i.t. pretreated mice ((a) and 6(b), respectively). The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean (***P < .005; compared to saline + IMO group). The number of animal used for each group was 8.
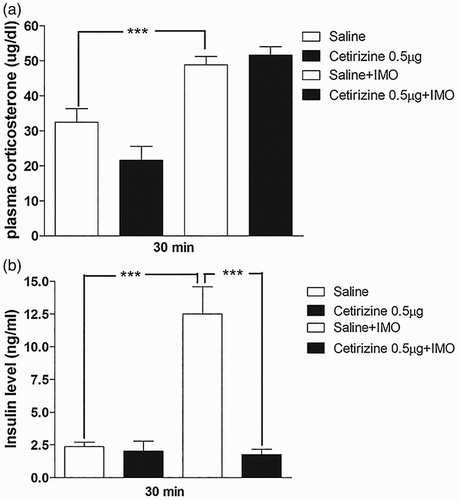
Effects of histamine receptor (H1) antagonist cetirizine administered i.t. on plasma corticosterone and insulin levels induced by IMO
As i.t. pretreated cetirizine caused an elevation of the blood glucose level as shown in (b), we further investigated the plasma corticosterone and insulin levels at 30 min after IMO initiated, in i.t. pretreated with cetirizine (0.5 µg). As shown in (a), cetirizine did not affect the corticosterone level. However, cetirizine significantly decreased the plasma insulin level ((b)). It indicates that histamine receptor (H1) antagonist increases the plasma glucose level after 30 min of IMO via an insulin-mediated pathway.
Figure 7. Effect of cetirizine administered i.t. on plasma corticosterone and insulin in the IMO model. Mice were pretreated i.t. with 0.5 µg of cetirizine for 10 min. Then, the mice were enforced into IMO for 30 min and returned to the cage. Plasma corticosterone and insulin levels were measured at 30 min after IMO in cetirizine i.t. pretreated mice ((a) and 7(b), respectively). The blood was collected from tail-vein. The vertical bars indicate the standard error of the mean (***P < .005; compared to saline + IMO group). The number of animal used for each group was 8.
Discussion
Numerous previous studies reported that central histamine receptors are related to glucose regulation. In the molecular level, histamine stimulates glycogenolysis, gluconeogenesis from lactate and ureagenesis in rat hepatocytes (Cheng et al. Citation2000). In addition, many previous studies reported histamine receptors are related with glucose regulation in stress. Kuzmin et al. reported that histamine H2 receptor antagonist (ranitidine) pretreated intravenously suppresses stress-induced noradrenaline secretion without affecting adrenaline (Citation1999). Histamine plays a role in mediating the stress-induced release of hormones such as adrenocorticotropic hormone (ACTH) and the central release of noradrenaline and serotonin (Brown et al. Citation2001). Central histaminergic neurons participate in the release of ACTH, prolactin, adrenal catecholamines and renin by stress (Carrasco & Van de Kar Citation2003), which may directly or indirectly affect blood glucose homeostasis.
In our previous study, we reported that the spinally located histamine H2 receptor activation and histamine receptors H1 or H3 blockade play a modulatory role in blood glucose regulation in D-glucose fed mice model (Sim et al. Citation2014). In the present study, we found that the elevation of the blood glucose level induced by IMO was attenuated by i.c.v. treatment with α-methylhistamine (a histamine H3 receptor agonist). However, 2-pyridylethylamine (a histamine H1 receptor agonist), cetirizine (a histamine H1 receptor antagonist), dimaprit (a histamine H2 receptor agonist), ranitidine (a histamine H2 receptor antagonist), carcinine (a histamine H3 receptor antagonist), VUF 8430 (a histamine H4 receptor agonist) or JNJ 7777120 (a histamine H4 receptor antagonist) have no effect. In addition to this, α-methylhistamine i.c.v. pretreatment did not affect the corticosterone level, but it reduces the insulin level after IMO. This finding indicates that the increase in the corticosterone level leads to increase in the blood glucose level during IMO to supply more energy to the body. Moreover, the elevation of insulin level appears to maintain a homeostatic regulation of the blood glucose in IMO. This finding indicates that the corticosterone and insulin are not involved in blood glucose attenuation by α-methylhistamine. Previous studies have shown that a single IMO application significantly increases the circulating level of corticosterone, glucose, insulin, glycerol and ketone bodies, which is the typical response to acute stress (Ricart-Jane et al. Citation2002). And also in our previous study, we similarly observed that the increase in the blood glucose level after acute IMO was accompanied by a rise in the plasma insulin level. The elevation of the insulin level appears to maintain a homeostatic regulation of the blood glucose in IMO (Sharma et al. Citation2015). In an earlier reported study, supraspinally administration with histamine H3 receptor agonist (proxyfan) significantly decreases the glucose level compared with control mice (Henry et al. Citation2011). These findings suggest that histamine H3 receptor activation in the brain appears to play an essential role in the regulation of the blood glucose level. In contrast to the results with involvement of histamine H3 receptor agonist in the regulation of the blood glucose level, i.c.v. administration of histamine H1, H2, H4 and H3 receptor antagonists appears not to be involved in the regulation of the blood glucose level.
In our previous study, we already demonstrated the role of spinally located histamine receptor in regulation of the blood glucose level in D-glucose fed mice model (Sim et al. Citation2014). In the present study, we found that i.t. treatment with cetirizine enhanced the blood glucose level, whereas 2-pyridylethylamine did not affect the elevation of the blood glucose level induced by IMO, suggesting that the inactivation of histamine H1 receptors located at the spinal cord exert a modulatory effect on the blood glucose regulation in a potent manner in the IMO model. In addition, we found that i.t. treatment with cetirizine did not affect the corticosterone level, but it decreases the insulin level induced by IMO, suggesting that the inactivation of H1 receptor and simultaneous down-regulation of insulin may cause hyperglycemic effect in the IMO model. Moreover, i.t. treatment with dimaprit and ranitidine did not affect the elevation of the blood glucose level, suggesting that histamine H2 receptors located on the spinal cord did not exert a modulatory effect on the blood glucose regulation in IMO model.
In addition, we found that i.t. treatment with α-methylhistamine attenuates the blood glucose level, whereas carcinine enhanced the elevation of the blood glucose level induced by IMO. Moreover, the attenuation of blood glucose is not dependent on corticosterone and insulin mechanism by i.t. treatment with α-methylhistamine. Blockade of H1 receptor and 5HT 2c receptor by antipsychotic may increase the body weight of fatty tissue and also may result in insulin resistance (Wirshing et al. Citation1998), whereas in our study, hyperglycemic effect of pretreatment with carcinine may be due to insulin mechanism. These data suggest that the activation or inactivation of histamine H3 receptors located on the spinal cord exerts a modulatory effect on the blood glucose regulation in the IMO model.
Finally, i.t. treatment with VUF 8430 attenuated the blood glucose level, whereas JNJ 7777120 did not affect the elevation of the blood glucose level induced by IMO, suggesting that the activation of histamine H4 receptors located at the spinal cord exerts a modulatory effect on the blood glucose regulation in a potent manner in the IMO model. Our study also suggests that corticosterone and insulin are not involved in the attenuation of blood glucose level by VUF 8430.
We concluded that the selective histamine receptors exert their hyperglycemic or hypoglycemic effects, at least by acting directly on the brain regions as well as on the spinal cord in the IMO model. However, the exact mechanism involved in up-regulation or down-regulation of the blood glucose level induced by some histamine receptor activators or inactivators supraspinally or spinally administered in the IMO model is currently obscure. Furthermore, histamine receptors (H1, H2, H3 and H4) belong to the same class sharing a similar pharmacological action and have different effects on modulating the blood glucose level in the IMO model. Additional studies to describe the exact mechanism associated with centrally administered histamine receptors-induced glucose level alteration in IMO model is required in the future.
Disclosure
No potential conflict of interest was reported by the authors.
Funding
This research was supported by Priority Research Centers (NRF-2009-0094071) Program through the National Research Foundation of Korea (NRF) funded the Ministry of Education, Science and Technology and Hallym University Fund (HRF-201403-014).
References
- Benzo CA. 1983. Minireview. The hypothalamus and blood glucose regulation. Life Sci. 32:2509–2515. doi: 10.1016/0024-3205(83)90231-X
- Brown RE, Stevens DR, Haas HL. 2001. The physiology of brain histamine. Prog Neurobiol. 63:637–672. doi: 10.1016/S0301-0082(00)00039-3
- Carrasco GA, Van de Kar LD. 2003. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 463:235–272. doi: 10.1016/S0014-2999(03)01285-8
- Chen Y, Kramar EA, Chen LY, Babayan AH, Andres AL, Gall CM, Lynch G, Baram TZ. 2013. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry. 18:485–496. doi: 10.1038/mp.2012.17
- Cheng JS, Lee KC, Wang JL, Tseng LL, Chou KJ, Tang KY, Jan CR. 2000. Histamine-induced increases in intracellular free Ca2+ levels in hepatoma cells. Chin J Physiol. 43:165–169.
- Gadek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. 2011. Effect of prior stress on interleukin-1beta and HPA axis responses to acute stress. Pharmacol Rep. 63:1393–1403. doi: 10.1016/S1734-1140(11)70703-4
- Glick D, Vonredlich D, Levine S. 1964. Fluorometric determination of corticosterone and cortisol in 0.02–0.05 milliliters of plasma or submilligram samples of adrenal tissue. Endocrinology. 74:653–655. doi: 10.1210/endo-74-4-653
- Haley TJ, McCormick WG. 1957. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x
- Henry MB, Zheng S, Duan C, Patel B, Vassileva G, Sondey C, Lachowicz J, Hwa JJ. 2011. Antidiabetic properties of the histamine H3 receptor protean agonist proxyfan. Endocrinology. 152:828–835. doi: 10.1210/en.2010-0757
- Hylden JL, Wilcox GL. 1981. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 217:212–215. doi: 10.1016/0006-8993(81)90203-1
- Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. 2010. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 85:33–39. doi: 10.1016/j.jri.2010.02.005
- Karlstedt K, Jin C, Panula P. 2013. Expression of histamine receptor genes Hrh3 and Hrh4 in rat brain endothelial cells. Br J Pharmacol. 170:58–66. doi: 10.1111/bph.12173
- Kuzmin AI, Zaretsky DV, Kalenikova EI, Zaretskaja MV, Medvedev OS, Chazov EI. 1999. The effect of histamine receptor antagonists on stress-induced catecholamine secretion: an adrenomedullary microdialysis study in the rat. Eur J Pharmacol. 378:311–316. doi: 10.1016/S0014-2999(99)00467-7
- Lux F, Brase DA, Dewey WL. 1988. Differential effects of subcutaneous and intrathecal morphine administration on blood glucose in mice: comparison with intracerebroventricular administration. J Pharmacol Exp Ther. 245:187–194.
- Mine T, Kojima I, Ogata E. 1991. Mechanism of glycogenolytic action of histamine in rat hepatocytes. Am J Physiol. 261:G1000–1004.
- Molderings GJ, Weissenborn G, Schlicker E, Likungu J, Gothert M. 1992. Inhibition of noradrenaline release from the sympathetic nerves of the human saphenous vein by presynaptic histamine H3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 346:46–50. doi: 10.1007/BF00167569
- Nishibori M, Itoh Y, Oishi R, Saeki K. 1987. Mechanism of the central hyperglycemic action of histamine in mice. J Pharmacol Exp Ther. 241:582–586.
- Ohnuma H, Yamatani K, Igarashi M, Sugiyama K, Manaka H, Tominaga M, Sasaki H. 1996. Intracerebroventricular injection of methylatropine suppresses insulin response to oral glucose load in rats. J Auton Nerv Syst. 57:43–48. doi: 10.1016/0165-1838(95)00101-8
- Ricart-Jane D, Rodriguez-Sureda V, Benavides A, Peinado-Onsurbe J, Lopez-Tejero MD, Llobera M. 2002. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism. 51:925–931. doi: 10.1053/meta.2002.33353
- Sandoval DA, Obici S, Seeley RJ. 2009. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov. 8:386–398. doi: 10.1038/nrd2874
- Seo YJ, Kwon MS, Shim EJ, Park SH, Choi OS, Suh HW. 2006. Changes in pain behavior induced by formalin, substance P, glutamate and pro-inflammatory cytokines in immobilization-induced stress mouse model. Brain Res Bull. 71:279–286. doi: 10.1016/j.brainresbull.2006.09.012
- Sharma N, Sim YB, Park SH, Lim SM, Kim SS, Jung JS, Hong JS, Suh HW. 2015. Effect of sulfonylureas administered centrally on the blood glucose level in immobilization stress model. Korean J Physiol Pharmacol. 19:197–202. doi: 10.4196/kjpp.2015.19.3.197
- Sim YB, Park SH, Kang YJ, Jung JS, Ryu OH, Choi MG, Suh HW. 2012. Interleukin-1beta (IL-1beta) increases pain behavior and the blood glucose level: possible involvement of sympathetic nervous system. Pharmacol Biochem Behav. 102:170–176. doi: 10.1016/j.pbb.2012.04.006
- Sim YB, Park SH, Kim SS, Kim CH, Kim SJ, Lim SM, Jung JS, Ryu OH, Choi MG, Suh HW. 2014. The modulatory role of spinally located histamine receptors in the regulation of the blood glucose level in d-glucose-fed mice. Korean J Physiol Pharmacol. 18:41–46. doi: 10.4196/kjpp.2014.18.1.41
- Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD, Bitner RS. 2009. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 1250:41–48. doi: 10.1016/j.brainres.2008.11.018
- Suh HW, Song DK, Huh SO, Kim YH. 2000. Involvement of dynorphin in immobilization stress-induced antinociception in the mouse. Eur Neuropsychopharmacol. 10:407–413. doi: 10.1016/S0924-977X(00)00102-4
- Surwit RS, Schneider MS, Feinglos MN. 1992. Stress and diabetes mellitus. Diabetes Care. 15:1413–1422. doi: 10.2337/diacare.15.10.1413
- Tokita S, Takahashi K, Kotani H. 2006. Recent advances in molecular pharmacology of the histamine systems: physiology and pharmacology of histamine H3 receptor: roles in feeding regulation and therapeutic potential for metabolic disorders. J Pharmacol Sci. 101:12–18. doi: 10.1254/jphs.FMJ06001X4
- Uresin Y, Erbas B, Ozek M, Ozkok E, Gurol AO. 2004. Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J Renin Angiotensin Aldosterone Syst. 5:93–96. doi: 10.3317/jraas.2004.017
- Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC. 1998. Novel antipsychotics and new onset diabetes. Biol Psychiatry. 44:778–783. doi: 10.1016/S0006-3223(98)00100-0
