Abstract
The nicotinic acetylcholine receptor (nAChR) is a member of the ligand-gated ion channel (LGIC) family and is composed of five subunits arranged around a central pore. Expressed sequence tag screening and traditional cloning strategies revealed five full-length cDNAs encoding nAChR subunit homologs (Pajα3, Pajα10, Pajα11, Pajα12, and Pajβ1) in the Morotoge shrimp, Pandalopsis japonica. The nAChR subunits exhibited common structural characteristics, including a signal peptide sequence, a large N-terminal extracellular domain with conserved motifs for ligand binding (loops A–F), and a transmembrane (TM) domain with four hydrophobic TM motifs (TM1–TM4). Based on the conserved GEK motifs located just before TM2, all five nAChR subunits from P. japonica appear to be cation-selective ion channels. Among the five subunits, Pajα3 and Pajβ1 clustered together with insect core groups, whereas Pajα10, Pajα11, and Pajα12 were classified as a divergent group. Three distinct transcripts were identified in Pajα3, presumably due to alternative splicing between TM3 and TM4, which may be involved in channel formation with other subunits. All five nAChR subunits were expressed predominantly in neuronal tissues, including the brain, sinus gland/X-organ complex, thoracic ganglia, and abdominal ganglia, with no significant differences in subunit expression levels among the neuronal tissues. The five shrimp nAChR subunits could not be functionally expressed in Xenopus oocytes, but coexpression of Pajβ1 and rat α4 subunit (Rα4) formed functional channels responding to acetylcholine. Functional expression of vertebrate α subunit (Rα4) with invertebrate β1 subunit (Pajβ1) will expand our knowledge regarding the structural characteristics and molecular gating mechanism of invertebrate nAChRs.
Introduction
The nicotinic acetylcholine receptor (nAChR), a member of the Cys-loop ligand-gated ion channel (LGIC) superfamily, mediates chemical neurotransmission in the nervous system and at neuromuscular junctions (Jones & Sattelle Citation2010). The functional nAChR is composed of five subunits arranged around a central pore (Jones & Sattelle Citation2010). In vertebrates, a total of 17 subunits have been identified (α1–α10, β1–β4, γ, δ, and ε), and nAChRs with various subunit combinations exhibit different pharmacological characteristics and physiological functions (Jones & Sattelle Citation2010; Karlin Citation2002; Millar & Gotti Citation2009; Tomizawa & Casida Citation2001). Mammalian nAChRs are potential targets for several therapeutic approaches, including the treatment of nicotine addiction, Alzheimer's disease, schizophrenia, and Parkinson's disease as well as the control of pain, and thus their functional assembly and gating mechanism have been extensively investigated (Arneric et al. Citation2007; Gotti et al. Citation2006). Invertebrate nAChRs also have potential for pharmaceutical or industrial applications. However, relatively little is known about nAChRs from invertebrates.
Insect nAChRs are among the best studied of the invertebrate receptors, mainly because of the agricultural and pathological importance (Matsuda et al. Citation2001; Millar & Denholm Citation2007). Recent genome projects have revealed the total numbers of nAChR subunits in several insect species, including Drosophila melanogaster, Anopheles gambiae, Apis mellifera, Bombyx mori, and Tribolium castaneum (Dupuis et al. Citation2012; Jones et al. Citation2005; Jones et al. Citation2006; Jones & Sattelle Citation2007, Citation2010; Millar Citation2003; Shao et al. Citation2007; Tomizawa & Casida Citation2001). Insect nAChRs exhibit unique characteristics, which are different from those of vertebrates. Insects have fewer nAChR subunits (10–12 subunits) compared with mammals (17 subunits). In addition, only one or two β subunits are present in insects, whereas four β subunits have been identified in mammals (Jones & Sattelle Citation2010). However, each insect nAChR subunit has transcriptional variants produced by either alternative splicing or RNA editing, which may contribute to diverse nAChRs in insect species (Dupuis et al. Citation2012).
Although a number of invertebrate nAChR subunit nucleotide sequences have been determined, functional studies have been limited because of the inability to express physiologically functional invertebrate nAChRs using heterologous systems such as Xenopus laevis oocytes or cell lines (Dupuis et al. Citation2012; Millar Citation1999). Although the Sgα1 subunit from the locust Schistocerca gregaria and Mpα1 and Mpα2 from the aphid Myzus persicae formed homopentameric channels when expressed in Xenopus laevis oocytes (Amar et al. Citation1995; Marshall et al. Citation1990; Sgard et al. Citation1998). Invertebrate subunits have failed to form heteromeric functional channels, which is contrast to vertebrate nAChRs. Alternatively, the co-expression of insect α subunits with a vertebrate neuronal β subunit has been successful in heterologous expression systems such as Drosophila S2 cells and Xenopus laevis oocytes (Bertrand et al. Citation1994; Lansdell & Millar Citation2004; Lansdell et al. Citation1997). However, there have been no reports of the functional expression of an insect β subunit with either vertebrate or invertebrate subunits (Yao et al. Citation2009). As both α and non-α subunits contribute to the affinity of nAChRs for nicotinic ligands, the β subunit must be included for the functional expression of invertebrate nAChRs (Lansdell & Millar Citation2000b).
In the present study, we identified five full-length cDNAs encoding nAChR subunits from the crustacean Pandalopsis japonica. A comparative structural analysis and a constructed phylogenetic tree indicated that one α and one β subunit are closely related to the insect core group, whereas three α subunits appear to be part of a divergent group. Tissue distribution profiles showed that all nAChR subunits from P. japonica are expressed in neuronal tissues. Finally, hybrid expression of Pajβ1 with rat α4 was successful using the Xenopus oocyte expression system, which will be useful for studying the role of the β subunit in invertebrate nAChRs.
Materials and methods
Animals
We purchased P. japonica from a local seafood market, and inter-molt animals were used for tissue collection. Xenopus laevis were purchased from Xenopus One (Whitmore Lake, MI) and acclimatized at 20°C for 2 weeks prior to oocyte harvest. Xenopus care and handling were in accordance with institutional guidelines (Wu & Gerhart Citation1991). The experimental frogs underwent no more than two surgeries, separated by at least one month. Frogs were anesthetized by immersion in 0.2% MS-222 (Sigma, St Louis, MO), and stage V or VI oocytes were surgically removed and collected for cRNA injection.
Bioinformatics analysis of nAChR subunits from P. japonica
Partial contigs encoding nAChR subunits were obtained by bioinformatics screening. Construction of a cDNA sequence database of neuronal tissues from P. japonica was described previously (Kim et al. Citation2012). The contigs encoding nAChR homologs were obtained by similarity searches using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast) with cDNA sequences of nAChR from Tribolium castaneum (NCBI Accession #: ABS86906, ABS86916, and ABS86914). The open reading frames (ORFs) of nAChR subunits from P. japonica were analyzed with ORF Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/). Multiple protein sequence alignment and similarity analysis were performed with ClustalW2 (http://www.ebi.ac.uk/tool/msa/clustalw2), and the results are presented using GeneDoc (http://www.nrbsc.org/gfx/genedoc). A phylogenetic diagram was generated by Molecular Evolutionary Genetics Analysis (MEGA5.1; http://www.megasoftware.net) with the neighbor-joining algorithm. The data were bootstrapped with 1000 replicates. Signal peptides and transmembrane (TM) domains were predicted using CBS prediction servers (http://www.cbs.dtu.dk/services).
Cloning of five full-length cDNAs encoding nAChR subunits from P. japonica
The full-length cDNA sequence of each subunit was obtained by the rapid amplification of cDNA 5ʹ or 3ʹ ends (5ʹ or 3ʹ RACE), as described previously (Lee et al. Citation2011; Matz et al. Citation1999). Total RNA was isolated from neuronal tissues, including the brain and thoracic ganglia, using RNAiso Plus reagent (Takara Bio Inc., Otsu, Japan). To eliminate contamination by genomic DNA, all isolated RNAs were treated with RNase-free Dnase I (Takara Bio Inc.). RNA concentrations were measured spectrophotometrically at 260 nm (Nanodrop Technologies, Wilmington, DE), and purity was verified by electrophoresis in 1% agarose gels. Isolated RNAs were aliquoted and stored at –70°C until use. The cDNA was synthesized as described previously (Lee et al. Citation2011). First-strand cDNA was synthesized from neuronal tissue total RNA (2 μg) using 1 μL of M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and 1 μL of 20 μM random hexamer or oligo-dT primer in a 20-μL reaction mixture at 37°C for 50 min. Synthesized cDNA was quantified spectrophotometrically, aliquoted, and stored at –20°C for later PCR. The 5ʹ and 3ʹ RACE mixtures and conditions were similar to those described for the previous experiments, except for the reverse sequence-specific primers (Supplemental Table) (Lee et al. Citation2011; Matz et al. Citation1999). The amplified products were analyzed by electrophoresis in 1.5% agarose gels and stained with ethidium bromide. PCR products of the expected size were purified using a Gel Extraction Kit (Bioneer, Daejeon, Korea) and ligated into pGEM-T Easy vector (Promega, Madison, WI). Vectors containing cloned inserts were transformed into E. coli DH5α and incubated overnight at 37°C. Positive clones were screened by PCR using M13 forward and reverse primers, and were sequenced in both directions (Supplemental Table). The full-length sequences were confirmed by RT-PCR using two sequence-specific primers (Supplemental Table). All primers used this experiment were designed using the IDTSciTools program (http://eu.idtdna.com/analyzer/Applications/OligoAnalyzer/) and were synthesized by Bioneer (Daejeon, Korea), Genotech (Daejeon, Korea), or Macrogen (Seoul, Korea).
Measurement of five nAChR subunit mRNAs from P. japonica
The mRNA expression of five Paj nAChR subunit genes was determined by end-point RT-PCR in various tissues, including the gonad, hepatopancreas, gill, hindgut, tail muscle, epidermis, X-organ/sinus gland complex (XO/SG), thoracic ganglia, abdominal ganglia, and brain. Aliquots of 2 μg of total RNA were used for cDNA synthesis according to the same procedure as described for cloning, except random hexamer was used as the reverse primer rather than oligo(dT). The PCR mixture and conditions were also similar to those used in cloning, except for the annealing temperature of each primer set (Supplemental Table). As an internal control, 18S rRNA was amplified (Supplemental Table). After 30 cycles of PCR, the expected amplicons were detected by agarose gel electrophoresis.
SYBR Premix Ex Taq (Takara Bio, Inc.) and a DNA Engine Choromo4 Real-time Detector (Bio-Rad Laboratories, Hercules, CA) were used for quantitative PCR analysis of five Paj nAChR subunit transcripts. Standard curves were constructed with a series of 10-fold diluted samples as described previously (Kim et al. Citation2008), confirming the efficiency of the primers (Supplemental Table). The PCR mixture (20 μL) included 10 μL of 2 × SYBR Green premix Ex Taq™ (Takara Bio, Inc.), 200 ng of cDNA template, and 2 μL of sequence-specific forward and reverse primers (4 μM). PCR conditions were the same as those for end-point RT-PCR, except 40 cycles were performed rather than 30 cycles. Two 18S rRNA primers were used for the internal control (Supplemental Table). Copy numbers of each transcript were calculated from the obtained Ct values and standard curve, as described previously (Kim et al., Citation2008). Copy numbers were then normalized relative to the 18S rDNA. Statistical analysis of the transcript copy numbers was performed with SigmaPlot software 10.0 (Systat Software, Richmond, CA).
Heterogeneous expression of shrimp nAChRs using the Xenopus oocyte expression system
The full-length cDNAs encoding the ORFs of the nAChR subunits were amplified from neuronal tissue cDNA using gene-specific primers (Supplemental Table). PCR products of the expected sizes were purified with a Gel Extraction Kit (Bioneer) and subcloned into pGEM-T Easy vector (Promega). The nucleotide sequences of the cloned nAChR subunits were confirmed by sequencing. Two rat nAChR subunits, Rα4 and Rβ2, in PSGEM2 expression vector were generous gifts from Dr Roger Papke (University of Florida, Gainesville, FL). The cRNA was transcribed in vitro from linearized DNA templates using an mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX), as described previously (Kim & McIntosh Citation2012). Synthesized cRNAs were purified with an RNeasy Mini Kit (Qiagen, Valencia, CA), quantified, and stored at –80°C until used for injection.
Oocytes were prepared according to established procedures (Cartier et al. Citation1996). The oocytes were stored for 1–2 days in buffer containing ND-96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 1.8 mM CaCl2, pH 7.1–7.5) with Pen/Strep/Gent consisting of 100 U/mL penicillin G (Sigma), 100 μg/mL streptomycin (Sigma), and 100 μg/mL gentamycin (Life Technologies, Gaithersburg, MD). The cRNA was injected with a nano-injector (Nanoject II; Drummond Scientific, Broomall, PA) fitted with micropipettes pulled from glass capillaries. The pipette tips were broken to an outer diameter of 22–25 μm and back-filled with mineral oil before mounting on the nano-injector (Cartier et al. Citation1996). The cRNA was drawn into the micropipette, and 50–75 nL containing 10 ng of cRNA of each subunit were injected into each oocyte. The injected oocytes were incubated for 48–96 h at 17°C in ND96 incubation medium before voltage clamping.
Voltage clamp recording
Two to three days after injection, ACh-gated currents were measured with a two-electrode voltage clamp amplifier. The oocyte chamber, consisting of a cylindrical well (∼30 μL volume), was gravity perfused at a rate of ∼2 mL/min with ND-96A buffer (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 1.8 mM CaCl2, pH 7.4–7.6) containing 1 μM atropine and 0.1 mg/mL BSA. The perfusion medium could be switched to acetylcholine (ACh) using a distributor valve (Smart Valve; Cavro Scientific Instruments, San Jose, CA) and a series of three-way solenoid valves (Neptune Research, Lake Park, FL). Glass microelectrodes, pulled from fiber-filled borosilicate capillaries (1 mm outer diameter × 0.75 mm inner diameter; WPI, Sarasota, Florida) and filled with 3 M KCl, served as voltage and current electrodes. Resistances were 0.5–5 MΩ for voltage and 0.5–2 MΩ for current electrodes (Cartier et al. Citation1996). The membrane potential was clamped at –70 mV. Data acquisition and activities of the distributor and solenoid valves were automatically controlled by an in-house virtual instrument constructed with the graphical programming language LabVIEW (National Instruments, Austin, TX). To apply a pulse of ACh to the oocyte, the perfusion fluid was switched to one containing ACh for 1 s. This was done automatically every 2 min. The peak amplitudes of the ACh-gated current responses were measured by an in-house virtual instrument written in LabVIEW (National Instruments). The inward current were measured by averaging three stable peak amplitudes and the inward current in response to 0.1 mM ACh in oocyte represents the average ± S.E at least three oocytes. All recordings were performed at room temperature (∼22°C).
Results
Isolation of five full-length cDNAs encoding nAChR subunits from P. japonica
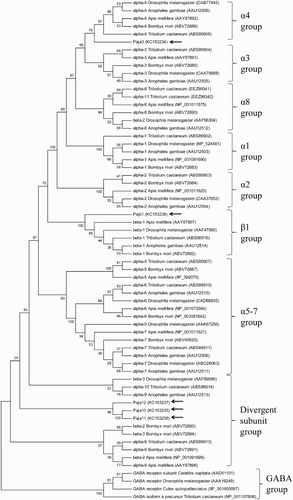
Based on the results of bioinformatics screening of expressed sequence tag (EST) sequences and traditional cloning strategies, five different full-length cDNAs encoding nAChR subunits were isolated from P. japonica. RT-PCR using sequence-specific primers confirmed that each sequence corresponded to a single transcript (Supplemental Table). To understand the evolutionary relationships and proper nomenclature of the nAChR subunits from P. japonica, phylogenetic analysis was performed using all subunits from five insect genomes (). Based on the previous classification derived from sequence similarity to the Drosophila subunits (Jones & Sattelle Citation2010), nAChR subunits from arthropods can be divided into seven core groups (α1s, α2s, α3s, α4s, α5–7s, α8s, and β1s) and a divergent group (). Among the five subunits from P. japonica, one sequence clustered with the α3s and α4s group members; it was named Pajα3 (GenBank Accession No: KC153234) because it showed greater sequence similarity to α3s (72% similarity to Tcas α3) than to α4s (66% similarity to Tcas α4). Another sequence was most closely related to the β1 group and was named Pajβ1 (GenBank Accession No: KC153238). Three other sequences (Pajα10, GenBank Accession No: KC153235; Pajα11, GenBank Accession No: KC153236; and Pajα12, GenBank Accession No: KC153237) were divergent group members, which do not belong to any of the seven insect core groups (). Four GABA subunits from insect species were clustered as the outgroup ().
Figure 1. Phylogenetic analyses of Pajα3, Pajα10, Pajα11, Pajα12, and Pajβ1 compared with subunits from other insect species. The diagram was generated by the neighbor-joining method with MEGA 5.1 using 1000 bootstrap replicates. Species names and GenBank Accession numbers are shown. Pajα3, Pajα10–12, and Pajβ1 are indicated by arrows.
The two subunits belonging to core groups (Pajα3 and Pajβ1) exhibited a highly conserved domain organization (). Pajα3 cDNA (1980 bp) encoded a protein consisting of 588 amino acids, which showed the highest degree of sequence similarity to the α3 subunit (Tcasα3) from Tribolium castaneum (72%). Pajβ (1542 bp) encoded a protein without Cys-pair residues in the C-loop and exhibited 82% similarity to Tcasβ1, the β1 subunit from T. castaneum (). Signal peptide sequences were identified in both Pajα3 and Pajβ1, with putative cleavage sites between residues 22 and 23 and residues 35 and 36, respectively. The large extracellular domain and TM domains were divided by the pre-TM1 RRR motif (Alves et al. Citation2011), and the extracellular domain contained six distinct regions (loops A–F) for ligand binding (). Four TM motifs (TM1–TM4) and a GEK motif near TM2 were also well conserved in the TM domains of both subunits, suggesting that the isolated nAChRs may be cation channels (Jensen et al. Citation2005; Karlin Citation2002). In the core group subunits, the N-terminal extracellular domain showed high sequence similarity (79–89%), whereas relatively low similarity was observed in the TM domains (45–78%). One major difference between Pajα3 and Pajβ1 was seen in the YxCC motif (Cys-Cys pair) involved in ligand binding in the C-loop, which is essential for Ach binding (Karlin, Citation2002).
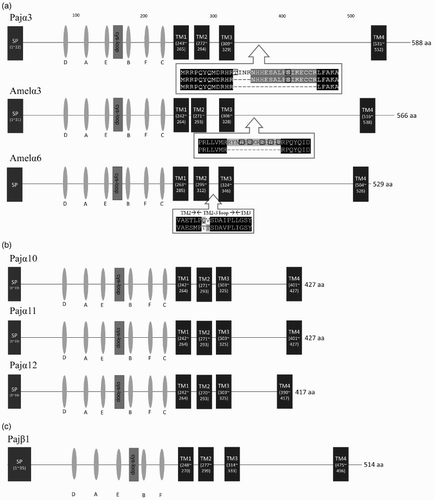
Three of the nAChR subunits from P. japonica were aligned with other divergent group members (). These subunits, Pajα10 (1281 bp), Pajα11 (1281 bp), and Pajα12 (1251 bp), encoded proteins of 427, 427, and 417 residues, respectively. They showed a conserved domain organization, as in the core group subunits, including a signal peptide sequence, an extracellular domain with six loops for ligand binding, and four TM domains. However, the subunits in the divergent group exhibited a lower sequence similarity compared with core group members (). Pajα10 and Pajα11 showed 32% similarity to Tcasα10 from T. castaneum, whereas Pajα12 exhibited 28% similarity to Tcasα10. Based on their nucleotide sequence identity (86%) and overall structural characteristics, Pajα10 and Pajα11 appear to have been generated by a recent gene duplication event (). Compared with those in the core group members, subunits in the divergent group were much smaller representing 100 residues less than those in the core group ( and ). This is attributable mainly to the shorter TM3–TM4 intracellular loop and carboxyl-terminus end after TM4 in the divergent group subunits. The length of the TM3–TM4 intracellular loop in the divergent group was 65–75 residues, compared with 141–191 residues in the core group, which may affect functional expression (). Different from other insect subunits in the divergent group, Pajαs showed well-conserved motifs, including a GEK motif for cation selectivity and Cys-pair residues in the C-loop, suggesting that they may form a functional channel when expressed in combination with the appropriate subunits ().
Figure 2. Deduced protein sequences of P. japonica α3 and β1 nAChR subunits from insect species. Conserved amino acids are indicated (black columns). Signal peptide sequences and transmembrane domains (TM boxes) were predicted by CBS Prediction server analysis. The positions of the loops (LpA–F), the two cysteines forming the Cys-loop, and an RRR motif are indicated. A GEK motif is boxed, and the Cys-pair, characteristic of α subunits, is marked by an arrow. GenBank accession numbers: T. castaneum Tcasα3 (ABS86904), Tcasα4 (ABS86906), Tcasβ1 (ABS86916); A. mellifera Amelα3 (AAY87891), Amelα4 (AAY87892), Amelβ1 (AAY87897); B. mori Bmorα3 (ABV72685), Bmorα4 (ABV72686), Bmorβ1 (ABV72692); P. japonica Pajα3 (KC153234), Pajβ1 (KC153238).
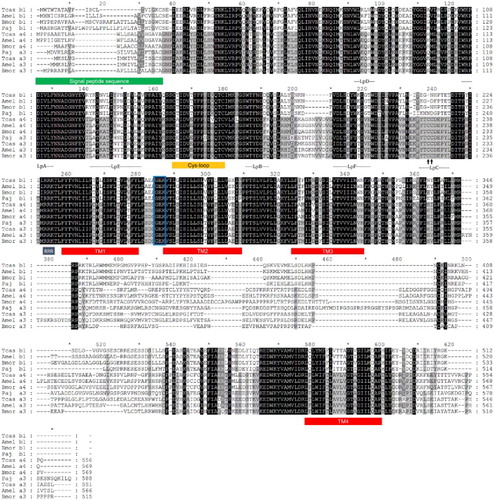
Figure 3. Deduced protein sequences of P. japonica α10–12 and nAChR subunits from insect species. Conserved amino acids are indicated (black columns). Signal peptide sequences (underlined) and transmembrane domains (TM boxes) were predicted by CBS Prediction server analysis. The positions of the loops (LpA–F), the two cysteines forming a Cys-loop, and an RRR motif are indicated. A GEK motif is boxed, and the Cys-pair, characteristic of α subunits, is marked by an arrow. GenBank accession numbers: T. castaneum Tcasα9 (ABS86913), Tcasα10 (ABS86914); D. melanogaster Dmelβ3 (AAF89090); A. gambiae Agamα9 (AAU12513); A. mellifera Amelα9 (AAY87896), Amelβ2 (NP_001091699); B. mori Bmorα9 (ABV72691), Bmorβ2 (ABV72693), Bmorβ3 (ABV72694); P. japonica Pajα10 (KC153235), Pajα11 (KC153236), Pajα12 (KC153237).

Figure 4. Schematic representation of the protein structures of Pajα3, Pajα10–12, and Pajα1. The boxes represent domains or motifs, and the ovals indicate loops. The signal peptide sequence (SP box), Cys-loop (Cys-loop box), and transmembrane domains (TM boxes) are indicated. (a) Pajα3 has three splicing variants with differences between TM3 and TM4. Amelα3 and Amelα6 also have splicing variants. Splicing variant amino sequences were shown. Putavie phosphorylation sites were indicated by black boxes. (b) Compared with the other subunits, Pajα10–12 have a small intercellular loop. (c) Loop C for Ach binding is lacking in Pajα1.
Alternatively spliced variants of Pajα3
Three different transcriptional isoforms were identified in Pajα3 (). They appear to be splice variants with sequence diversity in the long intracellular loop between TM3 and TM4. A splice site was detected between amino acids 367(R) and 368(L), which would produce three transcripts with different numbers of residues (Pajα3, Pajα3 + 15, and Pajα3 + 19). Similarly, two splice variants were identified in Agamα3 from A. mellifera, in which an additional 13 amino acid residues were included in the long intracellular loop between TM3 and TM4, as in Pajα3 (). These results suggest that Pajα3 is the ortholog of the insect α3 subunit (Jones et al. Citation2006). Although alternatively spliced isoforms were also identified in Amelα6 from A. mellifera, the splice site differed from that of the α3 subunit, indicating that splicing patterns and biological roles differ between the groups ().
Tissue distribution analysis of the five nAChR subunits
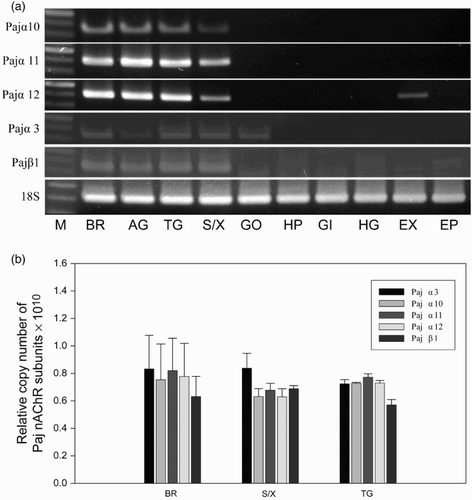
The expression patterns of the five nAChR subunit mRNAs in various tissues were determined by end-point RT-PCR ((a)). The amplification of 18S rRNA in all tissues confirmed the integrity of the cDNA. Based on the PCR products, the five nAChR subunits are expressed in neuronal tissues, including the brain, abdominal and thoracic ganglia, and XO/SG. In addition, the expression of Pajα3 and Pajα12 was also detected in the gonad and tail muscle, respectively ((a)). Quantitative RT-PCR was performed to determine whether there are correlations among different subunits ((b)). The expression levels of the five subunits did not differ significantly among the tissues, and there were no correlations among the different subunits for the formation of functional channels ((b)). We failed to synthesize primers to distinguish between the three alternatively spliced variants of Pajα3 and were unable to compare their expression levels (data not shown).
Figure 5. Expression of Pajα3, Pajα10–12, and Pajβ1 in various tissues. (a) End-point RT-PCR was performed (30 amplification cycles), and the products were separated in 1.5% agarose gels. The 18S rRNA was used as a control. (M, size marker; BR, brain; AG, abdominal ganglion; TG, thoracic ganglion; SG/XO, sinus gland X-organ; GO, gonad; HP, hepatopancreas; GI, gill; HG, hindgut; EX, tail muscle; EP, epidermis). (b) Relative copy number of five nAChR subunits among neuronal tissues from P. japonica. Data were normalized to the copy number of 18S rRNA. Statistical significance was accepted only when P < 0.05. (BR, brain; S/X, sinus gland X-organ; AG, abdominal ganglion.)
Functional expression of Pajβ1 subunit with rat α4 subunit on the surface of Xenopus oocytes
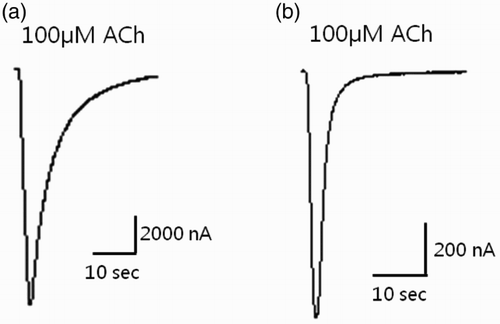
To determine the subunit composition of the functional P. japonica nAChR channel, all possible combinations of the five nAChR subunit cRNAs were injected into Xenopus oocytes, which is one of the most commonly used systems for functional expression of nAChRs (Millar Citation2009). Unfortunately, no response was detected to the ACh pulses from any combination of the five isolated nAChR subunits, indicating that no functional channels formed in the Xenopus oocyte system. Therefore, the expression of various hybrids of the five shrimp nAChR subunits with those from rat (Rα2–Rα6 and Rβ2–4) was examined. Among the different co-expressed combinations, Rα4 and Pajβ1 formed functional channels that responded to the ACh pulse (). The inward current in response to 100 μM ACh in oocytes expressing Rα4/Pajβ1 was extremely high (3699.6 ± 960.63 nA; n = 7). The amplitude of the current through Rα4/Pajβ1 was only 20% lower than that through Rα4β2 (5014.6 ± 767.93 nA; n = 7), which is one of the most common nAChRs in vertebrate neurons. To our knowledge, this is the first report of the functional expression of an arthropod β subunit, which will be important for studying the structure and function of nAChRs in invertebrates.
Discussion
Classification and characteristics of nAChR subunits in arthropods
Five different cDNAs encoding nAChR subunits (four α subunits and one β subunit) were identified in the decapod crustacean P. japonica. All of the identified subunits from P. japonica showed well-conserved domain organizations, including a signal peptide sequence, an N-terminal extracellular domain with conserved residues for ACh binding (loops A–F), and four hydrophobic putative TM domains (TM1–TM4) with a highly variable intracellular loop between TM3 and TM4 ( and 3). Based on the GEK peptide motif preceding TM2 (Jensen et al. Citation2005), the five subunits from P. japonica were postulated to form a cation-selective ion channel. Despite the similarity in overall structure among the subunits, each subunit has unique features that would enable the formation of various functional nAChRs with different pharmacological and physiological characteristics. This would allow the development of specific agonists or antagonists, which may be valuable as insecticides or drugs. The neonicotinoids such as imidacloprid, nitenpyram, thiamethoxam, and dinotefuran are a new class of insecticides that act selectively on insect nAChRs (Tomizawa & Casida Citation2003). These nAChR agonists bind with high affinity to insect nAChRs and with low affinity to vertebrate nAChRs (Matsuda et al. Citation2001; Millar & Denholm Citation2007). Electrophysiological studies using different nAChR agonists have demonstrated differences between the pharmacological profiles of invertebrate versus vertebrate nAChR subtypes (Millar & Lansdell Citation2010). Specific residues in insect nAChR α and β subunits contribute to neonicotinoid selectivity (Yao et al. Citation2008; Zhang et al. Citation2008). As in insect species, understanding nAChRs and their ligands in crustaceans can provide important benefits. The present study contributes to the understanding of the structural and functional characteristics of nAChRs in invertebrates.
In addition to the five subunits identified in the present study, the P. japonica genome is expected to encode many more nAChR subunits. In vertebrates, 17 nAChR subunits (α1–α10, β1–β4, γ, δ, and ε) have been cloned and studied (Corringer et al. Citation2000). Insect genome projects have identified 10–12 nAChR subunits (Dupuis et a.l. Citation2012; Jones et al. Citation2005; Jones et al. Citation2006; Jones & Sattelle Citation2007; Citation2010; Millar Citation2003; Shao et al. Citation2007; Tomizawa & Casida Citation2001). A search of the GenBank database (www.ncbi.nlm.nih.gov) revealed more than 15 sequences within the Daphnia pulex genome that have similarity to nAChR subunits. It is expected that decapod genomes may have a similar number of different nAChR subunits. The majority of the subunits appear to be α subunits, and arthropods may possess only 1–3 β subunits. Although the numbers of α subunits are similar between mammals (α1–α10) and insects (1α–α9 or α10), relatively fewer β subunits have been identified in insects (β1–β3) than in mammals (β1–β4). The β2 subunit of D. melanogaster belongs to the α5 group, which has evolved in a species-specific manner, and its biological implications are unknown (). In fact, members of only one group (β1s) appear to be true orthologs of each other in insect species, suggesting that only a single β subunit may be involved in the common functional channel (). In vertebrates, different β subunits combine with various α subunits to form different nAChR channels. Among the four β subunits in mammals, β1 forms a heteropentameric form in neuromuscular junctions (Millar & Gotti Citation2009), and β2 and β4 are expressed in neuronal tissues and form functional channels (Millar & Gotti Citation2009) Although β3 in mammals does not form a functional channel by itself with α subunits, co-expression with the β2 subunit results in the formation of unique functional channels with α subunits, especially with α6 (Millar & Gotti Citation2009). These results suggest that more pharmacologically and physiologically diverse nAChR channels are possible in mammals than in arthropods owing to the higher number of different β subunits in mammals. It would be interesting to understand the structural characteristics of β subunits expressed with α subunits; no such studies have been described in the literature to date.
The nAChR subunits from arthropod genomes were further divided into two groups: the core group and the divergent group ( and ). Members of the core group included α1–α8 and β1, which are highly conserved and well classified by sequence similarity ( and ). The α1 and α2 subgroup members were most closely related to each other, and the α3 and α4 subgroups, including Pajα3, were clustered together. Successful functional expression of α1 and α2 members has been reported in heterologous systems. The subunits Dα2 (the fruit fly D. melanogaster), Sgα1 (the locust S. gregaria), and Mpα1 and Mpα2 (the aphid M. persicae) formed homomeric nAChRs when expressed in Xenopus oocytes (Amar et al.Citation1995; Marshall et al. Citation1990; Sawruk et al. Citation1990; Sgard et al. Citation1998). These results indicate that α1 and α2 group members are able to form functional channels as homopentamers, whereas α3 and α4 subgroup members are not, despite their sequence similarity ().
Table 1. Summary of complete insect nAChR families with Pajα3, β1, α10, α11, and α12.
We identified three different isoforms generated by alternative splicing in the Pajα3 gene, two of which are longer than the other by 15 and 19 residues within the TM3–TM4 intracellular loop (). Unlike those from vertebrates, several nAChR subunits from arthropods are produced by posttranscriptional modifications, which may contribute further to protein diversity (Sattelle et al. Citation2005). Splice sites and patterns appear to be different for each subunit. The Amelα3 gene also encodes two variants (long and short forms) with TM3–TM4 intracellular loops different in length by 13 amino acid residues (Jones et al. Citation2006). The two longer Pajα3 forms have an extra 19 and 15 amino acid residues and 2 and 1 extra putative phosphorylation sites, respectively ((a)). This may affect the receptor properties as phosphorylation of the TM3–TM4 intracellular loop regulates several aspects of receptor function, including desensitization and aggregation, and may affect the actions of ligands (Borges & Ferns Citation2001; Hopfield et al. Citation1988; Jones & Sattelle Citation2010). Different forms generated by alternative splicing have also been observed in the α4 and α6 nAChR subunits (Gao et al. Citation2007; Jones et al. Citation2005; Jones et al. Citation2006; Lansdell & Millar Citation2000a; Rinkevich & Scott Citation2009). Some subunits may produce not only isoforms but also truncated transcripts in several insects (Jones et al. Citation2006; Saragoza et al. Citation2003). Based on the splice site and amino acid sequence similarity, Pajα3 is an ortholog of the α3 group in arthropods, and the biological implications of this should be further elucidated. In addition, RNA editing has been identified in nAChRs from insect species (Grauso et al. Citation2002), and this would increase the level of protein diversity. RNA editing has been largely studied in α6 nAChR subunits of different insect species (Jin et al. Citation2007; Jones & Sattelle Citation2010; Rinkevich & Scott Citation2009). The present study did not find evidence for RNA editing in the expression of P. japonica nAChR subunits.
Members of the α8 subgroup were clustered with the α3 and α4 subgroups. As described above, we renamed this as the α8 group, rather than the β2 group, in insects because only one β subunit (Dβ2 from D. melanogaster) has been identified and all other members are α subunits ( and ). The loss of the double cysteine residue of loop C in Dβ2 from D. melanogaster might have been driven by a species-unique evolutionary process. The α5–α7 subgroup members were clustered together and were distinct from other core group members (). They are so closely related to one another that α5 subgroup members do not form a monophyletic cluster. No subunits belonging to the α5–α7 subgroup were identified in decapods, and the biological significance of this is unclear in the absence of functional channel studies. Compared with core group members (66–82%), nAChR subunits in the divergent subunits including Pajα10–12 showed low levels of sequence similarity (27–32%). In addition, the divergent subunits possessed relatively short TM3–TM4 intracellular loops (Jones & Sattelle Citation2010) and short C-terminal extracellular domains (). Although the biological implications of these observations are not clear, the length of the TM3–TM4 intracellular loop domain is associated with the levels of cell surface and intracellular assembled receptors in mammals and influences functional properties (Kracun et al. Citation2008). Studies of divergent subunit functions have been hindered by the failure to obtain functional nAChR channels composed of divergent group subunits (Dupuis et al. Citation2012; Jones et al. Citation2009). Further studies are required to determine the pharmacological properties and functional roles of these subunits.
Functional expression of nAChRs from P. japonica
In the present study, we also constructed a functional nAChR in Xenopus oocytes with rat Rα4 subunit and Pajβ1. In addition to homopentameric nAChR channels of α1 and α2 subunits, several insect nAChR subunits form functional receptors when expressed with vertebrate β2 subunits or homomers in X. laevis oocytes (Bertrand et al. Citation1994; Lees et al. Citation2014; Sgard et al. Citation1998). However, invertebrate β subunits have not been expressed previously in heterologous expression systems. Although the hybrid nAChR obtained in the present study is not a native form, this is the first report of the functional expression of invertebrate β subunits using this system, and will be useful in future studies of the structural and functional characteristics of heteropentameric nAChRs in invertebrates.
Crystal structures of nAChRs from the electric organ of the marine ray Torpedo marmorata (Unwin Citation1996) and the mollusk Lymnaea stagnalis (Smit et al. Citation2001) have provided general information about the gating mechanism induced by ligand binding. The nAChR agonist binding site is formed by loops A–C in α subunits together with loops D–F present in either non-α subunits or homomer-forming α subunits (Arias Citation2000; Grutter & Changeux Citation2001; Prince & Sine Citation1998). The loops located at the interface are not well conserved among nAChRs and probably contribute to the diversity of subunit combinations (Brejc et al. Citation2001; Grutter & Changeux Citation2001). The residues in the six loops may be necessary, but not sufficient, for agonist binding. The residues in the loops interact directly with agonists, and substitutions in these residues always have a crucial influence on agonist binding (Liu et al. Citation2008). In the Torpedo nAChR model, agonist binding to nAChRs induces a major rearrangement of the C-loop within the ligand-binding pocket and disrupts a salt bridge between an arginine residue at the end of the β10 strand and a glutamate residue in the β1–β2 linker (Gay & Yakel Citation2007). However, the model did not fit well for heteromeric nAChRs, including α4β2 or α3β2. In the present study, the Rattus norvegicus α4 and β2 (Rα4 and Rβ2) subunits were used because homomeric nAChRs could not be formed. These subunits have been shown to form functional nAChRs when expressed in combination with one α and one β subunit (Duvoisin et al. Citation1989; Papke et al. Citation1989). As the β subunit also contributes to ligand binding and gating of nAChRs, further characterization of this hybrid channel will provide insights into the potential ligands and gating mechanism of invertebrate nAChRs
Supplemental data
Supplemental data for this article can be accessed 10.1080/19768354.2015.1109547
Supplemental Table. Primers used for Paj-α3, β1, α10, α11, and α12.docx
Download MS Word (15.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alves DS, Castello-Banyuls J, Faura CC, Ballesta JJ. 2011. An extracellular RRR motif flanking the M1 transmembrane domain governs the biogenesis of homomeric neuronal nicotinic acetylcholine receptors. FEBS Lett. 585:1169–1174. doi: 10.1016/j.febslet.2011.03.028
- Amar M, Thomas P, Wonnacott S, Lunt GG. 1995. A nicotinic acetylcholine receptor subunit from insect brain forms a non-desensitising homo-oligomeric nicotinic acetylcholine receptor when expressed in Xenopus oocytes. Neurosci Lett. 199:107–110. doi: 10.1016/0304-3940(95)12033-Z
- Arias HR. 2000. Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors. Neurochem Int. 36:595–645. doi: 10.1016/S0197-0186(99)00154-0
- Arneric SP, Holladay M, Williams M. 2007. Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol. 74:1092–1101. doi: 10.1016/j.bcp.2007.06.033
- Bertrand D, Ballivet M, Gomez M, Bertrand S, Phannavong B, Gundelfinger ED. 1994. Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate beta 2 subunit and Drosophila alpha subunits. Eur J Neurosci. 6:869–875. doi: 10.1111/j.1460-9568.1994.tb00997.x
- Borges LS, Ferns M. 2001. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J Cell Biol. 153:1–12. doi: 10.1083/jcb.153.1.1
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. 2001. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 411:269–276. doi: 10.1038/35077011
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. 1996. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 271:7522–7528. doi: 10.1074/jbc.271.13.7522
- Corringer PJ, Le Novere N, Changeux JP. 2000. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431
- Dupuis J, Louis T, Gauthier M, Raymond V. 2012. Insights from honeybee (Apis mellifera) and fly (Drosophila melanogaster) nicotinic acetylcholine receptors: from genes to behavioral functions. Neurosci Biobehav Rev. 36:1553–1564. doi: 10.1016/j.neubiorev.2012.04.003
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. 1989. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 3:487–496. doi: 10.1016/0896-6273(89)90207-9
- Gao JR, Deacutis JM, Scott JG. 2007. The nicotinic acetylcholine receptor subunit Mdalpha6 from Musca domestica is diversified via post-transcriptional modification. Insect Mol Biol. 16:325–334. doi: 10.1111/j.1365-2583.2007.00730.x
- Gay EA, Yakel JL. 2007. Gating of nicotinic ACh receptors; new insights into structural transitions triggered by agonist binding that induce channel opening. J Physiol. 584:727–733. doi: 10.1113/jphysiol.2007.142554
- Gotti C, Zoli M, Clementi F. 2006. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 27:482–491. doi: 10.1016/j.tips.2006.07.004
- Grauso M, Reenan RA, Culetto E, Sattelle DB. 2002. Novel putative nicotinic acetylcholine receptor subunit genes, Dalpha5, Dalpha6 and Dalpha7, in Drosophila melanogaster identify a new and highly conserved target of adenosine deaminase acting on RNA-mediated A-to-I pre-mRNA editing. Genetics. 160:1519–1533.
- Grutter T, Changeux JP. 2001. Nicotinic receptors in wonderland. Trends Biochem Sci. 26:459–463. doi: 10.1016/S0968-0004(01)01921-1
- Hopfield JF, Tank DW, Greengard P, Huganir RL. 1988. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 336:677–680. doi: 10.1038/336677a0
- Jensen ML, Schousboe A, Ahring PK. 2005. Charge selectivity of the Cys-loop family of ligand-gated ion channels. J Neurochem. 92:217–225. doi: 10.1111/j.1471-4159.2004.02883.x
- Jin Y, Tian N, Cao J, Liang J, Yang Z, Lv J. 2007. RNA editing and alternative splicing of the insect nAChR subunit alpha6 transcript: evolutionary conservation, divergence and regulation. BMC Evol Biol. 7:98. doi: 10.1186/1471-2148-7-98
- Jones AK, Buckingham SD, Brown LA, Sattelle DB. 2009. Alternative splicing of the Anopheles gambiae nicotinic acetylcholine receptor, Agamalphabeta9, generates both alpha and beta subunits. Invert Neurosci. 9:77–84. doi: 10.1007/s10158-009-0089-7
- Jones AK, Grauso M, Sattelle DB. 2005. The nicotinic acetylcholine receptor gene family of the malaria mosquito, Anopheles gambiae. Genomics. 85:176–187. doi: 10.1016/j.ygeno.2004.09.001
- Jones AK, Raymond-Delpech V, Thany SH, Gauthier M, Sattelle DB. 2006. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 16: 1422–1430. doi: 10.1101/gr.4549206
- Jones AK, Sattelle DB. 2007. The cys-loop ligand-gated ion channel gene superfamily of the red flour beetle, Tribolium castaneum. BMC Genomics. 8:327. doi: 10.1186/1471-2164-8-327
- Jones AK, Sattelle DB. 2010. Diversity of insect nicotinic acetylcholine receptor subunits. Adv Exp Med Biol. 683:25–43. doi: 10.1007/978-1-4419-6445-8_3
- Karlin A. 2002. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 3:102–114. doi: 10.1038/nrn731
- Kim HW, McIntosh JM. 2012. alpha6 nAChR subunit residues that confer alpha-conotoxin BuIA selectivity. FASEB J. 26:4102–4110. doi: 10.1096/fj.12-204487
- Kim KS, Kim BK, Kim HJ, Yoo MS, Mykles DL, Kim HW. 2008. Pancreatic lipase-related protein (PY-PLRP) highly expressed in the vitellogenic ovary of the scallop, Patinopecten yessoensis. Comp Biochem Physiol B Biochem Mol Biol. 151: 52–58. doi: 10.1016/j.cbpb.2008.05.009
- Kim M, Jeon JM, Oh CW, Kim YM, Lee DS, Kang CK, Kim HW. 2012. Molecular characterization of three crustin genes in the morotoge shrimp, Pandalopsis japonica. Comp Biochem Physiol B Biochem Mol Biol. 163:161–171. doi: 10.1016/j.cbpb.2012.05.007
- Kracun S, Harkness PC, Gibb AJ, Millar NS. 2008. Influence of the M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br J Pharmacol. 153:1474–1484. doi: 10.1038/sj.bjp.0707676
- Lansdell SJ, Millar NS. 2000a. Cloning and heterologous expression of Dalpha4, a Drosophila neuronal nicotinic acetylcholine receptor subunit: identification of an alternative exon influencing the efficiency of subunit assembly. Neuropharmacology. 39:2604–2614. doi: 10.1016/S0028-3908(00)00111-8
- Lansdell SJ, Millar NS. 2000b. The influence of nicotinic receptor subunit composition upon agonist, alpha-bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology. 39:671–679. doi: 10.1016/S0028-3908(99)00170-7
- Lansdell SJ, Millar NS. 2004. Molecular characterization of Dalpha6 and Dalpha7 nicotinic acetylcholine receptor subunits from Drosophila: formation of a high-affinity alpha-bungarotoxin binding site revealed by expression of subunit chimeras. J Neurochem. 90:479–489. doi: 10.1111/j.1471-4159.2004.02499.x
- Lansdell SJ, Schmitt B, Betz H, Sattelle DB, Millar NS. 1997. Temperature-sensitive expression of Drosophila neuronal nicotinic acetylcholine receptors. J Neurochem. 68:1812–1819. doi: 10.1046/j.1471-4159.1997.68051812.x
- Lee SO, Jeon JM, Oh CW, Kim YM, Kang CK, Lee DS, Mykles DL, Kim HW. 2011. Two juvenile hormone esterase-like carboxylesterase cDNAs from a Pandalus shrimp (Pandalopsis japonica): cloning, tissue expression, and effects of eyestalk ablation. Comp Biochem Physiol B Biochem Mol Biol. 159:148–156. doi: 10.1016/j.cbpb.2011.03.004
- Lees K, Jones AK, Matsuda K, Akamatsu M, Sattelle DB, Woods DJ, Bowman AS. 2014. Functional characterisation of a nicotinic acetylcholine receptor alpha subunit from the brown dog tick, Rhipicephalus sanguineus. Int J Parasitol. 44:75–81. doi: 10.1016/j.ijpara.2013.11.002
- Liu Z, Han Z, Liu S, Zhang Y, Song F, Yao X, Gu J. 2008. Amino acids outside of the loops that define the agonist binding site are important for ligand binding to insect nicotinic acetylcholine receptors. J Neurochem. 106:224–230. doi: 10.1111/j.1471-4159.2008.05359.x
- Marshall J, Buckingham SD, Shingai R, Lunt GG, Goosey MW, Darlison MG, Sattelle DB, Barnard EA. 1990. Sequence and functional expression of a single alpha subunit of an insect nicotinic acetylcholine receptor. EMBO. J 9:4391–4398.
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. 2001. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 22:573–580. doi: 10.1016/S0165-6147(00)01820-4
- Matz M, Shagin D, Bogdanova E, Britanova O, Lukyanov S, Diatchenko L, Chenchik A. 1999. Amplification of cDNA ends based on template-switching effect and step-out PCR. Nucleic Acids Res. 27:1558–1560. doi: 10.1093/nar/27.6.1558
- Millar NS. 1999. Heterologous expression of mammalian and insect neuronal nicotinic acetylcholine receptors in cultured cell lines. Biochem Soc Trans. 27:944–950. doi: 10.1042/bst0270944
- Millar NS. 2003. Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem Soc Trans. 31:869–874. doi: 10.1042/bst0310869
- Millar NS. 2009. A review of experimental techniques used for the heterologous expression of nicotinic acetylcholine receptors. Biochem Pharmacol. 78:766–776. doi: 10.1016/j.bcp.2009.06.015
- Millar NS, Denholm I. 2007. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert Neurosci. 7:53–66. doi: 10.1007/s10158-006-0040-0
- Millar NS, Gotti C. 2009. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 56:237–246. doi: 10.1016/j.neuropharm.2008.07.041
- Millar NS, Lansdell SJ. 2010. Characterisation of insect nicotinic acetylcholine receptors by heterologous expression. Adv Exp Med Biol. 683:65–73. doi: 10.1007/978-1-4419-6445-8_6
- Papke RL, Boulter J, Patrick J, Heinemann S. 1989. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 3:589–596. doi: 10.1016/0896-6273(89)90269-9
- Prince RJ, Sine SM. 1998. Epibatidine binds with unique site and state selectivity to muscle nicotinic acetylcholine receptors. J Biol Chem. 273:7843–7849. doi: 10.1074/jbc.273.14.7843
- Rinkevich FD, Scott JG. 2009. Transcriptional diversity and allelic variation in nicotinic acetylcholine receptor subunits of the red flour beetle, Tribolium castaneum. Insect Mol Biol. 18:233–242. doi: 10.1111/j.1365-2583.2009.00873.x
- Saragoza PA, Modir JG, Goel N, French KL, Li L, Nowak MW, Stitzel JA. 2003. Identification of an alternatively processed nicotinic receptor alpha7 subunit RNA in mouse brain. Brain Res Mol Brain Res. 117:15–26. doi: 10.1016/S0169-328X(03)00261-4
- Sattelle DB, Jones AK, Sattelle BM, Matsuda K, Reenan R, Biggin PC. 2005. Edit, cut and paste in the nicotinic acetylcholine receptor gene family of Drosophila melanogaster. Bioessays. 27:366–376. doi: 10.1002/bies.20207
- Sawruk E, Schloss P, Betz H, Schmitt B. 1990. Heterogeneity of Drosophila nicotinic acetylcholine receptors: SAD, a novel developmentally regulated alpha-subunit. EMBO J. 9:2671–2677.
- Sgard F, Fraser SP, Katkowska MJ, Djamgoz MB, Dunbar SJ, Windass JD. 1998. Cloning and functional characterisation of two novel nicotinic acetylcholine receptor alpha subunits from the insect pest Myzus persicae. J Neurochem. 71:903–912. doi: 10.1046/j.1471-4159.1998.71030903.x
- Shao YM, Dong K, Zhang CX. 2007. The nicotinic acetylcholine receptor gene family of the silkworm, Bombyx mori. BMC Genomics. 8:324. doi: 10.1186/1471-2164-8-324
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, et al. 2001. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 411:261–268. doi: 10.1038/35077000
- Tomizawa M, Casida JE. 2001. Structure and diversity of insect nicotinic acetylcholine receptors. Pest Manag Sci. 57:914–922. doi: 10.1002/ps.349
- Tomizawa M, Casida JE. 2003. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 48:339–364. doi: 10.1146/annurev.ento.48.091801.112731
- Unwin N. 1996. Projection structure of the nicotinic acetylcholine receptor: distinct conformations of the alpha subunits. J Mol Biol. 257:586–596. doi: 10.1006/jmbi.1996.0187
- Wu M, Gerhart J. 1991. Raising Xenopus in the laboratory. Methods Cell Biol. 36:3–18. doi: 10.1016/S0091-679X(08)60269-1
- Yao X, Song F, Chen F, Zhang Y, Gu J, Liu S, Liu Z. 2008. Amino acids within loops D, E and F of insect nicotinic acetylcholine receptor beta subunits influence neonicotinoid selectivity. Insect Biochem Mol Biol. 38:834–840. doi: 10.1016/j.ibmb.2008.05.009
- Yao X, Song F, Zhang Y, Shao Y, Li J, Liu Z. 2009. Nicotinic acetylcholine receptor beta1 subunit from the brown planthopper, Nilaparvata lugens: A-to-I RNA editing and its possible roles in neonicotinoid sensitivity. Insect Biochem Mol Biol. 39:348–354. doi: 10.1016/j.ibmb.2009.02.001
- Zhang Y, Liu S, Gu J, Song F, Yao X, Liu Z. 2008. Imidacloprid acts as an antagonist on insect nicotinic acetylcholine receptor containing the Y151M mutation. Neurosci Lett. 446:97–100. doi: 10.1016/j.neulet.2008.09.039