ABSTRACT
Plant-derived multiple monoclonal antibody (mAb) CO17-1A × BR55 (mAbP CO17-1A × BR55) produced in transgenic tobacco plants were cross-pollinated with mAb CO17-1A and mAb BR55. Human anti-colorectal cancer multiple mAb CO17-1A × BR55 was cloned using pBI121 vector. Mice were given a single intraperitoneal injection of azoxymethane (AOM) with 10 mg/kg body weight. Starting 1 week after the injection, mice received 2% dextran sulfate sodium (DSS) in the drinking water for 1 week. In addition, the mice were injected intraperitoneal with mAbs dissolved in phosphate buffered saline (100 μg/mouse) twice per week for 4 weeks. Apoptotic cell death, expression of pro-apoptotic proteins, activity of inflammatory cytokines and ERK pathway phosphorylation were assayed by Western blot and TUNEL kit. mAbP CO17-1A × BR55 meaningfully and efficiently suppressed the development of AOM/DSS-induced colorectal inflammation and colorectal tumors, as determined by a reduced activation of inflammatory cytokines, number of colorectal tumor-induced mouse, number of tumor per mouse colon than other mAbs. Cell death by apoptosis was much increased in the mAbP CO17-1A × BR55-treated tumor compared with negative control. Apoptotic cell death and expression of pro-apoptotic proteins including Bax and cleaved caspase-3 were highest in treatment with mAbP CO17-1A × BR55. In addition, mAbP CO17-1A × BR55 was meaningfully decreased the expression of inflammatory cytokines, including COX-2, iNOS, p50 and p65, but the expression of PPARγ was significantly increased compared with AOM/DSS-induced carcinogenesis negative control. Moreover, mAbP CO17-1A × BR55 meaningfully repressed the ERK1/2 phosphorylation in AOM/DSS-induced colorectal tumors. Therefore, our results suggest that multiple mAbP CO17-1A × BR55 have meaningful effects of anti-inflammation related with the anti-carcinogenesis in AOM/DSS-induced colorectal tumor by inhibition of ERK1/2 phosphorylation.
Introduction
Colorectal carcinoma (CRC) is one of the most common cancers worldwide (Yoo et al. Citation2011). CRC occurs mainly in the inside of the rectum or colon. Variety of CRC animal models is an important tool in studying the complex development and pathogenesis of CRC. The studies using animal model cannot substitute human clinical trials, but they must be suitably used in preclinical studies. Actually, these models are capable tools in furthering our understanding of novel chemopreventive and chemotherapeutic strategies in tumor. The azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced CRC animal models have proved to dramatically shorten the latency time for induction of CRC and has become an outstanding model for chemopreventive studies and studying colon carcinogenesis. Therefore, this study used the AOM/DSS-induced CRC animal models for studying chemopreventive characteristic of plant-derived antibodies. Environmental factors that induced inflammation have been suggested to augment the growth of CRC. Several studies have shown that inflammatory bowel disease is associated with colorectal cancer (Seril et al. Citation2002a, Citation2002b). These studies indicate that inflammation plays an important role in colon carcinogenesis (Osawa et al. Citation2003).
Peroxisome proliferator-activated receptor γ (PPARγ), known as a ligand-activated transcription factor, is expressed in several tissues and cell types, including kidney, pancreas, adipose, liver tissue and colon (Dubuquoy et al. Citation2006). Further, a previous study indicates that PPARγ plays an important role in regulating inflammatory responses in the bowels (Bassaganya-Riera et al. Citation2004). PPARγ, known as essential modulators, can regulate nuclear factor-κB (NF-κB) activation in anti-inflammation (Yong et al. Citation2016). Several studies have reported that PPARγ can suppress inflammatory expression by direct interaction with NF-κB, p65 and p50 subunits (Ricote & Glass Citation2007). In addition, a previous study reported that cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are significantly associated with NF-κB in AOM/DSS-induced colonic carcinoma (Tanaka et al. Citation2003).
Recently, advancement of cancer chemopreventive tools including suppression to invasive cancer or natural/synthetic agent for the prevention has been documented as a field with large potential to anti-cancer therapy (Mann et al. Citation2005). Moreover plants are being developed as engineering platform for a production of pharmaceutical proteins, and have advantages such as the ability to assemble, low costs and ability to modify monoclonal antibodies (mAbs) (Hehle et al. Citation2011). In recent years, using of monoclonal antibodies (mAbs) directly related to tumor-associated antigen is a therapeutic strategy. Currently a study reported that EpCAM (also known as CO17-1A) was produced from a recombinant plant, and it induced an immune response in BALB/C mice (Brodzik et al. Citation2008). The monoclonal antibody CO17-1A (mAb CO17-1A) is antigen GA733 as well-known highly expressed in human CRCs (Koprowski et al. Citation1979). In high-risk patient of colorectal cancer, the mAb CO17-1A is efficacious for the prevention of micro metastasis and recurrent of colorectal cancer (Riethmuller et al. Citation1998). In addition, their previous studies have recently reported that recombinant mAb CO17-1A has been expressed in tobacco plant (Verch et al. Citation1998) and that recombinant mAb can be safely purified from tobacco plant (Ko et al. Citation2003). However, anti-inflammatory effects of plant-derived multiple monoclonal antibody CO17-1A × BR55 (multiple mAbP CO17-1A × BR55) related to anti-cancer in AOM/DSS-induced colorectal cancer animal model are unclear.
The mitogen-activated protein kinase (MAPK) family regulated cell death, proliferation and migration (Tarallo & Sordino Citation2004). The MAPK family constitutes the three major subgroups, namely c-Jun N-terminal of stress-activated protein kinase 1/2 (JNK 1/2), extracellular regulated kinase 1/2 (ERK1/2) and the p38 protein kinases (Jung et al. Citation2004). The ERK 1/2 associated with cell proliferation and survival (Marques-Fernandez et al. Citation2013). The activation of ERK1/2 can induce apoptosis via a caspases-dependent pathway in neurons (Anderson & Tolkovsky Citation1999). In addition, ERK1/2, known the regulating inflammatory signaling pathway in endothelium intimal hyperplasia (Peng et al. Citation2008), is an anti-inflammatory signal that suppresses expression of NK-κB-dependent inflammatory genes (Maeng et al. Citation2006).
Therefore, the purpose of this study was to investigate the anti-inflammatory effects related to anti-cancer effects of plant-derived multiple monoclonal antibody CO17-1A x BR55 (multiple mAbP CO17-1A × BR55) in AOM/DSS-induced colorectal cancer of mouse.
Materials and methods
Generation of transgenic plants and purification of plant-derived mAbs and multiple mAb
Human anti-breast cancer mAb BR55, human anti-colorectal cancer mAb CO17-1A and multiple mAb CO17-1A × BR55 were cloned using pBI121 vector. Plant transformation was piloted with both the binary vectors, and transgenic lines expressing mAb BR55, mAb CO17-1A and multiple mAb CO17-1A × BR55 were acquired as followed in a previous study (Ko et al. Citation2005). To purify of mAbs-form plants, 200 g transgenic tobacco plant leaves were homogenized in 1 L extraction buffer, and were centrifuged (15,000 g for 30 min at 4°C). The supernatant was filtered in Miracloth (Calbiochem) and the protein that triggered saturation in (NH4)2SO4 was collected by centrifugation. The final solution was then centrifuged at 25,000 g for 30 min at 4°C, and the supernatants were converted to a protein A column (GE Healthcare), and mAb was extracted according to the manufacturer’s recommendations and overnight dialysis against phosphate buffered saline (PBS) (pH 7.4). The mAbs were concentrated to 1 mg/ml by an Amicon Ultra spin column and stored at 80°C.
Anti-tumor activity study in AOM/DSS-induced colorectal cancer animal model
Six-week-old male ICR mice were purchased from SAMTAKO Co., (Kyunggi-do, Korea). The animals were fed sterilized pellet diet (Samtako, Osan, Korea) and sterilized water ad libitum. All studies were performed in accordance with the Guild for Animal Experimentation by the Wonkwang University and approved by the Institutional Animal Care and Use Committee of Wonkwang University (Approval No. WKU13-33). All efforts were made to minimize pain or discomfort of animals used. AOM was purchased from Sigma-Aldrich. DSS (MW 36,000–50,000) was purchased from MP Biomedicals, LLC (Aurora, OH) and was dissolved in water at a concentration of 2% (w/v). Mice were given a single intraperitoneal injection of AOM with 10 mg/kg body weight. Starting 1 week after the injection, mice received 2% DSS in the drinking water for 1 week. In addition, the mice were injected intraperitoneal with anti-EpCAM, multiple mAbp CO17-1A × BR55 and mAbp CO17-1A were dissolved in PBS (100 μg/mouse) twice per week for 4 weeks. At the end of the experiment, the mice were killed by cervical dislocation. The tumors were counted in colon, and histopathological examination was performed on paraffin-embedded sections after hematoxylin and eosin (H&E) staining.
Immunohistochemistry
All samples were fixed in formalin and embedded in paraffin for examination. Paraffin sections were deparaffinized and rehydrated, were washed in distilled water, and were exposed to heat-mediated antigen retrieval treatment. 2% hydrogen peroxide quenched the endogenous peroxidase activity by treatment of methanol for 15 min, and washed in PBS for 5 min. 3% normal horse serum was blocked the sections for 30 min and incubated with primary COX-2, iNOS, p50 and p65 (1:200 dilutions) left overnight at 4°C. After 24 h, sections were washed thrice by PBS for 5 min respectively and were incubated with biotinylated anti-mouse and anti-rabbit antibody at real temperature for 2 h. The slides were incubated with the avidin–biotin–peroxidase complex (ABC, Vector Laboratories, Inc., Burlingame, CA). The slide samples were washed and the peroxidase reaction was developed by diaminobenzidine and peroxide.
Detection of apoptotic cell death
Colon tissues were made a paraffin section. After the colon sections were washed twice with PBS at room temperature, propidium iodide (PI; Sigma) staining and TdT-mediated dUTP nick labeling (TUNEL) assays were achieved using the in situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions.
Western blot
Briefly, colorectal tumor tissues were homogenized with radioimmunoprecipitation assay buffer (Sigma), and then centrifuged with 13,000 rpm for 20 minutes at 4°C. Proteins (20 µg/Lane) were loaded on 10% SDS-PAGE gel and then transferred to polyvinylidene difluoride membrane (Millipore, NJ). The blots were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline for 2 h, and the membrane was incubated overnight with a primary antibody: ERK1/2, phosphorylation-ERK1/2 (p-ERK1/2), Akt, phosphorylation-Akt (p-Akt), BCl-2, Caspase-8, Caspase-9, Caspase-3, COX-2, iNOS, p50, p65, pIκBα, PPARγ and β-actin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C. Then it was incubated with the matching horseradish peroxidase (HRP) conjugated anti-mouse and anti-rabbit secondary antibody (Santa Cruz Biotechnology), and proteins were extracted by the enhanced chemiluminescence system (Pierce, Rockford, IL).
Statistical analysis
All data are accessible as mean (SD). Multi-group association was executed using one-way ANOVA and two-way ANOVA, followed by Tukey’s and Bonferroni post hoc pairwise comparison. P value < .05 was considered statistically important. All statistical investigations were executed using GraphPad Prism (Ver. 5.00; GraphPad Software Inc., La Jolla, CA). Therefore, this study results propose that multiple mAbP CO17-1A × BR55 have meaningful and excellent effects on apoptotic anti-cancer by suppression of ERK 1/2 phosphorylation than anti-EpCAM and mAbP CO17-1A in the AOM/DSS-induced colorectal tumor animal model.
Results
Obtaining purified multiple mAbP CO17-1A × BR55 from transgenic plant leaves
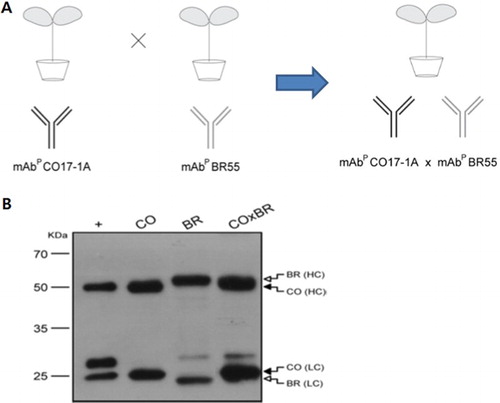
Transgenic tobacco plants were obtained the expression of vector carrying mAb BR55, mAb CO17-1A and multiple mAb CO17-1A × BR55. Cross-pollinating with mAb BR55 and mAb CO17-1A generated the transgenic tobacco plants expressing multiple mAb CO17-1A × BR55 ().
Figure 1. Generation of transgenic tobacco plants expressing anti-cancer mAb CO17-1A, mAb BR55 and multiple mAb CO17-1A × BR55, and its purification. (a) Schematic of transgenic plants generation expressing multiple mAb CO17-1A × BR55 by cross-pollinate of mAb CO17-1A and mAb BR55. (b) Western blot of purified mAbM CO17-1A (40 ng), mAbP CO17-1A (40 ng), mAbP BR55 and multiple mAbP CO17-1A × BR55. Heavy chain (50 kDa) was detected by anti-murine Fc conjugated HRP and light chain (25 kDa) was detected by anti-murine F (ab′) 2 conjugated HRP, respectively.
Multiple mAbP CO17-1A × BR55 suppress the AOM/DSS-induced colorectal tumor in mice colon
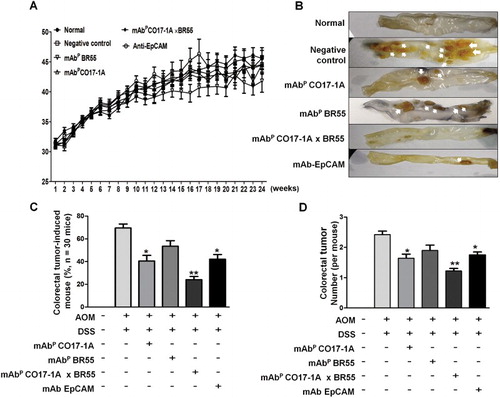
The effect of multiple mAbP CO17-1A × BR55 on AOM/DSS-induced colorectal tumor growth was analyzed in the AOM/DSS mouse model. Body weight was not significantly changed visibly ((a)). After the treatment of AOM (10 mg/kg) and 2% DSS to mice for 4 weeks, the colorectal tumor number was recorded. It has been revealed that induced colorectal tumor was significantly suppressed in all mice groups treated with mAbP CO17-1A, anti-EpCAM and multiple mAbP CO17-1A × BR55 ((b) and (c)), respectively. The induced tumor number was significantly decreased respectively 65% with multiple mAbP CO17-1A × BR55 treatment, 40% with mAbP CO17-1A treatment and 25% with anti-EpCAM compared with negative control ((c)). Especially, the number of mouse of induced colorectal tumor was higher in treatment of multiple mAbP CO17-1A × BR55 than mAbP CO17-1A and anti-EpCAM ((b) and (c)). Additionally, the number of induced colorectal tumor was meaningfully lower in multiple mAbP CO17-1A × BR55-treated colons ((d)).
Figure 2. Anti-efficacy of multiple mAbP CO17-1A × BR55 in AOM/DSS-induced colorectal tumor. (a) Body weight of mice was injected with AOM/DSS and mAbs for 24 weeks. (b) Inhibition of AOM/DSS-induced colorectal tumor in mAbs-treated mice. (c) Number of colorectal tumor was induced in mouse by AOM/DSS (n = 30) and (d) tumor per mouse (n = 30). **P < .01, *P < .05 indicates a statistically significant difference from the negative control group.
Multiple mAbP CO17-1A × BR55 induce the apoptotic cell death in AOM/DSS-induced colorectal tumor
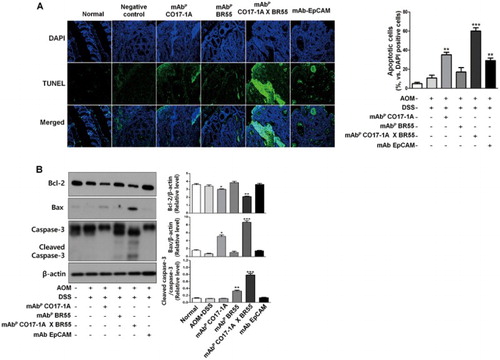
Furthermore, anti bodies including anti-EpCAM, mAbP CO17-1A and multiple mAbP CO17-1A × BR55 ((a)). Strong inhibition of colorectal tumor induction by plant-derived monoclonal antibodies including anti-EpCAM, mAbP CO17-1A and multiple mAbP CO17-1A × BR55 indicated that the inhibition of colorectal tumor growth was induced by apoptosis. Therefore, we measured the changes in apoptotic cell death through DAPI and TUNEL double staining and Western blot. The frequency and intensity of fluorescence was significantly increased in colorectal tumor treated with anti-EpCAM, mAbP CO17-1A and multiple mAbP CO17-1A × BR55. Moreover, in treatment of multiple mAbP CO17-1A × BR55, apoptotic cell death peaked more than that in mAbP CO17- and 1A anti-EpCAM ((a)). However, apoptotic cell death was weak in cells treated with mAbP BR55. This study subsequently examined the relationship between the expression of apoptotic regulatory proteins and the induction of apoptosis. Results showed that expression of anti-apoptotic proteins such as BCl-2 was meaningfully decreased by treatment with mAbP CO17-1A and multiple mAbP CO17-1A × BR55 compared to the negative control ((b)). In particular, the multiple mAbP CO17-1A × BR55 were markedly inhibited the Bcl-2 expression more than other mAbs ((b)). However, treatment of mAbP BR55 and anti-EpCAM did not affect the appearance of anti-apoptotic proteins. Pro-apoptotic proteins such as Bax and cleaved caspase-3 significantly increased after treatment with mAbp, pAbp and EpCAM, respectively ((b)). Moreover, multiple mAbP CO17-1A × BR55 induced higher pro-apoptotic regulatory protein expression than anti-EpCAM and mAbP CO17-1A ((b)).
Figure 3. Multiple mAbP CO17-1A × BR55 induce the apoptotic cell death in AOM/DSS-induced colorectal tumor of mice. (a) Apoptotic cell death was determined by TUNEL assay. The TUNEL-positive (green) cells are apoptotic cells (200× magnification). Apoptotic cells (TUNEL-positive cells) were estimated by direct counting of fragmented nuclei after TUNEL staining. (b) Apoptotic cell death was determined by Western blot. An equal amount of total protein (30 μg/lane) was subjected to 12% SDS-PAGE. Expression of cleaved Caspase-3, Bcl-2, Bax and β-actin were detected by Western blot using specific antibodies. Here, β-actin protein was used as an internal control. ***P < .001, **P < .01, *P < .05 indicate a statistically significant difference from the negative control group.
Multiple mAbP CO17-1A × BR55 inhibited the colorectal tumorigenic inflammation in AOM/DSS-induced colorectal tumor of mice colon
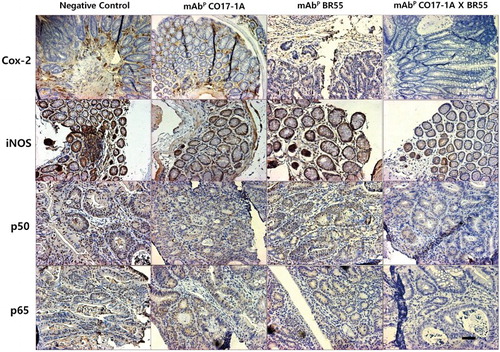
We subsequently investigated the relationship between the expression of inflammatory proteins and the reduction of inflammation. The examination by Western blot showed that expression of inflammatory proteins including COX-2, iNOS, p50 and p65 was meaningfully decreased by treatment with mAbP CO17-1A and multiple mAbP CO17-1A × BR55 compared to the negative control (). In particular, the multiple mAbP CO17-1A × BR55 markedly inhibited the inflammatory proteins expression more than other mAbs (). However, PPARγ was significantly increased after treatment with mAbp and EpCAM, respectively (). Moreover, multiple mAbP CO17-1A × BR55 induced higher PPARγ expression than anti-EpCAM and mAbP CO17-1A (). These results suggested that multiple mAbP CO17-1A × BR55 displayed highest anti-inflammation effects in induction of colorectal tumor in the AOM/DSS mouse model. The immunohistochemical analysis of colorectal tumor sections by anti-EpCAM, mAbP CO17-1A and multiple mAbP CO17-1A × BR55 markedly decreased the expression of inflammatory proteins, including COX-2, iNOS, p50 and p65, compared with negative control ().
Figure 4. Multiple mAbP CO17-1A × BR55 inhibit the inflammation in AOM/DSS-induced colorectal tumor. Inflammation was determined by Western blot. An equal amount of total protein (30 μg/lane) was subjected to 12% SDS-PAGE. Expression of COX-2, iNOS, p50, p65, pIκBα, PPARγ and β-actin were detected by Western blot using specific antibodies. Here, β-actin protein was used as an internal control. ***P < .001, **P < .01 and *P < .05 indicate a significant difference from the negative control group.
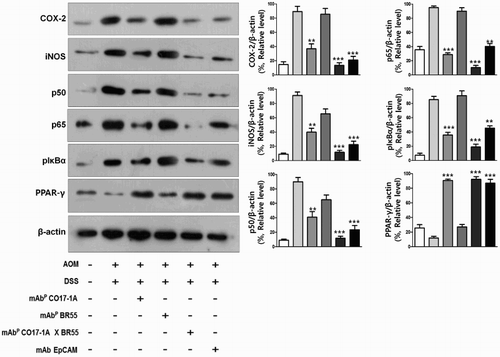
Figure 5. Multiple mAbP CO17-1A × BR55 regulates the inflammatory proteins in AOM/DSS-induced colorectal tumor tissues of mice. Expression of inflammatory proteins was determined by H&E staining and immunohistochemistry assay. Inflammatory proteins expressing cells were examined immunohistochemistry stain using the ABC kit. Each band is representative of three independent experimental results. Bar indicates 100 μm.
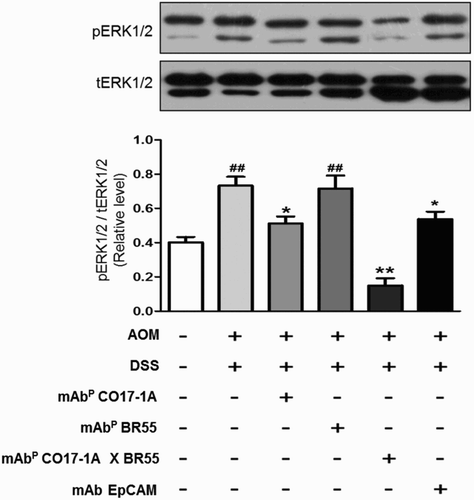
Multiple mAbP CO17-1A × BR55 downregulate the extracellular signal-regulated kinases 1 and 2 (ERK1/2) phosphorylation in AOM/DSS-induced colorectal tumors
We analyzed the markedly inhibitory effect of multiple mAbP CO17-1A × BR55 on ERK1/2 phosphorylation in colorectal tumors. The phosphorylation of ERK1/2 was significantly inhibited treatment of mAbs, including anti-EpCAM, mAbP CO17-1A and multiple mAbP CO17-1A × BR55 in AOM/DSS-induced colorectal tumors (). Particularly, treatment of multiple mAbP CO17-1A × BR55 efficiently and significantly suppressed the ERK1/2 activation more than other mAbs, including anti-EpCAM and mAbP CO17-1A in the AOM/DSS-induced colorectal tumor model ().
Figure 6. Multiple mAbP CO17-1A × BR55 inhibit the apoptosis and the ERK1/2phosphorylation in AOM/DSS-induced colorectal tumor. Apoptotic cell death was determined by Western blot. (a) An equal amount of total protein (30 μg/lane) was subjected to 12% SDS-PAGE. Expression of cleaved Caspase-3, Bcl-2, Bax and β-actin were detected by Western blot using specific antibodies. Here, β-actin protein was used as an internal control. (b) Expression of total ERK1/2 and phosphorylated ERK1/2 was detected by Western blot in AOM/DSS-induced colorectal tumors. **P < .01 and *P < .05 indicate a statistically significant difference from the negative control group. ##P < .01 indicates a statistically significant difference from the normal group.
Discussion
The study results indicated that the multiple mAbP CO17-1A × BR55-mediated inhibition of colorectal tumor induction in the AOM/DSS animal model is associated with inhibition of inflammation by downregulation of ERK1/2 phosphorylation.
CO17-1A, a human CRC-associated antigen, is highly expressed in CRC patients (Zaloudik et al. Citation2002). Several studies reported the anti-cancer effects of anti-EpCAM in colorectal cancer growth (Ko et al. Citation2005; Kwak et al. Citation2011). In this study we find that mAbP CO17-1A and multiple mAbPCO17-1A × BR55 from tobacco significantly inhibited the inflammation in AMO/DSS-induced colorectal tumor. Moreover, anti-tumor and anti-inflammatory effects of multiple mAbPCO17-1A × BR55 were significantly better than anti-EpCAM and mAbP CO17-1A alone. Chemopreventive/chemotherapeutic agent can reduce the overall growth of cancer cells by apoptosis (Kim et al. Citation2010). Therefore, we investigated the anti-inflammation and anti-colorectal tumor effects of multiple mAbP CO17-1A × BR55 in the AOM/DSS-induced colorectal tumor animal model.
We find that the development of colorectal tumor was significantly decreased by plant-derived mAbs in colon of the AOM/DSS animal model. A variety of colorectal tumors were regulated by the apoptosis pathway which is regulated by numerous anti-apoptotic proteins (e.g. BCl-XL, BCl-2), and pro-apoptotic proteins (e.g. caspase-3 and -9 and Bax) (Schoemaker et al. Citation2002). The apoptosis pathway was regulated by the ratio of pro- and anti-apoptotic proteins. The present study results showed that plant-derived mAbs inhibited the Bcl-2 expression. Moreover, multiple mAbP CO17-1A × BR55 markedly inhibited the expression of BCl-2 in AOM/DSS-induced colorectal tumor in mouse. Our Western blot results showed that multiple mAbP CO17-1A × BR55 meaningfully and effectively induce the apoptotic tumor suppression than anti-EpCAM and mAbP CO17-1A. In the contrast, pro-apoptotic proteins such as cleaved caspase-3 and caspase-9 were upregulated by plant-derived mAbs in AOM/DSS-induced colorectal tumor. In particular, multiple mAbP CO17-1A × BR55 are strongly upregulated the pro-apoptotic proteins, such as caspase-3 activation, caspase-9 activation and Bax activation, in colorectal tumor of the AOM/DSS animal model. Bax gene product dimerizes with Bcl-2 and controls cell death by activating caspase-3 (Seol et al. Citation2001). The previous study described that caspases are classified based on either initiator or effector caspases in apoptosis (Kim et al. Citation2010). Initiator caspases, caspase-8 and -9, are phosphorylated by a variety apoptotic signals (Kim et al. Citation2010). Activated or cleaved caspase-3 is an essential effector for induction of apoptosis (Xue et al. Citation2012). Previous studies’ results support our hypothesis that multiple mAbP CO17-1A × BR55 meaningfully and effectively induce the apoptosis of AOM/DSS-induced colorectal tumor than anti-EpCAM and mAbP CO17-1A.
Pro-inflammatory biomarkers significantly increased in the colonic carcinogenesis by AOM/DSS (Giner et al. Citation2016). DSS has been known to cause inflammatory damage in mouse colorectal carcinogenesis. Inflammation is prompted by factors including COX-2, iNOS, PPAR-γ and NF-κB and is an essential tumor promotor (Tanaka et al. Citation2003). In colorectal tumor, inflammation is attenuated by activation of PPARγ (Yamamoto-Furusho et al. Citation2011). In many studies it is appeared that PPARγ has the anti-cancer attribute in colorectal cancer by increasing the expression of cancer suppressor genes (Yamaguchi et al. Citation2008). PPARγ has a role as anti-inflammatory mediators by interfering with the modulation of NF-κB pathway. A previous study reported the important of NF-κB signaling pathway in the AOM/DSS-induced CRC model (Giner et al. Citation2011). The NF-κB is implicated in numerous diseases and physiological processes, particularly in regulation of inflammation. NF-κB was activated in various proto-oncogenes and carcinogens (Bharti et al. Citation2003). Previous research has reported various mechanisms by which NF-κB could contribute to the progress of CRC mouse model (Greten et al. Citation2004). Pro-inflammatory factors including COX-2 and iNOS were activated by NF-κB signaling. NF-κB was activated through IκBα phosphorylation during the promotion of colitis-associated carcinogenesis (Greten et al. Citation2004). Inflammatory cytokines including COX-2 and iNOS were increased in AOM/DSS-induced colorectal tumor. Expression of iNOS increased in DSS-induced mice colonic inflammation (Hokari et al. Citation2001). Risk of cancer development increase by inflammation is associated with upregulation of iNOS and COX-2 (Maeda & Akaike Citation1998). Several studies reported that iNOS and COX-2 expression increased in colorectal carcinogenesis (Takahashi et al. Citation2000). Expression of iNOS increased in AOM/DSS-induced mouse colon carcinogenesis (Seril et al. Citation2002b). COX-2 plays an important role as a pro-inflammatory mediator in the late phase of tumorigenesis (Li et al. Citation2015). We find that the inflammation was significantly decreased by plant-derived mAbs in AOM/DSS-induced colorectal tumor. In particular, treatments of multiple mAbP CO17-1A × BR55 significantly decreased the expression of COX-2, iNOS, p65, p50 and pIκBα, and meaningfully increased the expression of PPARγ as determined by immunohistochemistry stain and Western blot assay in AOM/DSS-induced colorectal tumor tissues.
The ERK1/2 pathway plays important roles in tumor initiation and progression (Krueger et al. Citation2009), while the inhibition of ERK pathway increases apoptosis in cancer cells (Shelton et al. Citation2004). Several studies reported that the ERK1/2 signaling pathway can induce growth inhibition and apoptosis of human prostate cancer cells (Chen et al. Citation2010) and that the ERK1/2 signaling pathway is activated in human CRC (Boulaaba et al. Citation2013). ERK1/2 phosphorylation inhibited the caspase-mediated apoptosis in cancer cells (Aoudjit & Vuori Citation2001). In addition, ERK is known to play roles in regulating inflammatory activities, such as transcriptional activity of NF-κB. A previous study described that phosphorylation of ERK was induced by DSS treatment in murine peritoneal macrophages (Kwon et al. Citation2007). The suppression of ERK activation reduced the DSS-induced inflammatory injury in mouse colon (Kobayashi et al. Citation2006). These study results support our result, which is ERK1/2 activation was meaningfully repressed by multiple mAbP CO17-1A × BR55 in AOM/DSS-induced colorectal tumor.
Therefore, this study results suggested that the multiple mAbP CO17-1A × BR55 have anti-inflammatory and anti-tumorigenic effects through inhibition of ERK1/2 activation in AOM/DSS-induced colorectal cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anderson CN, Tolkovsky AM. 1999. A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci. 19:664–673.
- Aoudjit F, Vuori K. 2001. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 152:633–643. doi: 10.1083/jcb.152.3.633
- Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. 2004. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 127:777–791. doi: 10.1053/j.gastro.2004.06.049
- Bharti AC, Donato N, Singh S, Aggarwal BB. 2003. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 101:1053–1062. doi: 10.1182/blood-2002-05-1320
- Boulaaba M, Tsolmon S, Ksouri R, Han J, Kawada K, Smaoui A, Abdelly C, Isoda H. 2013. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology. 65:927–936. doi: 10.1007/s10616-013-9564-4
- Brodzik R, Spitsin S, Golovkin M, Bandurska K, Portocarrero C, Okulicz M, Steplewski Z, Koprowski H. 2008. Plant-derived EpCAM antigen induces protective anti-cancer response. Cancer Immunol Immunother. 57:317–323. doi: 10.1007/s00262-007-0366-4
- Chen MF, Chen WC, Chang YJ, Wu CF, Wu CT. 2010. Role of DNA methyltransferase 1 in hormone-resistant prostate cancer. J Mol Medi (Berl). 88:953–962. doi: 10.1007/s00109-010-0640-3
- Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. 2006. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut. 55:1341–1349. doi: 10.1136/gut.2006.093484
- Giner E, Andujar I, Recio MC, Rios JL, Cerda-Nicolas JM, Giner RM. 2011. Oleuropein ameliorates acute colitis in mice. J Agric Food Chem. 59:12882–12892. doi: 10.1021/jf203715m
- Giner E, Recio MC, Rios JL, Cerda-Nicolas JM, Giner RM. 2016. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food Res. 60:242–255. doi: 10.1002/mnfr.201500605
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 118:285–296. doi: 10.1016/j.cell.2004.07.013
- Hehle VK, Paul MJ, Drake PM, Ma JK, van Dolleweerd CJ. 2011. Antibody degradation in tobacco plants: a predominantly apoplastic process. BMC Biotechnol. 11:128. doi: 10.1186/1472-6750-11-128
- Hokari R, Kato S, Matsuzaki K, Kuroki M, Iwai A, Kawaguchi A, Nagao S, Miyahara T, Itoh K, Sekizuka E, et al. 2001. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free Radic Biol Med. 31:153–163. doi: 10.1016/S0891-5849(01)00565-2
- Jung YS, Lee DH, Lim H, Yi KY, Yoo SE, Kim E. 2004. KR-31378 protects cardiac H9c2 cells from chemical hypoxia-induced cell death via inhibition of JNK/p38 MAPK activation. Japanese J Physiol. 54:575–583. doi: 10.2170/jjphysiol.54.575
- Kim EJ, Park SY, Lee JY, Park JH. 2010. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 10:96–107. doi: 10.1186/1471-230X-10-96
- Ko K, Steplewski Z, Glogowska M, Koprowski H. 2005. Inhibition of tumor growth by plant-derived mAb. Proc Natl Acad Sci USA. 102:7026–7030. doi: 10.1073/pnas.0502533102
- Ko K, Tekoah Y, Rudd PM, Harvey DJ, Dwek RA, Spitsin S, Hanlon CA, Rupprecht C, Dietzschold B, Golovkin M, et al. 2003. Function and glycosylation of plant-derived antiviral monoclonal antibody. Proc Natl Acad Sci USA. 100:8013–8018. doi: 10.1073/pnas.0832472100
- Kobayashi K, Arimura Y, Goto A, Okahara S, Endo T, Shinomura Y, Imai K. 2006. Therapeutic implications of the specific inhibition of causative matrix metalloproteinases in experimental colitis induced by dextran sulphate sodium. J Pathol. 209:376–383. doi: 10.1002/path.1978
- Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. 1979. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 5:957–971. doi: 10.1007/BF01542654
- Krueger F, Madeja Z, Hemberger M, McMahon M, Cook SJ, Gaunt SJ. 2009. Down-regulation of Cdx2 in colorectal carcinoma cells by the Raf-MEK-ERK 1/2 pathway. Cell Signal. 21:1846–1856. doi: 10.1016/j.cellsig.2009.07.020
- Kwak DH, Ryu JS, Kim CH, Ko K, Ma JY, Hwang KA, Choo YK. 2011. Relationship between ganglioside expression and anti-cancer effects of the monoclonal antibody against epithelial cell adhesion molecule in colon cancer. Exp Mol Med. 43:693–701. doi: 10.3858/emm.2011.43.12.080
- Kwon KH, Ohigashi H, Murakami A. 2007. Dextran sulfate sodium enhances interleukin-1 beta release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life Sci. 81:362–371. doi: 10.1016/j.lfs.2007.05.022
- Li W, Hua B, Saud SM, Lin H, Hou W, Matter MS, Jia L, Colburn NH, Young MR. 2015. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol Carcinogen. 54:1096–1109. doi: 10.1002/mc.22179
- Maeda H, Akaike T. 1998. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Mosc). 63:854–865.
- Maeng YS, Min JK, Kim JH, Yamagishi A, Mochizuki N, Kwon JY, Park YW, Kim YM, Kwon YG. 2006. ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell Signal. 18:994–1005. doi: 10.1016/j.cellsig.2005.08.007
- Mann JR, Backlund MG, DuBois RN. 2005. Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol. 2:202–210. doi: 10.1038/ncponc0140
- Marques-Fernandez F, Planells-Ferrer L, Gozzelino R, Galenkamp KM, Reix S, Llecha-Cano N, Lopez-Soriano J, Yuste VJ, Moubarak RS, Comella JX. 2013. TNFalpha induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death Dis. 4:e493. doi: 10.1038/cddis.2013.25
- Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, et al. 2003. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 124:361–367. doi: 10.1053/gast.2003.50067
- Peng CY, Pan SL, Huang YW, Guh JH, Chang YL, Teng CM. 2008. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-kappaB activity in vascular smooth-muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 378:579–588. doi: 10.1007/s00210-008-0328-1
- Ricote M, Glass CK. 2007. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 1771:926–935. doi: 10.1016/j.bbalip.2007.02.013
- Riethmuller G, Holz E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Funke I, Pichlmaier H, Hirche H, et al. 1998. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 16:1788–1794.
- Schoemaker MH, Ros JE, Homan M, Trautwein C, Liston P, Poelstra K, van Goor H, Jansen PL, Moshage H. 2002. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes: NF-kappaB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J Hepatol. 36:742–750. doi: 10.1016/S0168-8278(02)00063-6
- Seol JG, Park WH, Kim ES, Jung CW, Hyun JM, Lee YY, Kim BK. 2001. Potential role of caspase-3 and -9 in arsenic trioxide-mediated apoptosis in PCI-1 head and neck cancer cells. Int J Oncol. 18:249–255.
- Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY. 2002a. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci. 47:1266–1278. doi: 10.1023/A:1015362228659
- Seril DN, Liao J, Ho KL, Yang CS, Yang GY. 2002b. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis. 23:993–1001. doi: 10.1093/carcin/23.6.993
- Shelton JG, Steelman LS, White ER, McCubrey JA. 2004. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 3:372–379.
- Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. 2000. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 21:1319–1327. doi: 10.1093/carcin/21.7.1319
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. 2003. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x
- Tarallo R, Sordino P. 2004. Time course of programmed cell death in Ciona intestinalis in relation to mitotic activity and MAPK signaling. Dev Dyn. 230:251–262. doi: 10.1002/dvdy.20055
- Verch T, Yusibov V, Koprowski H. 1998. Expression and assembly of a full-length monoclonal antibody in plants using a plant virus vector. J Immunol Methods. 220:69–75. doi: 10.1016/S0022-1759(98)00149-5
- Xue M, Ge Y, Zhang J, Wang Q, Hou L, Liu Y, Sun L, Li Q. 2012. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS One. 7:e43483. doi: 10.1371/journal.pone.0043483
- Yamaguchi K, Cekanova M, McEntee MF, Yoon JH, Fischer SM, Renes IB, Van Seuningen I, Baek SJ. 2008. Peroxisome proliferator-activated receptor ligand MCC-555 suppresses intestinal polyps in ApcMin/+ mice via extracellular signal-regulated kinase and peroxisome proliferator-activated receptor-dependent pathways. Mol Cancer Ther. 7:2779–2787. doi: 10.1158/1535-7163.MCT-08-0173
- Yamamoto-Furusho JK, Penaloza-Coronel A, Sanchez-Munoz F, Barreto-Zuniga R, Dominguez-Lopez A. 2011. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) expression is downregulated in patients with active ulcerative colitis. Inflamm Bowel Dis. 17:680–681. doi: 10.1002/ibd.21322
- Yong JH, Seok JW, Yu JH, Choi Y, Song SJ, Kim A, Kim HJ, Kim JW. 2016. Glucocorticoid-mediated anti-inflammatory effect through NF kappa B is preserved in the absence of Dexras1. Anim Cells Syst. 20:1–6. doi: 10.1080/19768354.2016.1140676
- Yoo JM, Kim YJ, Lee SJ, Kim SH. 2011. Sequential administration of camptothecin sensitizes human colon cancer HCT116 cells to paclitaxel via p21Cip1/WAF1. Anim Cells Syst. 15:9–17. doi: 10.1080/19768354.2011.555187
- Zaloudik J, Li W, Jacob L, Kieny MP, Somasundaram R, Acres B, Song H, Zhang T, Li J, Herlyn D. 2002. Inhibition of tumor growth by recombinant vaccinia virus expressing GA733/CO17-1A/EpCAM/KSA/KS1-4 antigen in mice. Cancer Gene Ther. 9:382–389. doi: 10.1038/sj.cgt.7700452
