ABSTRACT
Age-related memory decline is closely associated with decreased neurogenesis and increased apoptosis in the hippocampus. Noradrenaline exerts its effect by selectively binding to and activating adrenergic receptors (ARs). Tamsulosin, α1-AR antagonist, is reported to have access to the brain and interact with α1-AR. In this study, the effects of tamsulosin on short-term and spatial learning memory in terms of neurogenesis and apoptosis were investigated using rats. Step-down avoidance test for short-term memory and radial 8-arm maze test for spatial learning memory were conducted. Neurogenesis was detected by 5-bromo-2’-deoxyuridine (BrdU) immunohistochemistry and apoptosis was evaluated by caspase-3 immunohistochemisty and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNE) staining. Western blot for protein kinase C (PKC), cAMP-responsive element-binding protein (CREB), brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB), phosphatidylinositol 3-kinase (PI 3-kinase), Akt, Bcl-2, and Bax was conducted. In the aged rats, short-term and spatial learning memory was declined. Hippocampal nerogenesis was suppressed and hippocampal apoptosis was enhanced in the aged rats. In addition, phosphorylation of PKCα, CREB, PI-3 kinase, and Akt was decreased in the hippocampus of old-aged rats. Tamsulosin activated PKC/CREB and PI-3 kinase/Akt pathways. With these pathways, BDNF-TrkB signaling enhanced hippocampal neurogenesis and suppressed apoptosis in the old-aged rats. As the results, tamsulosin improved performance of short-term and spatial learning memory in the aged rats.
1. Introduction
Age-related memory decline is closely associated with decreased neurogenesis and increased apoptosis in the hippocampus (Driscoll and Sutherland Citation2005; Kim et al. Citation2010). Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophic family involved in neuronal survival and differentiation. BDNF plays a crucial role in the learning process, and deficit or loss of BDNF in the hippocampus of aged rats contributes to learning and memory impairment (Yang et al. Citation2014). Aging-related hippocampal susceptibility to apoptosis was demonstrated by increased pro-apoptotic Bax expression, enhanced caspase-3 activity, and reduced anti-apoptotic Bcl-2 expression (Kaufmann et al. Citation2001; Jin et al. Citation2008).
Noradrenaline modulates hippocampal synaptic plasticity and deterioration of noradrenaline system may cause learning and memory impairment during aging (Knauber and Müller Citation2000). Noradrenaline exerts its influence by selectively binding to and activating adrenergic receptors (ARs); alpha1 (α1), alpha2 (α2) and beta (β) subtypes.
The effects of AR on learning and memory are inconsistent and not clearly defined. It was reported that α1-AR stimulation suppressed memory consolidation (Gibbs and Summers Citation2001), however, other studies reported that activation of α1-AR enhanced learning and memory (Puumala et al. Citation1998; Sirviö and MacDonald Citation1999). Even the same α1-AR agonist showed opposite effects depending on the dosage. Low dose of α1-adrenergic agonist improved spatial learning memory and memory consolidation, and high dose impaired spatial learning memory (Gibbs and Bowser Citation2010; Torkaman-Boutorabi et al. Citation2014). Torkaman-Boutorabi et al. (Citation2014) suggested that the α1-AR antagonist, prazosin, impaired the spatial learning memory. In contrast, Katsouri et al. (Citation2013) reported that chronic treatment with prazosin prevented memory impairment in Alzheimer's disease mice.
Tamsulosin, another α1-AR antagonist, is also reported to have access to the brain and interact with α1-AR (Sirviö and MacDonald Citation1999; Hellstrom and Sikka Citation2006). Tamsulosin was originally developed for the treatment of arterial hypertension and has been reported to be effective in the treatment of lower urinary tract symptoms, such as benign prostatic hyperplasia (Michel and de la Rosette Citation2004). In our previous studies, tamsulosin improved voiding function through inhibiting neuronal activity in the neuronal voiding centers (Kim et al. Citation2012, Citation2013). Tamsulosin improved short-term and spatial learning memory in the rats under normal conditions (Kim et al. Citation2015).
In the present study, we evaluated whether α1-AR antagonist tamsulosin was effective in improving learning and memory impairment by aging. In this study, the effects of tamsulosin on short-term and spatial learning memory in terms of neurogenesis and apoptosis were investigated using rats.
2. Materials and methods
2.1. Animals and treatments
Fisher 344 rats were used in this study. Four-month-old rats (n = 10, 310 ± 10 g) were used as the young-aged control group (YC), and 25-month-old rats (n = 40, 420 ± 20 g) were used as the old-aged groups. The old-aged groups were re-divided into 4 groups (n = 10 in each group): the old-aged control group, the old-aged and 0.01 mg/kg tamsulosin-treated group, the old-aged and 0.1 mg/kg tamsulosin-treated group, and the old-aged and 1 mg/kg tamsulosin-treated group. For the tamsulosin-treated rats, tamsulosin was orally administered at respective dose daily for 4 weeks. All rats received 50 mg/kg 5-bromo-2’-deoxyuridine (BrdU; Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally once a day for 7 days from the starting of the experiment. The experimental design was approved by the Institutional Care and Use Committee of Kyung Hee University Institutional Animal Care and Ethics Committee [KHUASP(SE)-14-047].
2.2. Step-down avoidance test
Short-term memory was determined using step-down avoidance test, as previously described (Kim et al. Citation2010; Lee et al. Citation2016). The rats were trained before the test on the 28th day of the starting experiment. The rats were placed on a 25 × 7 × 2.5 cm platform in width, length, and height. During the training, the rats received a 0.5 mA scramble foot shock for 2 s immediately upon stepping down. Two hours after training, the latency (sec) was measured. The latency was defined as the elapsed time until the rat stepped down and placed all four paws on the grid. A latency over 300 s was counted as 300 s.
2.3. Radial 8-arm maze test
Spatial learning memory was determined using radial 8-arm maze test, as previously described (Kim et al. Citation2010, Citation2016). Radial 8-arm maze apparatus consisting of a central octagonal plate (30 cm in diameter) and 8 radiating arms (50 cm in length and 10 cm in width) The apparatus was placed 1 m above the floor and has a small receptacle filled with water (3 × 1 cm in diameter and depth) at the end of the arms. The rats were trained three times before the test. Prior to the training sessions, the rats were deprived of water for 24 h and were able to explore the water for 5 min after each training session. The test was conducted on the 31st day of the starting experiment. The time to find the water at the end of the arm was calculated and the test was terminated when the rat found water in eight arms or exceeded eight min. Re-entry into the previously visited arms was considered as an error and the number of correct number was calculated before the first error occurred.
2.4. Tissue preparation
Tissue preparation was performed, as previously described (Kim et al. Citation2010; Lee et al. Citation2016). Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France) was use to anesthetize the rats immediately after radial 8-arm maze test. And then, 50 mM phosphate-buffered saline (PBS) was transcardially perfused with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). After removing the brains, freezing microtome (Leica, Nussloch, Germany) was used to make 40 μm thick coronal sections.
2.5. Brdu immunohistochemistry
BrdU immunohistochemistry was performed, as previously described (Kim et al., Citation2010; Lee et al., Citation2016). The sections were pretreated in 50% formamide-2 × standard saline citrate (SSC) at 65°C for 2 h, denatured in 2 N HCl at 37°C for 30 min, and rinsed two times in 100 mM sodium borate (pH 8.5). The sections were incubated with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany) overnight at 4°C, and the sections were incubated with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 h. The sections were incubated with an avidin-peroxidase complex (1:100; Vector Laboratories) for another 1 h, and then the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.03% 3,3’-diaminobenzidine (DAB), 40 mg/ml nickel chloride, and 0.03% hydrogen peroxide for 5 min for visualization.
The differentiation of BrdU-positive cells was detected on the same section using a mouse anti-neuronal nuclei (NeuN) antibody (1:1000; Chemicon Inc., Temecula, CA, USA). The sections were incubated with a biotinylated anti-mouse secondary antibody. The sections were mounted on gelatin-coated slides. The slides were covered by Permount® (Fisher Scientific, New Jersey, NJ, USA).
2.6. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining
TUNEL staining was performed using an In Situ Cell Death Detection Kit® (Roche), as previously described (Kim et al., Citation2010; Lee et al., Citation2016). The sections were incubated with proteinase K (100 μg/ml), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.03% DAB.
2.7. Caspase-3 immunohistochemistry
Caspase-3 immunohistochemistry was performed, as previously described (Kim et al., Citation2010; Lee et al., Citation2016). After incubating with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight, the sections were incubated with biotinylated mouse secondary antibody (1:200; Vector Laboratories) for another 1 h. The secondary antibody was amplified with the Vector Elite ABC kit® (1:100; Vector Laboratories). Antibody-biotin–avidin-peroxidase complex was visualized using 0.03% DAB.
2.8. Western blot analysis
Western blot was conducted, as previously described (Kim et al. Citation2016; Lee et al. Citation2016). The hippocampal tissues were homogenized on ice, and lysed in a lysis buffer. Protein (20 μg) was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse β-actin antibody (1:500; Santa Cruz Biotechnology), rabbit BDNF antibody (1:1000; Santa Cruz Biotechnology), rabbit tyrosine kinase B (TrkB) antibody (1:1000; Santa Cruz Biotechnology), mouse Bax antibody (1:1000; Santa Cruz Biotechnology), mouse Bcl-2 antibody (1:1000; Santa Cruz Biotechnology), rabbit cAMP-responsive element-binding protein (CREB) antibody (1:1000; Santa Cruz Biotechnology), rabbit phospho-CREB (p-CREB) antibody (1:1000; Santa Cruz Biotechnology), mouse phosphatidylinositol 3-kinase (PI 3-kinase) p85α antibody (1:1000; Santa Cruz Biotechnology), rabbit phospho-PI 3-kinase p85α (p-PI 3-kinase p85α) antibody (1:1000; Santa Cruz Biotechnology), rabbit protein kinase C alpha (PKCα) antibody (1:1000; Santa Cruz Biotechnology), rabbit phospho-PKCα (p-PKCα) antibody (Merck Millipore Corp., Darmstadt, Germany), rabbit Akt antibody (1:1000; Cell Signaling Technology Inc., Beverly, MA, USA), and rabbit phospho-Akt (p-Akt) antibody (1:1000; Cell Signaling Technology Inc.) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit antibody (1:2000; Vector Laboratories) for BDNF, TrkB, Akt, p-Akt, CREB, p-CREB, p-PI 3-kinase p85α, PKCα, and p-PKCα, and horseradish peroxidase-conjugated anti-mouse antibody for β-actin, Bax, Bcl-2 and PI 3-kinase p85α (1:2000; Amersham Pharmacia Biothech GmbH, Freiburg, Germany) were used as the secondary antibodies. Band detection was confirmed by enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology).
2.9. Data analysis
The detected bands were calculated densitometrically using Molecular AnalystTM, version .4.1 (Bio-Rad, Hercules, CA, USA). The numbers of TUNEL-positive, caspase-3-positive, and BrdU-positive cells in the hippocampal dentate gyrus were counted hemilaterally. Statistical analysis was performed using one-way analysis of variance (ANOVA) and Duncan's post-hoc test. The results were expressed as mean ± standard error of the mean (SEM). Significance was set at p < 0.05.
3. Results
3.1. Short-term and spatial learning memory
Short-term memory was evaluated using step-down avoidance test ((A)). The latency in the old-aged rats was shorter than the young-aged rats (p < 0.05). However, tamsulosin treatment increased the latency in the old-aged rats (p < 0.05).
Figure 1. Short-term and spatial learning memory. (A) Step-down avoidance test. (B) Radial 8-arm maze test. Left: Number of correct made before first error. Middle: Number of error made before eight successful performance. Right: Time for accomplishing eight successful performance. YC: Young-aged control group, OC: old-aged control group, TAM 0.01: old-aged and 0.01 mg/kg tamsulosin-treated group, TAM 0.1: old-aged and 0.1 mg/kg tamsulosin-treated group, TAM 1: old-aged and 1 mg/kg tamsulosin-treated group. ∗ represents p < 0.05 compared to the young-aged control group. # represents p < 0.05 compared to the old-aged control group.
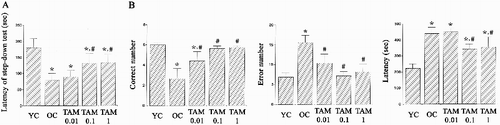
Spatial learning memory was evaluated using radial 8-arm maze test ((B)). The old-aged rats spent significantly longer time and made more error than the young-aged rats (p < 0.05). Their correct number was lower than the young-aged rats (p < 0.05). However, tamsulosin treatment increased the correct number and reduced the error number, resulting in shortening the latency to accomplish this task (p < 0.05).
3.2. Expressions of p-PKCα, p-CREB, BDNF, TrkB, and neurogenesis
PKCα and p-PKCα expressions were examined ((A)). In the old-aged rats, p-PKCα expression in the hippocampus was reduced than the young-aged rats (p < 0.05). However, tamsulosin treatment increased p-PKCα expression in the old-aged rats, resulting in increased p-PKCα/PKCα ratio (p < 0.05).
Figure 2. Expressions of protein kinase C alpha (PKCα), cAMP-responsive element-binding protein (CREB), brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB) in the hippocampus. (A) Phosphorylated PKCα (p-PKCα) and PKCα expressions. (B) Phosphorylated CREB (p-CREB) and CREB expressions. (C) BDNF and TrkB expressions. (D) Neurogenesis in the hippocampal dentate gyrus. Left: Photomicrographs of 5-bromo-2′-deoxyuridine (BrdU)-positive cells. The scale bars represent 1.5 mm in whole brain panel and 50 μm in other panels. Right: Number of BrdU-positive cells in each group. YC: Young-aged control group, OC: old-aged control group, TAM 0.01: old-aged and 0.01 mg/kg tamsulosin-treated group, TAM 0.1: old-aged and 0.1 mg/kg tamsulosin-treated group, TAM 1: old-aged and 1 mg/kg tamsulosin-treated group. ∗ represents p < 0.05 compared to the young-aged control group. # represents p < 0.05 compared to the old-aged control group.
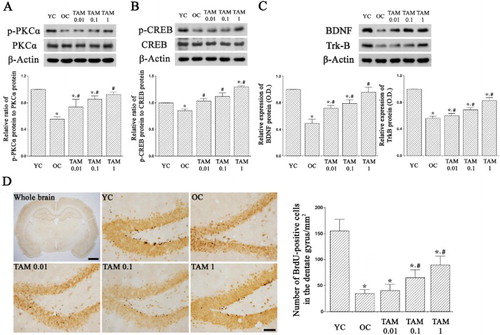
p-CREB and CREB expressions were examined ((B)). In the old-aged rats, p-CREP expression in the hippocampus was reduced than the young-aged rats (p < 0.05). However, tamsulosin treatment enhanced p-CREB expression in the old-aged rats, resulting in increased p-CREB/CREB ratio (p < 0.05).
DNF and TrkB expressions were examined ((C)). In the old-aged rats, BDNF and TrkB expressions in the hippocampus were reduced than the young-aged rats (p < 0.05). However, tamsulosin treatment increased BDNF and TrkB expressions in the old-aged rats (p < 0.05).
Photomicrographs of BrdU-positive cells in the hippocampal dentate gyrus are presented in (D). The number of BrdU-positive cells in the hippocampal dentate gyrus of old-aged rats were reduced than the young-aged rats (p < 0.05). However, tamsulosin treatment increased the number of BrdU-positive cells in the old-aged rats (p < 0.05).
3.3. Expressions of p-PI 3-kinase p85α, p-Akt, Bcl-2, and Bax
p-PI 3-kinase p85α and PI 3-kinase p85α expressions were examined ((A)). Old-aged rats showed reduced p-PI 3-kinase p85α expression in the hippocampus than the young-aged rats (p < 0.05). However, tamsulosin treatment increased p-PI-3 kinase p85α expression in the old-aged rats, resulting in increased p-PI 3-kinase p85α/PI 3-kinase p85α ratio (p < 0.05).
Figure 3. Expressions of phosphatidylinositol 3-kinase p85α (PI 3-kinase p85α), Akt, Bcl-2, and Bax in the hippocampus. (A)Phosphorylated p-PI 3-kinase p85α (p-PI 3-kinase p85α) and PI 3-kinase p85α expressions. (B) Pphosphorylated Akt (p-Akt) and Akt expressions. (C) Bcl-2 and Bax expressions. YC: Young-aged control group, OC: old-aged control group, TAM 0.01: old-aged and 0.01 mg/kg tamsulosin-treated group, TAM 0.1: old-aged and 0.1 mg/kg tamsulosin-treated group, TAM 1: old-aged and 1 mg/kg tamsulosin-treated group. ∗ represents p < 0.05 compared to the young-aged control group. # represents p < 0.05 compared to the old-aged control group.
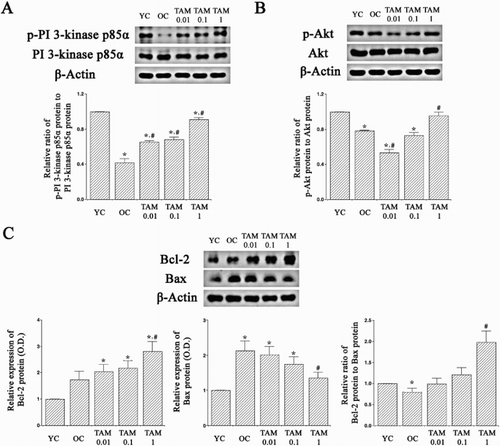
p-Akt and Akt expressions were examined ((B)). Old-aged rats showed reduced p-Akt expression in the hippocampus than the young-aged rats (p < 0.05). However, tamsulosin treatment increased p-Akt expression in the old-aged rats, resulting in increased p-Akt/Akt ratio (p < 0.05).
Bcl-2 and Bax expressions were examined ((C)). Bax and Bcl-2 expressions in the hippocampus of old-aged rats were higher than the young-aged rats. However, the ratio of Bcl-2 to Bax in the old-aged rats was lower than the young-aged rats, because Bax expression was higher than Bcl-2 in the old-aged rats (p < 0.05). However, tamsulosin treatment increased Bcl-2 expression and suppressed Bax expression, resulting in increased Bcl-2/Bax ratio in the old-aged rats (p < 0.05).
3.4. Numbers of caspase-3-positive and TUNEL-positive cells
Caspase-3 expression was examined ((A)). Caspase-3 expression was higher in the hippocampus of old-aged rats than the young-aged rats. (p < 0.05). On the other hand, tamsulosin treatment suppressed caspase-3 expression in the old-aged rats (p < 0.05).
Figure 4. Caspase-3 expression and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL)-positive cells. (A) Caspase-3 expression. Left: Photomicrographs of caspase-3-positive cells. The scale bars represent 1.5 mm in whole brain panel and 50 μm in other panels. Right: Caspase-3 expression in each group. (B) TUNEL-positive cells. Left: Photomicrographs of TUNL-positive cells. The scale bars represent 1.5 mm in whole brain panel and 50 μm in other panels. Right: Number of TUNEL-positive cells in each group. YC: Young-aged control group, OC: old-aged control group, TAM 0.01: old-aged and 0.01 mg/kg tamsulosin-treated group, TAM 0.1: old-aged and 0.1 mg/kg tamsulosin-treated group, TAM 1: old-aged and 1 mg/kg tamsulosin-treated group. ∗ represents p < 0.05 compared to the young-aged control group. # represents p < 0.05 compared to the old-aged control group.
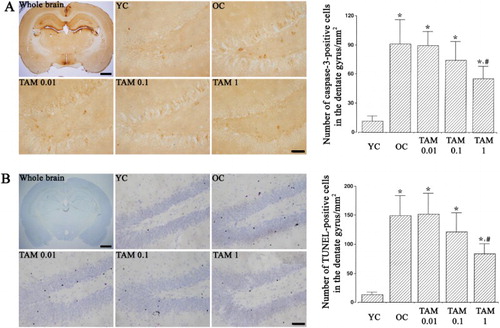
The number of TUNEL-positive cells was examined ((B)). The number of TUNEL-positive cells was higher in the hippocampus of old-aged rats than the young-aged rats. (p < 0.05). However, tamsulosin treatment suppressed the number of TUNEL-positive cells in the old-aged rats (p < 0.05).
4. Discussion
In this study, short-term and spatial learning memory was impaired in the aged rats. Subchronic treatment of prazosin improved performance of memory task in the aged rats (Knauber and Müller Citation2000). Previously, we reported that tamsulosin improved memory by activating N-methyl-D-aspartate (NMDA) receptor-mediated ion currents in addition to overactive bladder therapy (Kim et al. Citation2015). In our study, tamsulosin treatment showed improved short-term and spatial learning memory in the aged rats.
In this study, BDNF expression and neurogenesis in the hippocampus were decreased in the aged rats. Age-related decline in the hippocampal functions accompanied with suppressed neurogenesis (von Bohlen und Halbach O Citation2010). Age-induced reduction of neurogenesis was associated with diminished BDNF expression (Kim et al. Citation2010). BDNF and TrkB expressions were decreased in the hippocampus of aged rats (Kim et al. Citation2010; Yang et al. Citation2014). In our study, tamsulosin treatment increased BDNF and TrkB expressions and also enhanced hippocampal neurogenesis in the aged rats.
In this study, phosphorylation of PKCα, CREB, PI-3 kinase, and Ak in the hippocampus was decreased in the aged rats. PKC is involved in the neurotransmitter release, receptor regulation, cell proliferation, and synaptic remodeling (Pascale et al. Citation2007). Decreased hippocampal PKC activity deteriorated spatial memory, in contrast, PKC activator improved memory during normal aging process (Govoni et al. Citation2010). Among PKC isozymes, PKCα is involved in differentiation, regeneration, and learning ability (Pascale et al. Citation2005). Hongpaisan et al. (Citation2013) reported that reduced PKCα and PKCε expressions in the hippocampus of aged rats correlated with impaired learning and memory retention, and rescued PKCε and PKCα expressions normalized memory to the level of young rats.
PKC also mediates phosphorylation of CREB, and upregulation of CREB enhanced both short-term and long-term memory (Suzuki et al. Citation2011). Memory impairment in the aged rats was associated with decreased CREB phosphorylation in the hippocampus (Xu et al. Citation2010). Gain or loss of CREB function improved or impaired, respectively, the formation of long-term memory (Kida and Serita Citation2014).
TrkB directly activates PI 3-kinase, which normally presents in cytosol (Yuan and Yankner Citation2000). Phosphorylation of PI 3-kinase activated Akt, and this signaling pathway inhibited caspase-mediated apoptosis (Zhang et al. Citation2010). Phosphorylation of Akt promoted cell survival by inactivating apoptosis factors (Yuan and Yankner Citation2000). Especially, phosphorylation of Akt is known to up-regulate anti-apoptotic proteins such as Bcl-2 and Bcl-xL (Hsu et al. Citation2010). In our study, tamsulosin treatment facilitated phosphorylation of PKCα, CREB, PI-3 kinase, and Ak in the aged rats.
In this study, Bcl-2 and Bax expressions in the hippocampus were increased in the aged rats. Bax expression was more prominently enhanced, and then Bcl-2/Bax ratio was increased in the aged rats. While Bcl-2 is crucial for the maintenance of neuronal survival, Bax plays a critical role in neuronal cell death (Yuan and Yankner Citation2000). The ratio of Bcl-2 to Bax or vice versa is an important factor in deciding whether cells undergo apoptosis (Hsu et al. Citation2010; Kim et al. Citation2010). Bcl-2 expression in the hippocampus was increased with aging, and such up-regulation of Bcl-2 exerted neuroprotective effect in the aged rats by decreasing the Bax/Bcl-2 ratio (Kaufmann et al. Citation2001; Kim et al. Citation2010). In our study, of tamsulosin enhanced Bcl-2 expression and suppressed Bax expression, and consequently increased Bcl-2/Bax ratio in the aged rats.
In this study, the number of TUNEL-positive cells and caspase-3 expression in the hippocampus were increased in the aged rats. The number of TUNEL-positive cells and caspase-3 expression were increased in the brain of the aged rats (Martin et al. Citation2002; Kim et al. Citation2010). In our study, treatment of tamsulosin suppressed the number of TUNEL-positive cells and caspase-3 expression in the aged rats.
In conclusion, tamsulosin activated PKC/CREB and PI-3 kinase/Akt pathways. With these pathways, BDNF-TrkB signaling enhanced hippocampal neurogenesis and suppressed apoptosis in the old-aged rats. As the results, tamsulosin improved performance of short-term and spatial learning memory in the aged rats. Tamsulosin can be considered as a new therapeutic agent for improving memory function during aging process.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Sung-Eun Kim http://orcid.org/0000-0003-3742-0271
Additional information
Funding
References
- Driscoll I, Sutherland RJ. 2005. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 16:8787–121. doi: 10.1515/REVNEURO.2005.16.2.87
- Gibbs ME, Bowser DN. 2010. Astrocytic adrenoceptors and learning: α1-adrenoceptors. Neurochem Int. 57:404–410. doi: 10.1016/j.neuint.2010.03.020
- Gibbs ME, Summers RJ. 2001. Stimulation of α1-adrenoceptors inhibits memory consolidation in the chick. Eur J Neurosci. 14:1369–1376. doi: 10.1046/j.0953-816x.2001.01742.x
- Govoni S, Amadio M, Battaini F, Pascale A. 2010. Senescence of the brain: focus on cognitive kinases. Curr Pharm Des. 16:660–671. doi: 10.2174/138161210790883732
- Hellstrom WJ, Sikka SC. 2006. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 176:1529–1533. doi: 10.1016/j.juro.2006.06.004
- Hongpaisan J, Xu C, Sen A, Nelson TJ, Alkon DL. 2013. PKC activation during training restores mushroom spine synapses and memory in the aged rat. Neurobiol Dis. 55:44–62. doi: 10.1016/j.nbd.2013.03.012
- Hsu YY, Liu CM, Tsai HH, Jong YJ, Chen IJ, Lo YC. 2010. KMUP-1 attenuates serum deprivation-induced neurotoxicity in SH-SY5Y cells: roles of PKG, PI3 K/Akt and Bcl-2/Bax pathways. Toxicology. 268:46–54. doi: 10.1016/j.tox.2009.11.021
- Jin Y, Yan EZ, Li XM, Fan Y, Zhao YJ, Liu Z, Liu WZ. 2008. Neuroprotective effect of sodium ferulate and signal transduction mechanisms in the aged rat hippocampus. Acta Pharmacol Sin. 29:1399–1408. doi: 10.1111/j.1745-7254.2008.00848.x
- Katsouri L, Vizcaychipi MP, McArthur S, Harrison I, Suárez-Calvet M, Lleo A, Lloyd DG, Ma D, Sastre M. 2013. Prazosin, an α1-adrenoceptor antagonist, prevents memory deterioration in the APP23 transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 34:1105–1115. doi: 10.1016/j.neurobiolaging.2012.09.010
- Kaufmann JA, Bickford PC, Taglialatela G. 2001. Oxidative-stress-dependent up-regulation of Bcl-2 expression in the central nervous system of aged Fisher-344 rats. J Neurochem. 76:1099–1108. doi: 10.1046/j.1471-4159.2001.00118.x
- Kida S, Serita T. 2014. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull. 105:17–24. doi: 10.1016/j.brainresbull.2014.04.011
- Kim TW, Choi HH, Chung YR. 2016. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J Exerc Rehabil. 12:156–62. doi: 10.12965/jer.1632644.322
- Kim SE, Ko IG, Hwang L, Choi IY, Shin MS, Kim CJ, Kim KH. 2013. An animal study to compare the degree of the suppressive effects on the afferent pathways of micturition between tamsulosin and sildenafil. J Biomed Sci. 20:81. doi: 10.1186/1423-0127-20-81
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. 2010. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 45:357–365. doi: 10.1016/j.exger.2010.02.005
- Kim CH, Ko IG, Kim SE, Shin MS, Kang YH, Cho JW, Shin KM, Kim CJ, Lim BV, Kim KH. 2015. Alpha1-adrenoceptor antagonists improve memory by activating N-methyl-D-aspartate-induced ion currents in the rat hippocampus. Int Neurourol J. 19:228–236. doi: 10.5213/inj.2015.19.4.228
- Kim SE, Shin MS, Kim CJ, Park JH, Chung KJ, Jung H, Kim KH, Lee JH, Ko IG. 2012. Effects of tamsulosin on urinary bladder function and neuronal activity in the voiding centers of rats with cyclophosphamide-induced overactive bladder. Int Neurourol J. 16:13–22. doi: 10.5213/inj.2012.16.1.13
- Knauber J, Müller WE. 2000. Subchronic treatment with prazosin improves passive avoidance learning in aged mice: possible relationships to α1-receptor up-regulation. J Neural Transm. 107:1413–1426. doi: 10.1007/s007020070005
- Lee SH, Ko IG, Kim SE, Hwang L, Jin JJ, Choi HH, Kim CJ. 2016. Aqueous extract of Cordyceps alleviates cerebral ischemia-induced short-term memory impairment in gerbils. J Exerc Rehabil. 12:69–78. doi: 10.12965/jer.1632586.293
- Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, Horrobin DF, Campbell VA, Lynch MA. 2002. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem. 277:34239–34246. doi: 10.1074/jbc.M205289200
- Michel MC, de la Rosette JJ. 2004. Efficacy and safety of tamsulosin in the treatment of urological diseases. Expert Opin Pharmacother. 5:151–160. doi: 10.1517/14656566.5.1.151
- Pascale A, Amadio M, Govoni S, Battaini F. 2007. The aging brain, a key target for the future: The protein kinase C involvement. Pharmacol Res. 55:560–569. doi: 10.1016/j.phrs.2007.04.013
- Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, Govoni S, Alkon DL, Quattrone A. 2005. Neuronal ELAV proteins enhance mRNA stability by a PKCα-dependent pathway. Proc Natl Acad Sci USA. 102:12065–12070. doi: 10.1073/pnas.0504702102
- Puumala T, Greijus S, Narinen K, Haapalinna A, RiekkinenSrP, Sirviö J. 1998. Stimulation of alpha-1 adrenergic receptors facilitates spatial learning in rats. Eur Neuropsychopharmacol. 8:17–26. doi: 10.1016/S0924-977X(97)00040-0
- Sirviö J, MacDonald E. 1999. Central α1-adrenoceptors: Their role in the modulation of attention and memory formation. Pharmacol Ther. 83:49–65. doi: 10.1016/S0163-7258(99)00017-0
- Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, et al. 2011. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 31:8786–8802. doi: 10.1523/JNEUROSCI.3257-10.2011
- Torkaman-Boutorabi A, Danyali F, Oryan S, Ebrahimi-Ghiri M, Zarrindast MR. 2014. Hippocampal α-adrenoceptors involve in the effect of histamine on spatial learning. Physiol Behav. 129:17–24. doi: 10.1016/j.physbeh.2014.02.009
- von Bohlen und Halbach O. 2010. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Aging Neurosci. 2:36.
- Xu J, Rong S, Xie B, Sun Z, Deng Q, Wu H, Bao W, Wang D, Yao P, Huang F, Liu L. 2010. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J Gerontol A Biol Sci Med Sci. 65:933–940. doi: 10.1093/gerona/glq094
- Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L. 2014. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res. 264:82–90. doi: 10.1016/j.bbr.2014.02.005
- Yuan J, Yankner BA. 2000. Apoptosis in the nervous system. Nature. 407:802–809. doi: 10.1038/35037739
- Zhang L, Qu Y, Tang J, Chen D, Fu X, Mao M, Mu D. 2010. PI3 K/Akt signaling pathway is required for neuroprotection of thalidomide on hypoxic-ischemic cortical neurons in vitro. Brain Res. 1357:157–165. doi: 10.1016/j.brainres.2010.08.007
