ABSTRACT
Previously we reported that CFL-1, the single LRR-type F-box protein in the Caenorhabditis elegans genome, affected defecation behavior and daumone response. CFL-1 is highly homologous to the FBXL20 in mammals, which regulates synaptic vesicle release by targeting its substrate Rim1 for ubiquitin-mediated degradation. The worm homolog of Rim1 is UNC-10, a presynaptic membrane protein that triggers synaptic vesicle fusion through interaction with RAB-3 GTPase. To examine if CFL-1 exerts its modulatory effect on the defecation and daumone response via ubiquitination of UNC-10, we performed RNAi knock-down of CFL-1 in the unc-10(e102) mutant background. We noticed additive increase in defecation interval when the activities of both CFL-1 and UNC-10 were compromised. Also, the degree of dauer formation upon daumone treatment in unc-10 mutants treated with CFL-1 RNAi decreased further than the level observed in untreated mutants or wild type N2 worms with CFL-1 RNAi knock-down. Our data suggest that CFL-1 affects defecation frequency and daumone response in C. elegans through the ubiquitination of UNC-10.
Introduction
Proteolysis plays divers roles in cellular maintenance, not only in removing damaged or mis-folded proteins but also in the regulation of physiological processes such as cell cycle and apoptosis. The ubiquitin-proteasome system (UPS) features the most evolutionarily conserved proteolytic device found in almost all living cells (Jung et al. Citation2009). Among three enzyme complexes of UPS (i.e. E1, E2 and E3), E3 ligase is in charge of connecting ubiquitin-loaded E2 to target proteins. The ability to discern correct target comes from the diverse panoply of E3 ligases, among which the SCF (SKP1-CUL1-F-box) family portrays the largest and most characterized group (Willems et al. Citation2004). In the SCF complex, the subunit containing an F-box motif is responsible for the recognition of the substrate to be ubiquitinated. These F-box proteins interact with ubiquitination targets through their C-terminal structure, and are further divided into subtypes such as FBXW (with WD repeats), FBXL (with cystein-containing leucine-rich-repeat; LRR_cc) or FBXO (without obvious domain).
The SCF-type E3 complex is also found in the nematode Caenorhabditis elegans and participates in various cellular processes (Papaevgeniou & hondrogianni Citation2014). C. elegans genome encodes more than 20 SKP homologs and up to hundreds of F-box proteins (Kipreos & Pagano Citation2000). In stark contrast to such diversity, C. elegans appears to encode only one F-box protein with the authentic LRR_cc domain (Kim et al. Citation2012). The solitary presence of FBXL in C. elegans is intriguing regarding the presence of more than 20 FBXLs in mammals and the diverse functions carried out by them (Ho et al. Citation2006).
Previously we reported that this novel C. elegans FBXL, which we named CFL-1, is highly homologous to mammalian FBXL20 (Kim et al. Citation2012). Despite the evolutionary distance between nematode and mammals, the homology was extended beyond the F-box motif and LRR domain. Compromised CFL-1 activity affected the defecation frequency and daumone response, suggesting that CFL-1 may participate in the neuronal signaling as does its mammalian homolog FBXL20 via prompt degradation of synaptonemal proteins (Yao et al. Citation2007). In this regard, it would be intriguing to assess the involvement of UNC-10, the worm homolog of FBXL20 target Rim1, in defecation control and daumone response in C. elegans. To this end, we examined the effect of modulating CFL-1 activity in the unc-10 mutant background. Our data suggest that CFL-1 subjects distinct set of proteins including UNC-10 to ubiquitination in the regulation of defecation and daumone response.
Methods and materials
Worm maintenance
C. elegans was maintained on MYOB plates (Church et al. Citation1995) seeded with OP50 bacteria, according to the standard culture protocol (Brenner Citation1974).
RNAi knock-down of CFL-1 activity
To generate the RNA interference (RNAi) construct of cfl-1 transcript, 1.2 kb region of the cDNA spanning both the F-box and LRR repeat was polymerase chain reaction amplified using primers 5′-TACGACGCTTTCACCAGCTC-3′ (forward) and 5′-TGATCCGTTGGTGGAGTGAC-3′ (reverse), then inserted into the L4440 plasmid. Both recombinant and original L4440 plasmid were transformed into Escherichia coli HT115 strain. Feeding RNAi was performed according to the protocol of Kamath and Ahringer (Citation2003) with minor modifications (Min & Lee Citation2007). Briefly, 200 μl of overnight HT115 culture was seeded onto MYOB and induced with isopropyl β-D-1-thiogalactopyranoside for 48 h. L4 worms were placed on the HT115 lawn and cultured at 16°C. F1 worms were retrieved after 48 h and transferred onto fresh RNAi plate to produce F2 worms, which was subjected to required analyses.
Measurement of defecation frequency
F2 generation worms were picked from RNAi plate (MYOB seeded with HT115 containing original or recombinant L4440) at the young adult stage and placed onto a fresh MYOB plate that is lightly seeded with OP50 (e.g. 40 μl of overnight culture per plate). Worms were allowed to settle for 10 min at room temperature before starting defecation analysis. Movement of each individual worm was monitored under the dissection microscope, and time was measured starting from the first incident of defecation up to the 11th defecation. Average time interval between defecation motor program was calculated by dividing the recorded time by 10. Each set of experiment recruited 10 worms that were individually monitored.
Dauer formation assay
About 20 adult F2 generation worms were picked from RNAi plate (nematode growth media without peptone, seeded with HT115 containing original or recombinant L4440) and transferred onto a fresh RNAi plate containing daumone (the heptanoid type, kind gift from Dr Y-K Paik at Yonsei University), dissolved in EtOH and added to the media at final concentrations of 38 μM (Jeong et al. Citation2005). Worms placed on the daumone plate were allowed to lay eggs at 20 °C for 2 h. After removing adults, the plates were incubated for 3 days at 25°C. The rate of dauer formation was calculated by dividing the number of dauer by total number of worms hatched on the plate.
Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS Statistics 22.0). Two-group comparisons were performed by Mann–Whitney U-test (Fay & Proschan Citation2010).
Results and discussions
Changes in the defecation frequency and dauer formation in unc-10 mutant worms
In the previous study, restricted expression of GFP::cfl-1 promoter fusion reporter at the anus and chemosensory amphid neurons has led us to investigate if CFL-1 is involved in functions associated with these areas. Indeed, we observed changes in the defecation frequency and daumone response in wild type N2 worms when CFL-1 activity was down-regulated by RNAi (Kim et al. Citation2012). Such results are reminiscent of the fact that FBXL20, the mammalian homolog of CFL-1, is involved in the release of synaptic vesicle at the active zone via ubiquitination of Rim1protein (Yao et al. Citation2007). If CFL-1 partakes a similar function in C. elegans, the most likely target of CFL-1 mediated ubiquitination would be UNC-10, the worm counterpart of Rim1 (Koushika et al. Citation2001; Gracheva et al. Citation2008).
To see if modulation of UNC-10 also affects defecation and daumone response in C. elegans, we employed the unc-10(e102) loss-of-function mutant strain where a G to A substitution disrupted splicing acceptor at the C-terminal (Koushika et al. Citation2001). First the defecation frequency of unc-10(e102) mutants (kind gift from Prof. Joohong Ahan, Hanyang University, Korea) was compared with that of the wild type N2. As shown in (A), time intervals between defecation in mutant worms were slightly longer than the wild type, to a degree similar to that of the N2 worms with CFL-1 RNAi knock-down (Kim et al. Citation2012).
Figure 1. Comparisons of defecation behavior and daumone response in unc-10 mutant and wild type N2. (A) The e102 strain of unc-10 mutants showed increase in average time interval between defecations compared with that of the wild type N2. Each bar represents average value (in seconds) calculated from 10 worms ± SD (*p < .001). A representative data from three independent experiments is shown. (B) The proportion of dauer formation among unc-10(e102) mutants upon treatment with 38 μM daumone was lower than that of the wild type N2. Bars represent average values from three plates ± SD (*p < .001). A representative data from two independent experiments are shown.
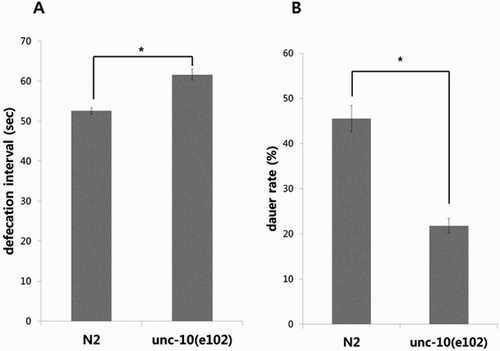
Next we analyzed the response towards daumone in unc-10(e102) mutants, and found that the rate of dauer formation was approximately twofold lower than that of the wild type N2 strain ((B)). Previously mutations in unc-10 and unc-13 loci were reported to be associated with aging control in C. elegans, but the underlying mechanism has not been addressed (Shen et al. Citation2007). Our data suggest that UNC-10 may affect aging by participating in the response towards dauer-inducing pheromone at the synapse.
Additional decrease in defecation frequency and daumone response in unc-10 mutant worms with CFL-1 knock-down
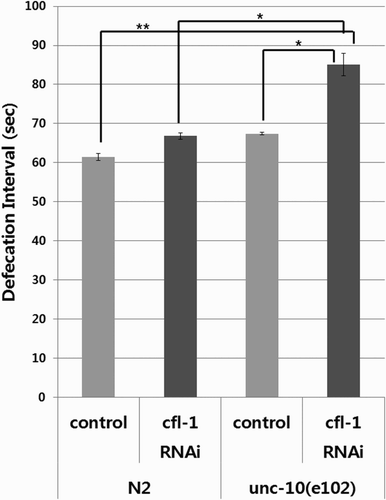
In an attempt to examine the effect of simultaneously subsiding CFL-1 and UNC-10 on defecation behavior, we treated unc-10(e102) mutant worms with cfl-1 RNAi construct. The increment in time interval between defecations in unc-10 mutants with CFL-1 knock-down was more pronounced than in worms defective in CFL-1 or UNC-10 alone (). Still, the degree of increase (i.e. about 37%) was rather small compared with that of the IP3 receptor-associated mutants such as itr-1 and shn-1 (Walker et al. Citation2002; Jee et al. Citation2004) indicating that factors other than active zone proteins are required for the control of defecation frequency.
Figure 2. Effect of CFL-1 knock-down on defecation frequency in the wild type and unc-10 mutant worms. The unc-10(e102) mutants with CFL-1 knock-down showed additional decrease in defecation frequency compared with worms defective in CFL-1 or UNC-10 alone. Bars represent average values from three plates ± SD (*p < .05, **p < .001). A representative data from two independent experiments are shown.
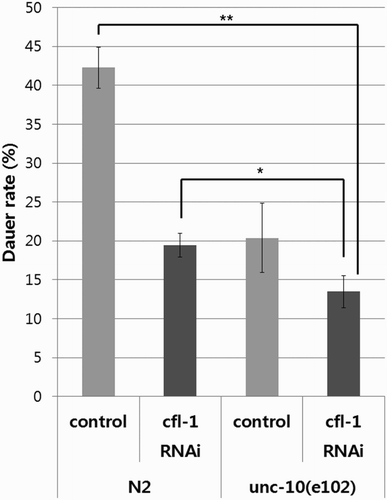
Next we tested if modulation of CFL-1 activity in the unc-10(e102) mutant background also exerts additive effect on daumone response. The rate of dauer formation upon treatment with 38 μM daumone in unc-10 mutants treated with cfl-1 RNAi was approximately 30% lower than that of the CFL-1 knock-down wild type (). Dauer rate in the double-defective worms was also lower than that of the untreated unc-10(e102), although the difference was not statistically significant due to large variations in data from the group with unc-10 mutation alone.
Figure 3. Effect of CFL-1 knock-down on daumone-induced dauer formation in the wild type and unc-10 mutant worms. Knocking down CFL-1 in the unc-10(e102) mutants caused further decrease in the rate of dauer formation upon treatment with 38 μM daumone compared with worms defective in CFL-1 or UNC-10 alone. Bars represent average values from three plates ± SD (*p < .05, **p < .001). A representative data from two independent experiments are shown.
Taken together, our results suggest that CFL-1 participate in the regulation of defecation and pheromone response via ubiquitination of UNC-10. How disrupting both CFL-1 and UNC-10 create additive effects in these processes is not clear at the moment, but may have to do with the fact that defective UNC-10 protein is produced from the mis-spliced RNAs in the unc-10(e102) mutants. Alternatively, CFL-1 may interact with targets other than UNC-10 that are also associated with defecation and/or daumone sensing.
Diverse F-box proteins may govern controlled degradation of active zone proteins at the synapse
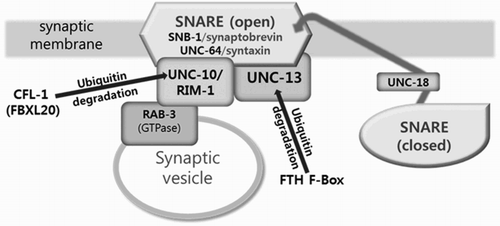
UNC-10 interacts with another synaptonemal protein UNC-13 at the active zone to trigger the discharge of synaptic vesicle (Weimer et al. Citation2006). UNC-13 has been shown to interact with a novel FTH-type F-box protein in a yeast 2-hybrid screening experiment (Polinsky et al. Citation2006), suggesting the involvement of an E3 ligase in its degradation. Interestingly, the mutation of unc-10 shortened the lifespan of worms while unc-13 mutation caused delay in aging (Shen et al. Citation2007). Such opposite function in aging control is quite intriguing since expression of both UNC-13 and UNC-10 are negatively controlled in daf-2 and daf-16 mutants. We hypothesize that differential control of UNC-10 and UNC-13 function would be possible if each protein is engaged with separate E3 ligase for their degradation at the synaptic active zone (). If this is the case, CFL-1 (and perhaps the novel FTH-type F-box protein for UNC-13) would add to the list of F-box proteins participating in the timely degradation of active zone proteins at the synapse, for example the FBXO type FSN-1 (mammalian homolog of Fbxo45) which is in charge of the ubiquitination of ALK receptor tyrosine kinase (Liao et al. Citation2004).
Figure 4. A hypothetical representation of interaction between F-box proteins and active zone proteins in C. elegans. The closed SNARE complex is modified into the open state upon activation by UNC-18, and associates with UNC-10 and UNC-13 on the presynaptic membrane. Once the contents of the synaptic vesicle are released by the engagement or RAB-3 GTPase and UNC-10, proteins in the synaptonemal complex are subjected to ubiquitin-mediated proteolysis by distinct set of F-box containing E3 ligases.
Regarding the evolutionary distance between nematodes and mammals, the conservation between pairings of FBXL20 to Rim1 and CFL-1 to UNC-10 is intriguing. As the single LRR-type F-box protein in C. elegans, CFL-1 may portray a prototype exerting functions that are allocated among multiple FBXLs in higher organisms. Further investigation on the molecular functions of CFL-1, in particular concerning the calmodulin-binding motif (Chen et al. Citation2011; Kim et al. Citation2012), would provide better understanding towards neuronal regulation and aging in C. elegans.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics. 77:71–94.
- Chen BB, Coon TA, Glasier JR, Mallampalli RK. 2011. Calmodulin antagonizes a calcium-activated SCF ubiquitin E3 ligase subunit, FBXL2, to regulate surfactant homeostasis. Mol Dell Biol. 31:1905–1920. doi: 10.1128/MCB.00723-10
- Church DL, Guan KL, Lambie EJ. 1995. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 121:2525–2535.
- Fay MP, Proschan MA. 2010. Wilcoxon–Mann–Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat Surv. 4:1–39. doi: 10.1214/09-SS051
- Gracheva EO, Hadwiger G, Nonet M, Richmond J. 2008. Direct interaction between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci Lett. 444:137–142. doi: 10.1016/j.neulet.2008.08.026
- Ho MS, Tsai PI, Chien CT. 2006. F-box proteins: the key to protein degradation. J Biomed Sci. 13:181–191. doi: 10.1007/s11373-005-9058-2
- Jee C, Lee J, Lee JI, Lee WH, Park B-J, Yu J-R, Park E, Kim E, Ahnn J. 2004. SHN-1, a Shank homologue of C. elegans, affects defecation rhythm via the inositol-1,4,5-trisphosphate receptor. FEBS Lett. 561:29–36. doi: 10.1016/S0014-5793(04)00107-3
- Jeong PY, Jing M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim K, Paik YK. 2005. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 433:541–545. doi: 10.1038/nature03201
- Jung T, Catalgol B, Grune T. 2009. The proteasomal system. Mol Asp Med. 30:191–296. doi: 10.1016/j.mam.2009.04.001
- Kamath RS, Ahringer J. 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 30:313–321. doi: 10.1016/S1046-2023(03)00050-1
- Kim SM, Jang SH, Son N, Han CT, Min KS, Lee HK, Hwang SY. 2012. A novel F-box protein with leucine-rich repeats affects defecation frequency and daumone response in Caenorhabditis elegans. Animal Cells Syst. 16:280–288. doi: 10.1080/19768354.2012.665612
- Kipreos ET, Pagano M. 2000. The F-box protein family. Genome Biol. 1:1–7. doi: 10.1186/gb-2000-1-5-reviews3002
- Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. 2001. A post-docking role for active zone protein Rim. Nature Neursci. 4:997–1005. doi: 10.1038/nn732
- Liao EH, Hung W, Abrams B, Zhen M. 2004. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 430:345–350. doi: 10.1038/nature02647
- Min K, Lee J. 2007. RNA interference in C. elegans: history, application and perspectives. Anim Cell Sys. 11:99–104.
- Papaevgeniou N, Chondrogianni N. 2014. The ubiquitin proteasome system in Caenorhabditis elegans and its regulation. Redox Biol. 2:333–347. doi: 10.1016/j.redox.2014.01.007
- Polinsky C, Houston C, Vado J, Shaikh A, Kohn RE. 2006. Synaptic protein UNC-13 interacts with an F-box protein that may target it for degradation by proteasomes. Acta Biochim Pol. 53:145–148.
- Shen LL, Yang W, Wang DY. 2007. Involvement of genes required for synaptic function in aging control in C. elegans. Neurosci Bull. 23:21–29. doi: 10.1007/s12264-007-0003-4
- Walker DS, Grower NJD, Ly S, Bradley GL, Batlis HA. 2002. Regulated disruption of inositol 1,4,5-trisphosphate signaling in Caenorhabditis elegans reveals new functions in feeding and embryogenesis. Mol Biol Cell. 13:1329–1337. doi: 10.1091/mbc.01-08-0422
- Weimer RM, Gracheva EO, Meyrignac O, Miller K, Richmond JE, Bessereau JL. 2006. UNC-13 and UNC-10/Rim localize synaptic vesicles to specific membrane domains. J Neurosci. 26:8040–8047. doi: 10.1523/JNEUROSCI.2350-06.2006
- Willems AR, Schwab M, Tyers M. 2004. A hitchhiker’s guide to the cullin ubiquitin ligases: SCF and its kin. Biochem Biophys Acta. 1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027
- Yao I, Takagi H, Ageta H, Kahyo T, Sato S, Hatanaka K, Fukuda Y, Chiba T, Morone N, Yuasa S, et al. 2007. SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell. 130:943–957. doi: 10.1016/j.cell.2007.06.052
