ABSTRACT
Resveratrol possesses a wide spectrum of pharmacological properties and has been an ideal alternative drug for the treatment of different cancers, including prostate cancer. However, the mechanisms by which resveratrol inhibits the growth of prostate cancer are still not fully elucidated. To understand the effect of resveratrol on the apoptosis and the epithelial-to-mesenchymal transition (EMT) of prostate cancer as well as its related mechanism, we investigated the potential use of resveratrol in PC-3 prostate cancer cells in vitro using real-time PCR, fluorescence-activated cell sorting, Western blotting, etc. Resveratrol suppresses the PC-3 prostate cancer cell growth and induces apoptosis. Resveratrol also influences the expression of EMT-related proteins (increased E-cadherin and decreased Vimentin expression). Finally, resveratrol also suppressed Akt phosphorylation in PC-3 cells. This study indicates that resveratrol may be a potential anti-cancer treatment for prostate cancer; moreover, it provides new evidence that resveratrol suppresses prostate cancer growth and metastasis.
1. Introduction
Prostate cancer is a major life-threatening disease in most countries (Snyder et al. Citation2013). There is an urgent need to discover novel and effective chemopreventive agents using clinically relevant models of prostate cancer.
Recent evidence implicates the induction of the epithelial-to-mesenchymal transition (EMT) in cancer progression (Thiery et al. Citation2009; Yilmaz & Christofori Citation2010; Chang et al. Citation2011). The transcription factors that regulate the EMT are expressed in numerous malignant cancers. Increasing evidence and research suggests that the EMT has important functions in the malignant transformation of prostate cancer, and it also induces cancer cells to invade and migrate (Hance et al. Citation2012; Lu et al. Citation2012). Therefore, the EMT may be a potential therapeutic target, and inhibition of the EMT may be a promising method to treat cancer.
Resveratrol (trans-3,4′,5-trihydroxystilbene, RES) is a natural polyphenol that has been identified in many plants, including grapes, berries, peanuts and many types of traditional Chinese medicine (Pirola & Frojdo Citation2008). Long-term basic and clinical studies have suggested that RES is an ideal alternative drug in the therapy of different diseases (Pervaiz & Holme Citation2009; Baur Citation2010; Fulda Citation2010). Recently, the anti-cancer activity of RES has been studied in various types of cancer, including myeloma, ovarian cancer, pancreatic cancer and breast cancer, via the regulation of multiple pathways (Sun et al. Citation2006; Shankar et al. Citation2011; Vergara et al. Citation2012). Some studies have indicated that RES could influence several processes and plays an important role in the regulation of various molecular targets, including nuclear factor (NF)-κB, the growth factor vascular endothelial growth factor (VEGF), the inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6, and the protein kinases p38 mitogen-activated protein kinase (MAPK) and Akt (Kundu & Surh Citation2008; Knobloch et al. Citation2011). However, the related molecular mechanism in prostate cancer remains unclear.
In this paper, we hypothesized that RES may have an inhibitory role in the EMT, a key inducer of metastasis in pancreatic cancer. In addition, RES potentially promotes apoptosis by regulating the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. To test this hypothesis, we detected the expression of apoptosis- and EMT-related molecules in PC-3 prostate cancer cells in the presence or absence of RES as well as the signaling pathways triggered by RES.
2. Material and methods
2.1. Reagents and cell culture
Antibodies against BAX, Bcl-2, cleaved-caspase 3, cleaved-caspase 9, E-cadherin, Vimentin and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). RES (purity >98%) was purchased from TCI (Shanghai) development Co., Ltd. (Shanghai, China). The AKT inhibitor was purchased from Selleck Chemical Biotechnology, Inc. (USA), and the AKT activator was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The PC-3 prostate cancer cell lines (obtained from the cell center of Kunming Animal Institute) were maintained in Dulbecco's modified Eagle medium (DMEM, Gibco®, Carlsbad, CA, USA) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% heat-inactivated fetal bovine serum and incubated in a 5% CO2 humidified atmosphere at 37°C. The cells were grown to 70% confluence prior to treatment.
2.2. Cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Beyotime, Shanghai, China) assay was used to determine cell viability. The MTT reagent was added, and the cells were incubated for an additional 4 h. After formazan crystals had formed, the MTT medium was then aspirated and replaced with 150 µl of solubilization solution, dimethylsulfoxide, per well to dissolve the formazan crystals. The plates were shaken for 5 min. The absorbance of each well was recorded on a microplate spectrophotometer at 570 nm (Molecular Devices Technologies, USA).
2.3. Cell migration assay
Cell migration was detected with a wound-healing assay. The PC-3 cells were seeded in six-well plates (1 × 105 cells/500 μl) and cultured overnight. A sterile pipette tip was used to produce a wound between the cells. The cellular debris was removed by washing with phosphate-buffered saline (PBS), and then RES (0, 12.5, 25 or 50 μM) was added to the cells for 24 h. Wound closure was monitored, imaged and analyzed using a light microscope (Olympus, Tokyo, Japan). The shortest distances between the edges of the cells migrating from both sides were measured.
2.4. Flow cytometry
We used the Annexin V-FITC Apoptosis Detection Kit and Cell Cycle Kit (Beyotime, Shanghai, China) to assess apoptosis and proliferation according to the manufacturer's instructions. Finally, the cells were analyzed by a flow cytometer (FACS PARTEC, Germany). Briefly, after treatment, the cells were harvested by trypsinization. After centrifugation, the cells were washed twice with cold PBS. Approximately 1 × 105 to 1 × 106 cells were resuspended in 300 µl of 1× binding buffer and transferred to a sterile glass flow cytometry tube. Then, 5 µl of Annexin V-FITC was added, and the sample was incubated in the dark for 30 min at room temperature. Then, the cells were incubated with 5 µl of propidium iodide in the dark and analyzed with a flow cytometer (FACS PARTEC, Germany).
2.5. Hoechst 33258 staining
The changes in nuclear morphology of apoptotic cells were examined by Hoechst 33258 staining and visualized under a fluorescence microscope. After treatment with and/or without RES, cells were incubated with Hoechst 33258 (Beyotime, Shanghai, China) for 30 min at room temperature in the dark. The cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan) after being washed with PBS. Cells that exhibited reduced nuclear size, chromatin condensation, intense fluorescence, and nuclear fragmentation were considered apoptotic.
2.6. Measurement of the mitochondrial membrane potential (ΔΨm)
Decreased mitochondrial membrane potential (ΔΨm) is an early hallmark event of apoptosis. The mitochondrial membrane potential (ΔΨm) was analyzed using the JC-1 assay (Beyotime, Shanghai, China). Briefly, a staining mixture (300 nM JC-1) was prepared according to the instructions enclosed in the JC-1 kit. The cells were incubated in the staining mixture for 30 min at 37°C. Then, the cells were washed twice with media and resuspended in fresh media. The ΔΨm was monitored with a fluorescence microscope (Olympus, Tokyo, Japan).
2.7. Immunofluorescence assay
After 24 h of treatment, the cells were fixed with 4% formaldehyde for 5 min, permeabilized with a 0.2% solution of Triton X-100 in PBS and blocked in 2% bovine serum albumin-PBS for 30 min. The nonspecific binding sites were blocked for 2 h with normal goat serum (Sigma-Aldrich, USA) diluted in 0.1% Triton-X-100-PBS. The cells were then incubated with a 1:200 dilution of the primary antibodies in blocking buffer overnight at 4°C. On the following day, the cells were incubated with an appropriate fluorophore-conjugated secondary antibody (Invitrogen, USA). DAPI was used to briefly stain the nucleus before the images were captured. The cells were subsequently imaged using a fluorescence or optical microscope (Olympus, Tokyo, Japan).
2.8. Real-time quantitative polymerase chain reaction (qPCR)
The total RNA was isolated from the cells using Trizol reagent (Takara, Dalian, China) according to the manufacturer's protocol. The RNA concentration was quantified by measuring the absorbance at 260 nm, and RNA purity was determined using the A260/A280 ratio. The total RNA (1 µg) from each tissue sample was reverse transcribed to cDNAs using a Prime Script RT Reagent Kit (Takara, Dalian, China) and the following reaction: 8 µl of 5× Prime Script Buffer (for real time), 2 µl of Prime Script RT Enzyme Mix, 0.1 nmol oligo dT Primer, 0.2 nmol random hexamers, 2 µg of total RNA and RNase-free deionized water to a final volume of 40 µl. The following reverse transcription program was performed: 15 min at 37°C, and 5 s at 85°C. The specific primers (Bax-F: 5′-CAGGATGCGTCCACCAAGAA-3′, R: 5′-CAAAGTAGAAGAGGGCAACCAC-3′; Bcl-2-F: 5′-ATGACTTCTCTCGTCGCTACCG-3′, R:5′-CATCCCAGCCTCCGTTATCC-3′; caspase 3-F: 5′-CATCCCAGCCTCCGTTATCC-3′, R: 5′-AGGGACTGGATGAACCACGAC-3′; caspase 9-F: 5′-AGAACGACCTGACTGCCAAGA-3′, R: 5′-GAGGATGACCACCACAAAGCA-3′; E-cadherin-F: 5′-ATCAAATCCAACAGGGACAAAGA-3′, R: 5′-TGACACGGCATGAGAATAGAGG-3′; and Vimentin-F: 5′-GCAGCCTCTATTCCTCATCCC-3′, R: 5′-TGTAGTTGGCAAAGCGGTCAT-3′) were designed with Primer Premier 5.0. All primers were synthesized by Sangong Biotechnology. The ABI 7300 (ABI, USA) apparatus was used for reverse transcription (RT)-PCR amplification and detection. The RT-PCR reactions were prepared in triplicate in 20 µl reaction mixtures as follows: 10 µl of 2× SYBR Premix Ex Taq II, 0.4 µM forward and reverse primers, respectively, 2 µl of the cDNA (50 ng) templates, and 6.4 µl of RNase-free water. A master mix without the cDNA template was used as a negative control. The RT-PCR cycling conditions were the same as those suggested in the instructions of the SYBR Premix Ex Taq II kit (Takara, Dalian, China). The melting curves were analyzed to ensure the specificity of the PCR products in the SYBR green reactions. The relative mRNA levels of the target genes were normalized to β-actin.
2.9. Western blotting assay
The proteins were electrophoretically resolved on a denaturing 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and electrotransferred (SDS-PAGE) onto nitrocellulose membranes. The membranes were initially blocked with 5% nonfat dry milk in tris-buffered saline for 2 h and then probed with antibodies against BAX, Bcl-2, caspase 3, caspase 9, E-cadherin, Vimentin, p-Akt, Akt and β-actin. After an overnight incubation with the primary antibodies at 4°C, the membranes were hybridized with secondary goat anti-mouse or goat anti-rabbit antibodies (Santa Cruz Biotechnology, CA, USA) for 2 h at room temperature. The immunopositive bands were developed using an enhanced chemiluminescence detection system (Pierce, Thermo Co. Ltd., USA) for horseradish peroxidase and exposed to autoradiography film (Bio-Rad, Co., Ltd, USA) to visualize the bands. β-actin was used as a loading control. The relative amounts of the various proteins were analyzed. The results were quantified using ImageJ software.
2.10. Statistical analysis
Each experiment was performed at least thrice. All results are expressed as the means ± standard deviation (SD). Statistical significance was assessed with an unpaired Student's t-test and one-way analysis of variance (ANOVA) (Tukey's post hoc test) using GraphPad Prism software Version 5.0a (GraphPad Software Inc., California, USA). *p < .05, ** p < .01, ***p < .001, compared with the control (0 μM) group.
3. Results
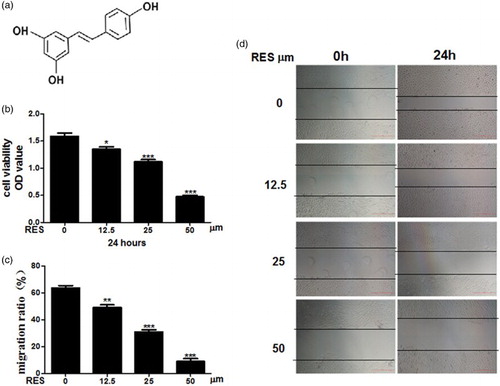
3.1. Resveratrol inhibits the growth and migration of PC-3 prostate cancer cells
The molecular structure of RES is presented in (a). The effect of RES on PC-3 cell proliferation and viability was determined using the MTT assay. The results showed that the viability of the PC-3 cells was dose dependently decreased in the presence of RES. The optimal inhibitory concentration was approximately 50 μM RES, which exhibited the most inhibition of PC-3 cell growth ((b)). Cell migration was determined using a wound-healing assay. As shown in (c) and (d), the control group exhibited marked cell migration toward the wound area 24 h after wounding, whereas the cells that were treated with different concentrations (12.5, 25 or 50 μM) of RES exhibited dose-dependent delays in wound closure. Based on these results, 50 μM RES was used to treat the cells in the subsequent experiments. The findings described above revealed that RES might be an effective inhibitor of prostate cancer cell proliferation and migration.
Figure 1. Resveratrol inhibits prostate cancer cells. (a) The molecular structure of resveratrol. (b) Cell proliferation was analyzed with the MTT assay. (c) Quantitative results of PC-3 cell migration. (d) Cell migration was evaluated using a wound-healing assay. Representative images are shown at 0 and 24 h post-wounding with ×40 magnification. Scale bars = 500 μm. Statistical significance was assessed by one-way ANOVA.
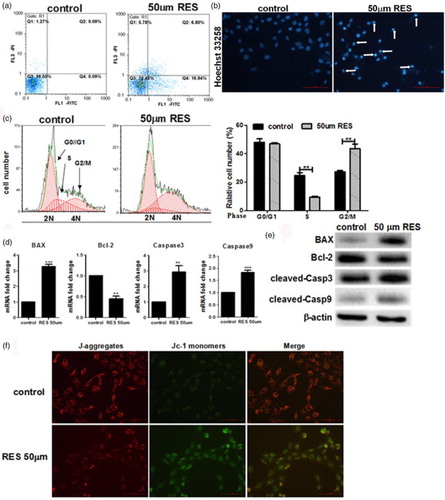
3.2. Resveratrol induces apoptosis in PC-3 prostate cancer cells
PC-3 cell apoptosis was investigated using an Annexin V/PI assay, a cell cycle assay, the expression of apoptosis-related genes and the mitochondrial membrane potential (ΔΨm). The AnnexinV/PI assays revealed a significant increase in the apoptotic population in the RES-treated group compared with the control group ((a)). Changes in nuclear morphology were tested by Hoechst 33258 staining. The normal nucleus showed a homogeneous staining and rounded shapes. Most cells showed an asymmetrical, highly bright fluorescence and the number of condensed nuclei increased in RES-treated group cells ((b)). The FACS cell cycle results revealed that the proportion of cells in G2/M phase was significantly increased after treatment with RES. These results suggest that RES increases the number of PC-3 cells in the G2/M phase and subsequently induces apoptosis ((c)). We also investigated the cleavage of caspase 3 and caspase 9 as well as the expression of the proapoptotic gene BAX and the antiapoptotic gene Bcl-2 using qPCR and Western blot assays. As shown in (d) and (e), the expression of the apoptotic gene Bax was significantly up-regulated in the RES-treated group, whereas the antiapoptotic Bcl-2 gene was markedly decreased. Similarly, the fluorescence microscopy results for the mitochondrial membrane potential revealed that the RES-treated cells exhibited more green JC-1 fluorescence than the control, indicating that the mitochondrial membrane potential was reduced ((f)). These data indicated that RES induces apoptosis in PC-3 prostate cancer cells.
Figure 2. Resveratrol induces apoptosis in prostate cancer cells. (a) Apoptosis was determined by Annexin V/PI double staining followed by flow cytometry. (b) Nucleic morphology were stained with Hoechst 33258; the white arrows represent location of apoptosis cells. (c) Flow cytometry analysis of the cell cycle in PC-3 cells and quantification of the number of cells in G0/G1, S and G2/M phases. (d, e) Real-time PCR and Western blot analyses of the apoptosis-related proteins. (f) Results of the ΔΨm test in the cells, ×200 magnification. Scale bars = 100 μm. Statistical significance was assessed by the unpaired Student's t-test.
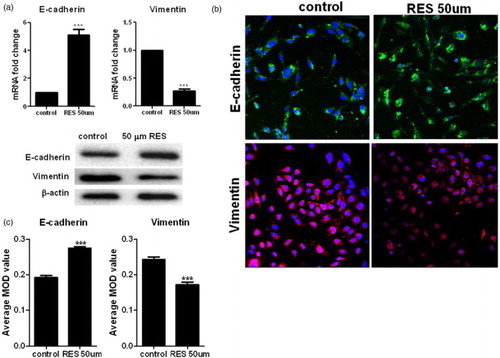
3.3. Resveratrol can suppress the EMT in PC-3 prostate cancer cells
To further determine whether RES can inhibit the EMT in PC-3 prostate cancer cells, the expression of the EMT phenotypic markers E-cadherin and Vimentin was detected using qPCR and Western blot analyses. The results from the qPCR and Western blots ((a)) demonstrate that the levels of the E-cadherin mRNA and protein were significantly increased in the RES-treated cells, whereas Vimentin levels were significantly decreased in the RES-treated cells compared with the control group. We also analyzed the RES-treated PC-3 cells using a fluorescence immunostaining assay and fluorescence microscopy. In the RES-treated cells, the fluorescence signal for E-cadherin was increased compared with control cells, and the Vimentin fluorescence signal was reduced ((b)). The mean optical densities of these proteins are provided in (c). These results further indicate that RES inhibits the EMT in PC-3 cells.
Figure 3. Resveratrol inhibits the EMT in prostate cancer cells. (a) Real-time PCR and Western blot analyses of the expression of E-cadherin and Vimentin in the cells. (b) Immunofluorescence detection of the E-cadherin and vimentin proteins in the PC-3 cells at ×200 magnification. Green and red indicates the protein of interest. Scale bars = 100 μm. (c) Quantification of E-cadherin and Vimentin expression using Image-Pro Plus 6.0 software. Statistical significance was assessed by the unpaired Student's t-test.
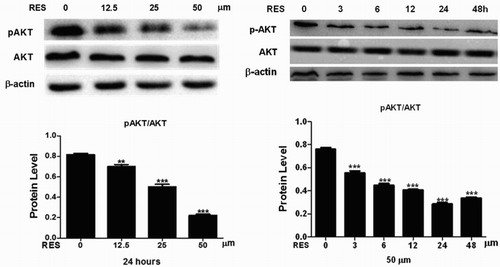
3.4. Resveratrol down-regulated the activation of the PI3K/Akt pathway
The serine/threonine kinase Akt, a downstream effector of PI3K, is involved in cell survival and antiapoptotic signaling in cancer. In this study, we observed that Akt phosphorylation was significantly decreased in the RES-treated PC-3 cells in a time- and dose-dependent manner; however, the levels of the total Akt protein were not altered in the Western blot assay (). This result indicates that RES inactivates the PI3K/Akt signaling pathway.
3.5. Effect of the PI3K/Akt signaling pathway on prostate cancer cell apoptosis and EMT
To assess whether the PI3K/Akt signaling pathway plays an essential role in prostate cancer growth and metastasis, the effect of an Akt inhibitor and an activator of PC-3 cell apoptosis and EMT were determined by FACS, qPCR and Western blot assays. The results showed that apoptosis was significantly promoted after the addition of the Akt inhibitor compared with the RES-treated group. However, apoptosis was significantly suppressed after the addition of the Akt activator compared with the RES-treated group, and changes similar to the control group were noted ((a)). Based on the qPCR results, the expression of the Bax, caspase 3 and caspase 9 mRNAs was increased when the Akt inhibitor was added, but the expression of the Bcl-2 mRNA was decreased compared with the RES-treated group ((b)). Accordingly, the expression of the Bax, caspase 3, caspase 9 and Bcl-2 proteins was consistent with their mRNA expression patterns ((c)).
Figure 5. Resveratrol promotes apoptosis and suppresses the EMT in PC-3 cells by suppressing the PI3K/Akt signaling pathway. (a) Apoptosis was determined by Annexin V/PI double staining followed by flow cytometry. (b, d) Real-time PCR and western blot analyses of the apoptosis-related proteins. (c, e) Real-time PCR and Western blot analyses of the EMT-related proteins. Statistical significance was assessed by one-way ANOVA.
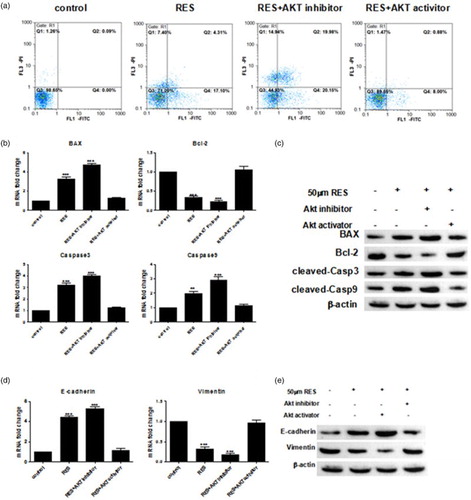
Furthermore, we determined the expression levels of the EMT-related genes after the cells were treated with RES with or without the Akt inhibitor and activator. As shown in (d), the Akt inhibitor up-regulated the mRNA level of the epithelial marker E-cadherin, whereas the expression of the mesenchymal marker Vimentin was significantly decreased. This finding implied that the addition of the Akt inhibitor aggravated the inhibitory effect of RES on the EMT. However, the Akt activator significantly reversed all these RES-induced effects, and the levels gradually returned to their former levels. As shown in (e), the protein levels of the EMT-related factors were consistent with the mRNA results. Taken together, these findings demonstrate that RES promotes apoptosis and suppresses the EMT in PC-3 cells by inhibiting the PI3K/Akt signaling pathway.
4. Discussion
In recent years, prostate cancer has been one of the most commonly diagnosed cancers in men in the US (Siegel et al. Citation2014). Despite the frequency of prostate cancer diagnoses, there are few prognostic biomarkers available for clinical use (Bhavsar et al. Citation2013; Tombal et al. Citation2014; Van der Kwast Citation2014). The EMT has an important role not only in embryonic development but also in tumor invasiveness and development (Colas et al. Citation2012). The EMT can allow cells to migrate, invade and metastasize, thus enhancing tumor development and progression (Franco-Chuaire et al. Citation2013).
Many studies have suggested that RES suppresses the growth of many cancer cells through multiple, intricate cellular signaling pathways, such as the STAT, NF-κB and Wnt signaling pathways (Vang et al. Citation2011; Aluyen et al. Citation2012; Whitlock & Baek Citation2012). Some studies also reported that RES inhibits the EMT process in pancreatic cancer cells by inhibiting the PI-3 K/Akt pathway (Li et al. Citation2013). RES inhibits the EMT in MCF-7 cells (Vergara et al. Citation2011). The PI-3 K/Akt signaling pathway has long been associated with pancreatic cancer metastasis. Tanno et al. (Citation2001) demonstrated that the active Akt-induced insulin-like growth factor I receptor expression markedly enhances the invasiveness of human pancreatic cancer cells. However, the mechanism by which RES suppresses tumor remains unclear and requires additional research.
In conclusion, the current study demonstrates that RES plays an important role in suppressing the proliferation and migration of prostate cancer cells in vitro by modulating apoptosis and the EMT via the PI3K/Akt signaling pathway. We also implied that the RES induce apoptosis in PC-3 cells via a BAX-dependent pathway. In our next research, we will focus on the type of apoptosis and what type of apoptosis is the crucial for RES induced in this cell. These results suggest that RES might be a potential anti-cancer agent for the treatment of prostate cancer and also provide a novel mechanistic theory for the therapeutic application of RES in prostate cancer patients.
Disclosure
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors thank the Colleague of the Department of Urology in Xiangya Hospital of Central South University for their excellent assistance and use of their many instruments.
Additional information
Funding
References
- Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, Bellanger RA. 2012. Resveratrol: potential as anticancer agent. J Diet Suppl. 9:45–56. doi: 10.3109/19390211.2011.650842
- Baur JA. 2010. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev. 131:261–269. doi: 10.1016/j.mad.2010.02.007
- Bhavsar T, McCue P, Birbe R. 2013. Molecular diagnosis of prostate cancer: are we up to age? Semin Oncol. 40:259–275. doi: 10.1053/j.seminoncol.2013.04.002
- Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al. 2011. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 13:317–323. doi: 10.1038/ncb2173
- Colas E, Pedrola N, Devis L, Ertekin T, Campoy I, Martinez E, Llaurado M, Rigau M, Olivan M, Garcia M, et al. 2012. The EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol. 14:715–720. doi: 10.1007/s12094-012-0866-3
- Franco-Chuaire ML, Magda Carolina SC, Chuaire-Noack L. 2013. Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin. 54:186–205.
- Fulda S. 2010. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov Today. 15:757–765. doi: 10.1016/j.drudis.2010.07.005
- Hance MW, Dole K, Gopal U, Bohonowych JE, Jezierska-Drutel A, Neumann CA, Liu H, Garraway IP, Isaacs JS. 2012. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J Biol Chem. 287:37732–37744. doi: 10.1074/jbc.M112.389015
- Knobloch J, Hag H, Jungck D, Urban K, Koch A. 2011. Resveratrol impairs the release of steroid-resistant cytokines from bacterial endotoxin-exposed alveolar macrophages in chronic obstructive pulmonary disease. Basic Clin Pharmacol Toxicol. 109:138–143. doi: 10.1111/j.1742-7843.2011.00707.x
- Kundu JK, Surh YJ. 2008. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 269:243–261. doi: 10.1016/j.canlet.2008.03.057
- Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu Q, Duan W, Yu S, Wang F, et al. 2013. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kappaB pathway. Curr Med Chem. 20:4185–4194. doi: 10.2174/09298673113209990251
- Lu T, Lin WJ, Izumi K, Wang X, Xu D, Fang LY, Li L, Jiang Q, Jin J, Chang C. 2012. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 26:1707–1715. doi: 10.1210/me.2012-1079
- Pervaiz S, Holme AL. 2009. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 11:2851–2897. doi: 10.1089/ars.2008.2412
- Pirola L, Frojdo S. 2008. Resveratrol: one molecule, many targets. IUBMB Life. 60:323–332. doi: 10.1002/iub.47
- Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, Srivastava RK. 2011. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 6:e16530. doi: 10.1371/journal.pone.0016530
- Siegel R, Ma J, Zou Z, Jemal A. 2014. Cancer statistics, 2014. CA Cancer J Clin. 64:9–29. doi: 10.3322/caac.21208
- Snyder A, Tepper JE, Slovin SF. 2013. Perspectives on immunotherapy in prostate cancer and solid tumors: where is the future? Semin Oncol. 40:347–360. doi: 10.1053/j.seminoncol.2013.04.009
- Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, Wei W. 2006. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 165:9–19. doi: 10.1016/j.cancergencyto.2005.06.016
- Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. 2001. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 61:589–593.
- Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial-mesenchymal transitions in development and disease. Cell. 139:871–890. doi: 10.1016/j.cell.2009.11.007
- Tombal B, Alcaraz A, James N, Valdagni R, Irani J. 2014. Can we improve the definition of high-risk, hormone naive, non-metastatic prostate cancer? BJU Int. 113:189–199. doi: 10.1111/bju.12469
- Van der Kwast TH. 2014. Prognostic prostate tissue biomarkers of potential clinical use. Virchows Arch. 464:293–300. doi: 10.1007/s00428-014-1540-7
- Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, et al. 2011. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 6:e19881. doi: 10.1371/journal.pone.0019881
- Vergara D, Simeone P, Toraldo D, Del Boccio P, Vergaro V, Leporatti S, Pieragostino D, Tinelli A, De Domenico S, Alberti S, et al. 2012. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol Biosyst. 8:1078–1087. doi: 10.1039/c2mb05486h
- Vergara D, Valente CM, Tinelli A, Siciliano C, Lorusso V, Acierno R, Giovinazzo G, Santino A, Storelli C, Maffia M. 2011. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 310:1–8. doi: 10.1016/j.canlet.2011.04.009
- Whitlock NC, Baek SJ. 2012. The anticancer effects of resveratrol: modulation of transcription factors. Nutr Cancer. 64:493–502. doi: 10.1080/01635581.2012.667862
- Yilmaz M, Christofori G. 2010. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 8:629–642. doi: 10.1158/1541-7786.MCR-10-0139