ABSTRACT
The reproductive cycle and releasing time for effective increase of resource of adult sea cucumber (Apostichopus japonicus) have been studied in the west coast of Korea. Adult sea cucumbers collected in Seocheon-gun April 2013 for first release and in the uncontaminated Taean-gun area in the west coast of Korea. Divers monthly collected the specimens in the released area from December 2013 to November 2014 in order to investigate the reproductive cycle of A. japonicas in Taean-gun. Random specimens were dissected to examine the gonadal developmental stages and discharge rates of the guts and gonads. The reproductive cycle of A. japonicus in both sexes are classified into the following six successive stages in the Taean-gun: (1) Stage I (recovery stage from December to February), (2) Stage II (early growing stage from February to March), (3) Stage III (late growing stage from March to April), (4) Stage IV (mature stage from April to July), (5) Stage V (partly spawned stage in July), and (6) Stage VI (spent stage from August to November). The estivation period of this species is from July to October in the Taean-gun region while surface water temperature is approximately 20–25.4°C. Thus, the optimum period to easily harvest them is from October to November in the uncontaminated Taean-gun area. It is also the best releasing time because the recovery stage starts from December to February in the Taean-gun region.
Introduction
The Apostichopus japonicus (Aspiodchirotida: Stichopodidae) referred to as sea cucumber is widely distributed along the coastal waters in East Asian countries such as Korea, China, and Japan (Park et al. Citation2013). In the coasts of Korea, this species is mainly found between large rocks and coarse gravels in subtidal zones (Park et al. Citation2007) at about up to 10 m in water depth. It is one of the most commercially important edible fishery products with its high nutritional value. The production levels of this species were recorded at 2491 tons in 1990, 1419 tons in 2000, 2936 tons in 2007, and 2259 tons in 2011 (KOSIS Citation2011). Taean-gun is the most abundant area for this species in the west of Korea, accounting for 30% of the annual production of sea cucumber before the largest ocean accident of Hebei Spirit oil spill occurred in the Taean-gun located in the west coast of Korea in December 2007. Thereafter, the outputs of sea cucumber in Taean-gun have been sharply reduced to 275 tons in 2008. The spilled oil has been completely removed and the water has been fully recorved in 2013.
A. japonicus has been designated as one of the important organisms in need of natural resources management. Thus, we participated in recovering project of A. japonicas in Taean-gun to improve sowing seed-releasing business and increase the incomes of fishermen. We also participated in making spawning grounds by throwing native rocks from February to March of 2013 in order to increase living surface area for juvenile and adult sea cucumbers and to provide a shelter from predators.
To restore sea cucumber A. japonicus in three districts in Taean-gun (Pado-ri and Uihyang-ri of Sowon-myun and Hwangchon-ri of Wonbuk-myun, where accident of Hebei Spirit oil spill occurred in December 2007), we released many sea cucumber adults in the three districts of Taean-gun in 2013. The released adults have been collected from Seocheon-gun and uncontaminated Taean-gun area which are considered one of the best breeds of A. japonicus.
The conditions of gonadal development after releasing remain unclear. In this research, we have studied the reproductive cycles and the conditions of gonadal development by collecting periodically the released ones. Another objective of this study is to provide basic information on the composition of spawning ground and development of islets of sea cucumber.
We considered many factors including sea temperature and salinity. Many areas have been studied on A. japonicus including reproduction (Sui Citation1989; Park et al. Citation2007), physiology (Lee & Park Citation1999; Gao Citation2008; Gao et al. Citation2009; Li et al. Citation2010; Kim et al. Citation2013; Oh et al. Citation2014), aquaculture (Sui Citation1988; Zhang et al. Citation1995; Uthicke Citation1999; Seo et al. Citation2009; Song Citation2009; MIFAFF Citation2013; Choi & Lee Citation2014; SSFRI NIFS Citation2014) and ecology (Park et al. Citation2013).
We have compared our observations with the above-described researches.
Materials and methods
Sampling
For histological studies of the reproductive cycle and the spawning period of sea cucumber A. japonicus, specimens in natural aqua farms were collected monthly by a woman diver in coastal waters off Taean-gun from December 2013 to November 2014. Collected live sea cucumbers in Taean-gun were transported to the laboratory of West Sea Fisheries Research Institute (WSFRI) in the National Institute of Fisheries Science (NIFS), total lengths of the captured sea cucumber was measured individually from mouth to anus using a ruler. Each individual was weighed using an electronic balance. Water temperatures and salinity were measured at 10:00 a.m. by Taean Aquaculture Research Institute in WSFRI.
Production of histological tissue section slides of gonadal tissues
To determine gonad developmental patterns in the one region of Taean-gun, we performed histological observations as follows. Samples of 480 individuals were used for histological examination quantitatively with a light microscope. Gonadal tissues were preserved in Bouin’s fixative for 24 h. After washing with running tap water for 24 h, tissues were dehydrated in alcohol and embedded in paraffin molds. Embedded tissues were sectioned at 5–7 μm thickness using a rotary microtome. Sections were mounted onto glass slides, stained with Hansen’s hematoxylin–0.5% eosin, and examined under a light microscope (Zeiss Axiovert 10 microscope).
Measurement of gonadosomatic index
To indirectly determine the spawning period, gonadosomatic index (GSI) of A. japonicus was calculated as the ratio of the fresh gonad weight to the fresh body weight according to the following formula:where GW is the gonad weight and BW is the body weight.
Releasing period
We collected adult sea cucumber A. japonicus (over 100 ± 7.9 g in body weight) from Seocheon-gun in April 2013 and from uncontaminated Taean-gun area in June 2013. Those are releases into the three districts of Taean-gun where the largest ocean accident of Hebei Spirit oil spill occurred in December 2007. The first releasing period was from 18 April to 20 April 2013 (3 days). The second releasing period was from 4 June to 5 June 2013 (2 days).
Specimen collection and determination of discharges of gonads and guts
Sea cucumbers A. japonicus with over 100 ± 7.9 g in body weight were used for this study. After specimens were transported alive to the laboratory, their lengths and body weights were immediately measured. The sea cucumbers were kept cool with ice to minimize discharges of guts and gonads in the vinyl packages. Vinyl packages (5 kg per package) were put into one large styrofoam container (20 kg) with ice water for transportation. We selected one case (5 kg) of vinyl package from each collected specimens to determine gonadal development and the discharge rate of gonads and guts. Individual sex was identified by dissecting the sea cucumber. We also determined the morphologies, GSI, and the discharge rates of the gonads and guts.
Statistical analyses
GSI values among different months have been compared by one-way analysis of variance (ANOVA) using a statistical package (SPSS ver. 17.0) with a significance level of α = 0.05. To determine which means are significantly different from one another, multiple comparisons have been conducted using Duncan’s multiple range test.
Results
Sea water temperatures and salinity concentrations during the study period
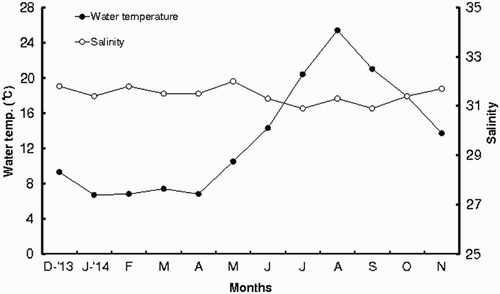
Monthly changes in the mean seawater temperature and salinity at the Taean-gun area from December 2013 to November 2014 are shown in . Monthly seawater temperature begins to increase from March with an average temperature of about 7.4°C. It reaches the highest in August with an average temperature of about 25.4°C. Then, It is gradually decreasing from September and reaching the lowest in January and February with an average temperature of around 6–7°C. As shown in , the mean salinities begin to increase from October with an average salinity of about 31.4 and reach the highest salinity in February with an average salinity of about 31.8. Thereafter, it starts decreasing and reaching the lowest in July with an average salinity of about 30.9 during the rainy season.
Gonad morphology and external color of gonad
The gonad of A. japonicus is a single structure consisting of numerous branched tubules arising from the gonad basis. It is attached to the anterior body wall. The gonaducts opened externally at the gonopore. During the gonad development, branched tubules extend into the perivisceral cavity and dominate the cavity. As gonads approached maturity, the sex of specimens can be determined by gonad color.
When female individuals are in the mature stage, ovaries are orange in color. Individual tubules have transparent thin tubule walls where oocytes are placed. Tubule length is one of the good indicators of reproductive maturity. Longest tubules are generally found at the mature stage. When male individuals are in the mature stage, testes appear milky white. Tubules have a uniform appearance. Tubules are packed with spermatozoa in the testes of mature males. Through the spawning season, simultaneous presence of both spawned and unspawned tubules indicates partial spawning that is a characteristic of A. japonicus.
Histological study
Histology study revealed that the gonad developmental stages designated by gonad tubule morphology and appearance are correlated with the six stages of gonadal development. Gonad developmental stages of females and males with histological analyses are described in the following sections.
A. Histology of ovaries
Based on morphological features and their staining responses to Hematoxylin/Eosin, developing oocytes are categorized as previtellogenic, vitellogenic, and mature oocytes. For histological analyses, we followed the gonadal stages of ovarian development of sea cucumber reported by Ramofafia et al. (Citation2000). In this study, the growing stage is subdivided into early and late growing stages according to oocyte development. For convenience, gonad developmental stages are classified into the following six successive stages: (1) recovery stage, (2) early growing stage, (3) late growing stage, (4) mature stage, (5) partly spawned stage, (6) spent stage. The criteria for defining each stage are as follows:
Stage I: recovery stage
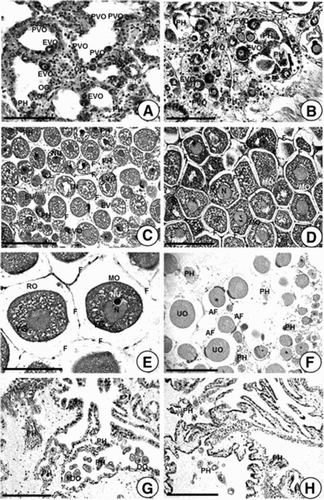
Ovaries in the recovery stage had thick walls ((A)). In this stage, several oogonia with 6.82–7.93 µm in diameter and previtellogenic oocytes with 13.0–23.0 µm in diameter have been observed along germinal epithelium. In particular, previtellogenic oocytes and a small number of early vitellogenic oocytes with 20.1–22.4 µm in diameter have been basophilically stained by Hematoxylin–eosin. However, a few relict oocytes and phagocytes are occasionally observed near the gonad wall ((A)). Individuals in the recovery stage have appeared from December 2013 to February 2014 when water temperatures were relatively low.
Figure 2. Photomacrographs showing gonadal phases of female A. japonicus as seen by light microscopy. (A) Section of ovarian tubules in Stage I (recovery stage); (B) Section of ovarian tubules in Stage II (early growing stage); (C) Section of ovarian tubules in Stage III (late growing stage); (D) Section of an ovarian tubule in Stage IV (mature stage); (E) Section of an ovarian tubule in the same stage (mature stage); (F) Section of tubules in Stage V (partly spawned stage; (G, H) Sections of tubules in Stage VI (spent stage). Abbreviations: AF, atretic follicle; DO, degenerating oocyte; EVO, early vitellogenic oocyte; F, follicle cell; LVO, late vitellogenic oocyte; MO, mature oocyte; N, Nucleus; NU, nucleolus; PH, phagocyte; PVO, previtellogenic oocyte; OG, oogonium; RO, ripe oocyte; UO, undischarged oocyte; YG, yolk granule. Scale bar 100 µm.
Stage II: early growing stage
In the early growing stage, oogonia and well-defined previtellogenic oocytes with 13.8–19.2 µm in diameter propagated along the germinal epithelium of the ovarian tubules. At this stage, previtellogenic oocytes and early vitellogenic oocytes were small and oval in shape, respectively. They have a large nucleolus in the nucleus. Early vitellogenic oocytes with 20.1–24.9 µm in diameter appear near the previtellogenic oocytes. Most cytoplasms of previtellogenic and early vitellogenic oocytes showed basophilic characteristics because the cytoplasms of oocytes are stained with hematoxylin. The ovarian (gonad) walls in the early growing stage are relatively thick. No free oocyte is present in the lumen of the tubule ((B)). Individuals in the early growing stage appeared between February and March in 2014 when water temperatures were very low.
Stage III: late growing stage
As early vitellogenic oocytes further developed, they enter late growing stage. This stage was characterized by active vitellogenesis of late vitellogenic oocytes near the ovarian (gonad) wall. At this time, late vitellogenic oocyte about 30.4–56.0 µm in diameter became round or oval in shape. It was surrounded by a follicle cell throughout the development. At this time, the gonad wall of the ovary is gradually reduced in thickness. Chemical components of the cytoplasms of late vitellogenic oocytes (basophilic components) are changed to gradually Eosinophilic components as gonadal development progresses. Some mature oocytes are present in the lumen of ovarian tubules ((C)). Individuals in the late growing stage were found between March and April in 2014 when water temperatures gradually increased.
Stage IV: mature stage
As late vitellogenic oocytes progress further, they enter the mature stage. The majority of ripe ova with 56.8–67.8 µm in diameter are round or oval in shape. Mature oocytes and a number of fully ripe ova in mature ovaries are densely packed with Eosinophilic oocytes or ova. The gonad wall is thin. Each mature oocyte or ovum remains within a follicle cell. The germinal vesicle starts to take up an eccentric position ((D, E)). Individuals in the mature stage have been founded from April to July in 2014 when water temperatures were relatively high.
Stage V: partly spawned stage
Not all ovarian tubules release all gametes during spawning. Commonly, partly spawned ovaries contain both spawned and unspawned tubules ((F)). Phagocytes are usually present in spawned and unspawned tubules. Spawned tubules have a reduced diameter with a wrinkled appearance. At this stage, the ovarian wall is gradually increased in thickness. In particular, relict oocytes about 14.4–25.0 µm in diameter and debris are observed in the lumen. Individuals in partly spawned stage have been found from June to August when water temperatures were high. Peak spawning have occurred in July in 2014.
Stage VI: spent stage
Spent ovaries were wrinkled and shrunken. Relict oocytes are occasionally present in the lumen ((G, H)) and phagocytes appear. The ovarian (gonad) wall was thick. Individuals in the spent stage were found from August to November in 2014 when water temperatures gradually decreased.
B. Histology of the testis
The pattern of testis growth in A. japonicus is also classified into six stages (). For histological analyses, we followed the gonadal stages according to the testicular developments of sea cucumber reported by Ramofafia et al. (Citation2000). For convenience, the testicular developmental stages are classified into the following six successive stages: (1) recovery stage, (2) early growing stage, (3) late growing stage, (4) mature stage, (5) partly spawned stage, and (6) spent stage. The criteria in defining each stage are described in the following sections.
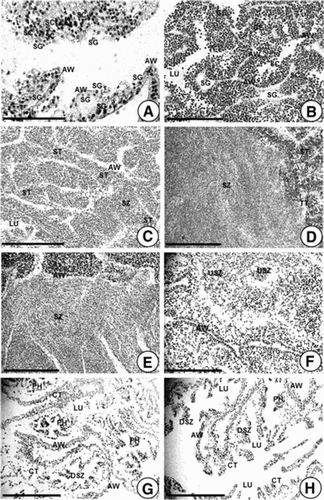
Figure 3. Photomicrographs showing gonadal phases of male A. japonicus as seen by light microscopy. (A) Section of testicular tubules in Stage I (recovery stage); (B) Section of testicular tubules in Stage II (early growing stage); (C) Section of tubules in Stage III (late growing stage); (D, E) Sections of tubules in Stage IV (mature stage); (F) Section of tubules in Stage V (partly spawned stage); (G, H) Sections of testicular tubules in Stage VI (spent stage). Abbreviations: AW, acinus wall; CT, connective tissue; DSZ, degenerating spermatozoa; GE, germinal ephithelium; LU, lumen; PH. phagocyte; SC, spermatocyte; SG, spermatogonium; ST, spermatid; SZ, spermatozoon; USZ, undischarged spermatozoon. Scale bar 100 µm.
Stage I: recovery stage
Testes in the recovery stage can only be identified by histology. The acinus (gonad) wall is lined with its basophilic layer of spermatogonia and primary spermatocytes ((A)). In particular, a layer of spermatogonia is present along the germinal epithelium. The acinus wall shows maximum thickness. Lumina in the testicular tubules are empty. Individuals in the recovery stage have been found from December in 2013 to February in 2014 when water temperatures were relatively low.
Stage II: early growing stage
In the early growing stage, a structural feature of growing testes is the numerous infolds of the germinal epithelium. The infolds of the germinal epithelium were lined by a dense layer of spermatogonia and spermatocytes in the lumina of testicular tubules ((B)). Individuals in the early growing stage have been found between February and March in 2014 when water temperatures were very low.
Stage III: late growing stage
In late growing stage, the germinal infolds are reduced. Spermatids and several spermatozoa are abundant in the lumina of testicular tubules ((C)). At this time, the thickness of the acinus wall is reduced. Individuals in the late growing stage have been found between March and April in 2014 when water temperatures gradually increased.
Stage IV: mature stage
In mature testes, the infolds of the germinal epithelium were commonly reduced or absent. The lumen is packed with spermatozoa ((D, E)). At this stage, a few spermatocytes could still be present along the germinal epithelium. The acinus wall was at the minimal thickness. Individuals in the mature testes have been found from April to July in 2014 when water temperatures were relatively high.
Stage V: partly spawned stage
Partly spawned testis contains testicular tubules that had spawned and those that had not spawned. Spawning activity is indicated by a diffuse arrangement of spermatozoa in the lumen. There is a reduction in the size of tubules, resulting in wrinkled and shrunken appearance. Aggregations of spermatozoa and the appearance of various phagocytes are present in the lumen ((F)). Individuals in the partly spawned stage have been found from June to August in 2014 when water temperatures were high. Peak spawning occurred in July 2014.
Stage VI: spent stage
Spent tubules were shrunken. They generally had empty lumen except for a few relict spermatozoa ((G) and (H)). Several phagocytes appear around the periphery. Spermatogonia are scattered along the germinal epithelium. The acinus walls are thin. Individuals in the spent stage have been found from August to November in 2014 when water temperatures gradually decreased.
Reproductive cycle with gonad developmental stages in both sexes
Based on monthly changes in morphological features of developing germ cells, the reproductive cycle in both sexes can be categorized into six successive stages. Histological analyses of A. japonicas have revealed that the gonadal developmental phases of both sexes show the same patterns of reproductive cycles during the year. Of the six stages in both sexes, the recovery stage is observed from December to February, and the early growing stage was observed from February to March. Then the late growing stage was observed from March to April. The mature stage was from April to July. The partly spawned stage was June. In addition, their occurrence frequencies over 85% in females and over 84% in males in July are almost the same. After the partly spawned stage, the spent stage of this species in females and males maintained for a long time from August to December.
GSI in females and males
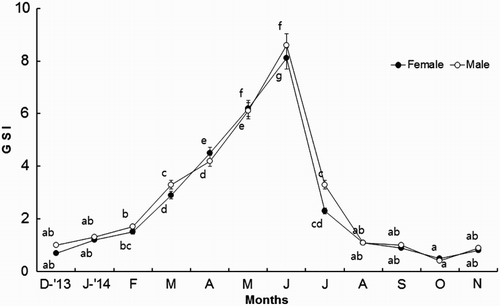
Adult sea cucumbers, A. japonicus were collected monthly at the released Taean coastal waters, Chungcheongnam-do Province, Korea from December 2013 to November 2014. To explore the spawning period indirectly, a total of 480 individuals were investigated for their gonadosomatic indices (GSI). The total length and body weight of samples are 15.0–25.5 cm (average 18.3 ± 3.1 cm) and 99.0–341.0 g (average 195.8 ± 76.9 g).
Monthly changes in GSI of female A. japonicus began to increase in March (4.5 ± 0.9), reaching the maximum in June (8.1 ± 1.4). Thereafter, it began to gradually decrease from July. Monthly changes in GSI of male A. japonicus begin to increase in March (3.3 ± 0.4), reaching the maximum in June (8.6 ± 1.0). Thereafter, it began to gradually decrease from July (). Overall, the variations in monthly changes in GSI values between male and female A. japonicus show a similar pattern (). However, the monthly changes in GSI values between male and female A. japonicus show significant differences ().
GSI comparison between the first release and the second release of adult sea cucumbers
The GSI values were measured before releasing the collected adult A. japonicus specimens into the three Taean-gun (Pado-ri and Uihyang-ri, Sowon-myun, and Hwangchon-ri, Wonbuk-myun). The GSI values of the first released adult sea cucumbers collected in April 2013 in Seocheon regions were 5.1–10.5 in females and 5.4–8.4 in males. However, the GSI values of the second released adult sea cucumbers collected in June 2013 in in uncontaminated Taean-gun regions were 0.9–2.1 in females and 0.9–1.7 in males. The three regions in Taean-gun belong to cold seawater zone in the west coast of Korea. Accordingly, the velocities of gonadal growth and development of sea cucumbers in Taean regions would be slower than those in Seocheon regions compared to the data. As expected, the GSI values of female and male A. japonicus in Seocheon regions are significantly (p < .05) higher than those in Taean regions ( and ).
Table 1. Water quality, GSI, and discharge ratio of guts and gonads of adult sea cucumber A. japonicus from the first release.
Table 2. Water quality, GSI, and discharge rate of guts and gonads of adult sea cucumber Apostichopus japonicus from the second release.
Environmental factors of water quality in the first and second releasing regions of the sea cucumbers in Taean-gun
Variations in water temperature and salinity, pH, and DO of seawater in the three regions during the first release were investigated in April 2013. As shown in , in the three regions (Padori, Uihyang-ri, and Hwangchon-ri), water temperatures, salinity, pH, and DO were 5.57–6.80°C, 32.33–32.57, 7.75–7.92, and 12.57–15.98 mg/L, respectively ().
The seawater temperature, salinity, pH, and DO of seawater in the three regions during the second release were investigated in June 2013. Because the second releasing period (from 4 to 5 June) of adult sea cucumbers was the main operation time in Taean-gun, after securing sea cucumber in the surrounding area, we released them to the three regions in June. For the second releasing, their mean body weights by regions were: 197.6–228.9 g in Pado-ri of Sowon-myun, 99.0–115.6 g in Uihyang-ri of Sowon-myun, and 129.3–134.6 g in Hwangchon-ri of Wonbuk-myun. Of the three regions, the largest individuals were released in Pado-ri of Sowon-myun. As shown in , water temperatures, salinity, pH, and DO were 11.12–12.04°C, 31.15–32.27, 7.88–7.95, and 7.51–9.36 mg/L, respectively ().
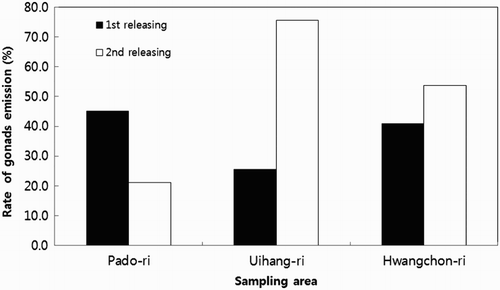
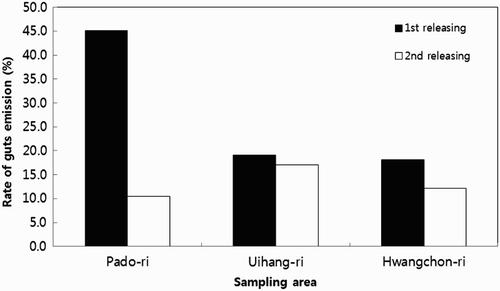
Discharging rates of gonads and guts during the first and second releasing of the sea cucumber in three regions
As shown in and , the discharging rates of gonads and guts of the sea cucumber of the first releasing of sea cucumber specimens in the three regions were 45.1% in Pado-ri, 29.8% in Uihyang-ri, and 40.9% in Hwangchon-ri. The discharging rates of guts and gonads of sea cucumbers of the second released specimens were 21.1% in Padori, 75.6% in Uihyang-ri, and 53.7% in Hwangchon-ri ( and ).
Discussion
Regarding gonadal development and maturation of marine invertebrates, Sastry (1966, 1968) has stated that exogenous factors (water temperature, food availability, salinity, day length, etc.) and endogenous factors (neuroendocrine activity) can control gonadal development and maturation in marine invertebrates. Water temperature and food availability seem to be particularly significant in the exogenous factors. These factors and other factors (salinity, day length, etc.) probably can interact with endogenous factors (neuroendocrine activity) in a complex manner to control the initiation of gametogenesis. Sastry (1968) has also stated that water temperature can act as a trigger to initiate germ cell growth. The water temperature required for activating the growth of germ cells at the beginning of oogenesis and spermatogenesis and attaining maturity ultimately can limit the annual period of gonad activity and gametogenesis in the natural environment. In this study, gamete differentiation of A. japonicus began in the winter–early spring seasons. It reaches maturity from April to July when the water temperature increases. The periods of food abundance and gonad development of marine invertebrates are often coincident. It has been reported that gonad growth and gametogenesis in the spring have coincided with a peak in food levels, although food concentrations have remained high throughout the summer months (Park et al. Citation2007).
The spawning of A. japonicus begins when water temperature is about 13–16°C in natural environment. However, it stopped at over 18°C. During the spawning period, gonad weights are about 10–20% of their total weights. It is well known that the minimum size for spawning is 58–60 g (MIFAFF Citation2013). The appropriate body weight of adult sea cucumber to be released may be over 200 g. In case of small-sized individuals (under 200 g), they can participate in reproduction. However, the spawning amounts may not be large and the egg qualities may not be good. Body weights of individuals used for artificial seed production in land are over 150 g. More than 200 g of the body weight of A. japonicus may be appropriate in order to enhance natural spawning of sea cucumber.
In this study, the GSI values of female and male individuals before release are different. The first released Seocheon region specimens on April show higher GSI value than the second released unspoiled Taean-gun specimens in June. The velocities of gonadal developments in Taean regions were later than those in Seocheon regions because Taean regions were in exceptionally cold water zone. Therefore, lower water temperatures in Taean regions might have lowered the velocities of gonadal developments compared to those in Seocheon regions in the west coast of Korea. MIFAFF (Citation2013) has reported that wild sea cucumbers in Korea and the Yonyungsung and Sandongsung regions in China are mature between May and August, the releasing time of A. japonicus in this study.
The sizes of guts were small and contracted during the estivation period of A. japonicus from July to September while water temperatures were above 20°C. It has been reported that the gut sizes of A. japonicus are increased from 3°C to 11°C from January to March during the winter season (Gao Citation2008; Gao et al. Citation2009). The guts of sea cucumbers are rapidly developed at seawater temperatures of 8–10°C (Sui Citation1988).
Individual discharged gonads and guts among the released will develop again. However, they might participate in reproduction next year after December. Water temperatures below 15°C, salinities of 28–34, pH 7. 9–8.4, and DO above 4–5 mg/L are optimum environmental conditions for the growth of sea cucumbers (Liao Citation1997; Yu et al. Citation2007; Song Citation2009). It is important to maintain the releasing conditions of sea cucumbers to increase survival and reproduction. It is important to maintain water temperature at 18–21°C and salinity at 30 (Li et al. Citation2010) for larval settlement as optimum environmental conditions. Accordingly, the release of adult sea cucumbers can be carried out at a proper time. While transportation and operation for releasing, some adults show a special discharge behavior under severe stress. Accordingly, the numbers of sea cucumbers that join the spawning populations this year will be decreased. We expect that the number of adult sea cucumbers that participate in spawning will increase next year.
It is desirable to transport the collected sea cucumbers in a package lowed seawater temperature. It is also important to have an extended period of cold seawater during the transport (Gyeongsangbuk-do Citation2013). In this study, after more than 10 hours of storage after the diver harvested the sea cucumbers, the storage package kept under 10°C with quality sea water. After collection, 20–30% of individuals extremely stressed when sea cucumbers are exposed to sunlight or transferred to storage tank, particularly in high-density storage, discharge their guts and gonads (MIFAFF Citation2013).
Sea cucumbers reared for 1–2 days in indoor storage tank showed slender body type. As shown our data, the discharging rate is very high in the released ones. Therefore, it is better to release the slender ones after the estivation instead of collecting elastic fatty body individuals early season April and June. When specimens of sea cucumbers should be transferred to another region after collection, a number of specimens will be put into storage tank. Therefore, after the estivation period when all individuals were slightly stressed, sea cucumbers started to come out of from shelters. It is an optimum period to easily collect them between October and November in Taean-gun region and between September and November in Seocheong region. We consider that October and November are the best releasing time for sea cucumber in the Taean-gun region.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Hae-Kyun Yoo http://orcid.org/0000-0001-7396-399X
Additional information
Funding
References
- Choi J, Lee SM. 2014. Growth of juvenile sea cucumber Apostichopus japonicus in integrated culture with rockfish Sebastes schlegeli or abalone Haliotis discus hannai. Kor J Fish Aqua Sci. 47:796–800.
- Gao F. 2008. Seasonal variations of nutritional composition, food resources, and digestive physiology in sea cucumber Apostichopus japonicus [PhD dissertation]. Chinese Academy of Sciences. (in Chinese).
- Gao F, Yang HS, Xu QA, Wang FY, Liu GB. 2009. Effect of water temperature on digestive enzyme activity and gut mass in sea cucumber Apostichopus japonicus (Selenka), with special reference to aestivation. Chi J Ocean Lim. 27:714–722. doi: 10.1007/s00343-009-9202-3
- Gyeongsangbuk-do. 2013. Guidebook for a basic design on specialized complex of sea cucumber aquaculture industry. Gyeongsangbuk-do. 1:72p.
- Kim TI, Park MW, Cho JK, Son MH, Jin YG. 2013. Survival and histological change of integumentary system of the juvenile sea cucumber, Apostichopus japonicus exposed to various salinity concentrations. Kor Soci Fish Mar Sci Edu. 25:1360–1365.
- KOSIS. 2011. Available from: http://kosis.kr.
- Lee CS, Park YJ. 1999. Influence of food and density on the growth and survival of sea cucumber, Stichopus japonicus. J Aquacul. 12:39–45.
- Li L, Qi L, Kong LF. 2010. Effects of environmental factors on larval settlement of sea cucumber, Apostichopus japonicus (Selenka). J Wor Aqua Soc. 41:936–941.
- Liao YL. 1997. Fauna Sinica, Phylum Echinodermata, Class Holothuroidea. Beijing: Science Press. (in Chinese).
- MIFAFF. 2013. Guidebook of sea cucumber aquaculture. MIFAFF. 134p.
- Oh M-H, Kwon IY, Kim TH. 2014. Biological performance evaluation of tubular subsurface cage system for sea cucumber, Apostichopus japonicus, grow-out by in-situ tests. Bull Kor Soc Fish Tech. 50:202–213. doi: 10.3796/KSFT.2014.50.2.202
- Park KJ, Park YJ, Kim SK, Choi SD, Kim YG, Choi NH. 2007. Histological study on the reproductive cycle of Stichopus japonicus in the west coast of Korea. J Aqua. 20:26–30.
- Park KJ, Ryu SO, Baek YS, Kim YS, Kang HW, Han HS. 2013. Substrate characteristics of sea cucumber Stichopus japonicus habitats in the west coast of Korea. Kor J Fish Aqua Sci. 46:886–891.
- Ramofafia C, Battaglene SC, Bell JD, Byrne M. 2000. Reproductive biology of the commercial sea cucumber Holothuria fuscogilva in the Solomon Islands. Mar Bio. 136:1045–1056. doi: 10.1007/s002270000310
- Seo JY, Kim DG, Kim GU, Cho SS, Park HG, Lee SM. 2009. Effect of different substrates in the rearing tank on growth and body composition of juvenile sea cucumber Apostichopus japonicus. J Aqua. 22:118–121.
- Song ZY. 2009. Relationship between sea cucumber Apostichopus japonicus aquaculture and dissolved oxygen. J Aqua. 12:15–16. (in Chinese).
- SSFRI NIFS. 2014. Guidebook of artificial seed production on sea cucumber. SSFRI. 53p.
- Sui XL. 1988. Culture and enhance of sea cucumber. Monograph. China Agriculture Publishing House, Beijing. (in Chinese)
- Sui XL. 1989. The main factors influencing the larval development and survival rate of the sea cucumber. Ocean Lim Sin. 20:314–321. (in Chinese).
- Uthicke S. 1999. Sediment bioturbation and impact of feeding activity of Holothuria (Halodeima) atra and Stichopus xhloronotus, two sediment feeding holothurians at Lizard Island, great barrier reef. Bull Mar Sci. 64:129–141.
- Yu RH, Li Q, Che S, Zhou JQ. 2007. Non social effects of pollution, high yield and healthy culture technique of sea cucumber (Apostichopus japonicus Selenka). Tran Ocean Lim. 4:157–160.
- Zhang B, Sun D, Wu Y. 1995. Preliminary analysis on the feeding habit of Apostichopus japonicus in the rocky coast waters off Lingshan Island. Mar Sci. 3:11–13.