ABSTRACT
The histone demethylase lysine-specific demethylase 4A (KDM4A/Jmjd2A) has diverse functions, including involvement in gene regulation and cell cycle, and plays an oncogenic role in cancer cells. The modulation of KDM4A through post-translational modifications remains unclear. Here, we show that small ubiquitin-like modifier (SUMO) 1-mediated modification of KDM4A was required for interaction with tumor suppressor p53. Our data revealed that KDM4A is mainly sumoylated at lysine residue 471. However, the SUMO modification resulted in little change in subcellular localization, demethylase activity, or protein stability of KMD4A. Intriguingly, co-immunoprecipitation data revealed that sumoylation-defective mutants of KDM4A had a lower binding ability with p53 compared to that of wild-type KDM4A, suggesting a positive role for sumoylation in the interaction between KDM4A and p53. Together, these data suggest that KDM4A is post-translationally modified by SUMO, and this sumoylation may be a novel regulatory switch for controlling the interplay between KDM4A and p53.
Introduction
Histone demethylases (HDMs) are crucial regulators of gene expression, cell cycle, chromosome structure, and genome stability (Cloos et al. Citation2008). Furthermore, these enzymes are de-regulated in a variety of cancers and developmental defects (Rotili and Mai Citation2011). HDM enzymes remove a methyl group from lysine residues of histones and non-histone proteins (Hamamoto et al. Citation2015). In higher eukaryotes, lysine demethylases are grouped into two classes. Class I lysine demethylases1 (KDM1) are flavin adenine dinucleotide (FAD)-dependent amine oxidases, which can act only on mono and dimethylated lysine. Class II (JmjC domain-containing histone demethylases) are Fe (II) and 2-oxoglutarate-dependent demethylases, which reverse lysine mono, di, and trimethylation (Anand and Marmorstein Citation2007). Recently, many studies found that the functions of HDMs are regulated by post-translational modifications (PTMs) such as methylation, phosphorylation, acetylation, ubiquitination, and sumoylation (Baba et al. Citation2011; Cheng et al. Citation2014; Toffolo et al. Citation2014). KDM4A can also be controlled via post-translational modifications (Tan et al. Citation2011). KDM4A/Jmjd2A is a JmjC domain-containing demethylase belonging to the KDM4 family that demethylates di and trimethylations of histone 3-lysine 9 (H3K9me3/2) and H3-Lys36 (H3K36me3/2) (Klose et al. Citation2006). KDM4A is a substrate of SKP1-CUL1-F-box (SCF) E3 ligase complexes (SCFFBXO22) and can be degraded via ubiquitin-mediated proteasomal degradation (Tan et al. Citation2011).
Sumoylation, an important posttranslational modification, involves a cascade of enzymatic processes catalyzed by the E1 SUMO-activating enzyme, the E2-conjugating enzyme (UBC9), and an E3 SUMO-ligase, which is involved in substrate target selection and promotes the transfer of SUMO from UBC9 to the target protein (Wilkinson and Henley Citation2012). SUMO encodes a small ubiquitin-like modifier and SUMO1, SUMO2/3, and SUMO4 have been identified in humans (Jürgen Dohmen Citation2004). Recently, a link between sumoylation and the demethylase enzyme has been demonstrated, e.g. sumoylation of KDM5B inhibited its function by preventing its occupancy at target genes (Bueno and Richard Citation2013).
Knockdown of KDM4A increases expression of the tumor suppressor protein p53, leading to apoptosis and reduced proliferation in colon cancer cell lines (Kim et al. Citation2012). One study revealed that SUMO modification plays a crucial role in regulation of the function of heterogeneous nuclear ribonucleoprotein K (hnRNP-K) as a positive regulator of p53 in response to DNA damage (Lee et al. Citation2012). Another report suggests that sumoylation of histone deacetylase 2 (HDAC2) interferes with DNA binding of p53 and alters expression of the p53 target (Wagner et al. Citation2012). KDM4A is a nuclear protein (Salifou et al. Citation2016) that is overexpressed in various cancer cells (Guerra-Calderas et al. Citation2015). In addition, SUMO1 was expressed at much higher levels in colon cancer cell lines compared to normal cells (Cubeñas-Potts and Matunis Citation2013). However, it is unclear how KDM4A may modulate expression of the p53 protein, and the molecular mechanisms regulating their interplay have yet to be defined. In the present study, we show that KDM4A can be modified by SUMO1 and this sumoylation facilitates the interaction between p53 and KDM4A. These data suggest a novel mechanism regarding control of p53 function via sumoylation of KDM4A.
Materials and methods
Cell culture and transfection
293 T cells (ATCC) and NIH3T3 cells were grown in Dulbecco's modified Eagle's medium (DMEM, Welgene, Seoul, Korea) with 4 mM L-glutamine and 10% fetal bovine serum. The 293 T and NIH3T3 cells were seeded and incubated in 100-mm dishes. Cells were transfected when 50% confluent using the Effectene transfection kit (Qiagen, Germantown, MD) according to the manufacturer's instructions. Cells were then harvested approximately 48 h after transfection.
Plasmids and antibodies
Full-length human KDM4A, KDM4B, and KDM4C were amplified by PCR from human HeLa cDNA produced in the synthesis reaction. The PCR product was then inserted into pCMV-3Tag-6 Flag epitope-tagging mammalian expression vectors (Stratagene, La Jolla, CA). The lysine (K) to arginine (R) mutants KDM4A K463R, KDM4A K471R, KDM4A K549R, KDM4A K999R, KDM4A K1036R, and KDM4A K3R (combination of K463R/K471R/K549R) plasmids were generated by PCR-based mutagenesis as previously described (Park et al. Citation2014). Human SUMO1 and SUMO1 lacking a C-terminal Gly–Gly (SUMO1-ΔGG) were amplified by PCR and subcloned into pCMV-3Tag-2A myc-tagging mammalian expression vectors (Stratagene). The following antibodies were used: anti-Flag (F3165) (Sigma-Aldrich, St Louis, MO), anti-Myc (ab9106) (Abcam, Cambridge, UK), anti-GAPDH (LF-PA0018) (AbFrontier, Seoul, Korea), anti-KDM4A (75–189) (NeuroMab, Davis, CA), anti-KDM4A (sc-81302) (Santa Cruz Biotechnologies, Dallas, TX), anti-H3K9me1 (ab9045) (Abcam), and anti-H3K9me3 (ab8898) (Abcam).
Immunoblot analysis
The cultured cells were washed and lysed with lysis buffer (150 mM NaCl, 10 mM Tris pH 7.5, 0.2% Triton X-100, 0.2 mM Na3VO4, 0.3% NP-40, 1 mM phenylmethylsulfonyl fluoride [PMSF]) containing protease inhibitors (Roche, USA). Cell lysate (50 μg protein) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene difluoride (PVDF) membrane (EMD Millipore, Billerica, MA).
Immunoprecipitation and in vivo sumoylation assay
Cultured cells were washed with phosphate-buffered saline (PBS) and extracted with lysis buffer (20 mM Tris-HCl pH 8, 137 mM NaCl, 10% glycerol, 1% NP-40, 2 mM ethylenediaminetetraacetic acid [EDTA]) containing protease inhibitors (Roche). Cell lysate protein (500 µg) was incubated with FLAG M2 agarose (Sigma) overnight at 4°C as previously described (Park et al. Citation2014), the beads were then washed five times with lysis buffer, and the immunoprecipitates were eluted by boiling the beads in 2× SDS sample buffer for 10 min. The immunoprecipitates were resolved by SDS-PAGE and transferred onto PVDF membrane for immunoblotting. For sumoylation assay, 10 mM N-ethylmaleimide (NEM) (Sigma), a reagent for the covalent modification of cysteine residues in proteins, was added to lysis buffer.
Immunofluorescence microscopy
Immunostaining was performed as previously described (Park et al. Citation2014). NIH3T3 cells grown on coverslips in 12-well plates were transfected with Flag-KDM4A-wild-type (WT), Flag-KDM4A (K471R), and Flag-KDM4A (K3R) expression plasmids. Cells were washed with cold PBS 48 h after transfection, fixed in 3.65% formaldehyde for 30 min at room temperature, and permeabilized with 0.2% Triton X-100 for 30 min. Cells were blocked with 5% bovine serum albumin (BSA) for 1 h. Cells were washed three times with PBS and then stained with anti-Flag (F3165), anti-H3K9me1 (ab9045), and anti-H3K9me3 (ab8898). Alexa Fluor488 (A11008) (Invitrogen, Carlsbad, CA) or Alexa Fluor568 (A11004) (Invitrogen)-conjugated secondary antibodies were used. A Carl Zeiss confocal microscope equipped with a 63× objective lens was used for image acquisition.
Results
KDM4A and KDM4B are post-translationally modified by SUMO1
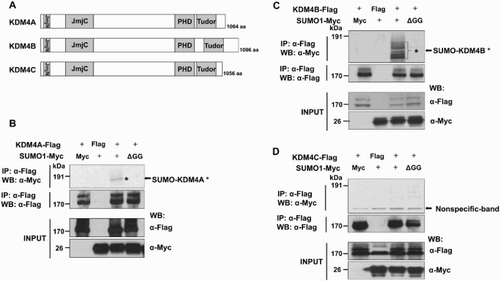
In order to determine the SUMO modification of the KDM4 family proteins, we searched the consensus sumoylation sequence φKxE (φ represents a hydrophobic amino acid) of all human KDM4 family proteins. Among the KDM4 family, we identified KDM4A and KDM4B as containing potential sumoylation sites identified using the SUMOplot analyses program (http://www.abgent.com/tool/sumoplot) ((A), data not shown). Based on these data, we determined the sumoylation of KDM4A, KDM4B, and KDM4C by SUMO1. Cells (293 T) were transiently transfected with Flag-KDM4A, Flag-KDM4B, or Flag-KDM4C expression plasmids with or without Myc-tagged SUMO1 (WT or ΔGG mutant). SUMO1-ΔGG is a mutated form of SUMO lacking the C-terminal glycine required for attachment to substrate. The slower-migrating band was visible when KDM4A and KDM4B were ectopically co-expressed with Myc-tagged SUMO1, but was barely detectable in KDM4C-overexpressing cells ((B–D)). In contrast, sumoylation of KDM4A and KDM4B was abrogated by SUMO1-ΔGG co-expression ((B) and 1(C)). Taken together, these data suggest that both KDM4A and KDM4B are post-translationally modified by SUMO1.
Figure 1. KDM4A and KDM4B are sumoylated by SUMO1. (A) Schematic representation of the KDM4 family proteins. PHD and Tudor domains are required for binding to methylated histone(s). (B), (C), (D) The 293 T cells were transiently co-transfected with Flag-KDM4A, Flag-KDM4B, or Flag-KDM4C with Myc-SUMO1 or Myc-SUMO1-ΔGG. After 48 h, cells were immunoprecipitated and detected by the Flag-tag. Asterisks indicate the SUMO-modified KDM4A and KDM4B proteins. IP, immunoprecipitate; WB, western blot
Lysine 471 is a major sumoylation site of KDM4A
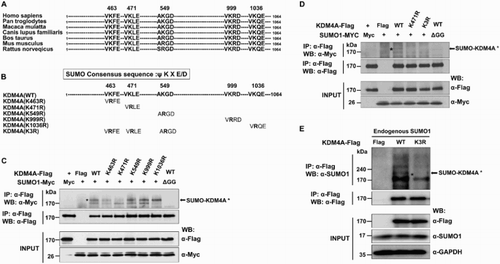
Interestingly, potential SUMO motifs present in KDM4A are conserved among vertebrates ((A)). In order to determine the lysine residues in KDM4A that are modified by SUMO1, we mutated K463, K471, K549, K999, and K1036 to R individually or in combination (K463/K471/K549), termed K3R ((B)). K463R, K549R, K999R, and K1036R did not alter SUMO modification of KDM4A, whereas mutation of K471R resulted in loss of detectable sumoylation ((C)). As predicted, the triple mutant, K3R, which includes the K471R mutation, completely abolished sumoylation of KDM4A ((D)). Subsequently, our in vivo sumoylation assay using anti-SUMO1 antibodies demonstrated that WT KDM4A can be modified by endogenous SUMO1 ((E)). In contrast, the slower-migrating bands were barely detectable in the sample expressing the Flag-KDM4A-K3R mutant ((E)). Together, these observations demonstrate that K471 is the major sumoylation site of KDM4A.
Figure 2. Lysine 471 is the major SUMO site in KDM4A. (A) Alignment of the KDM4A sequence from vertebrates showing a conservation of the potential sumoylation site at position K471. (B) Schematic representation of the position of the five potential sumoylation sites in KDM4A. Bolded ‘R’ indicates a point mutation from K to R. (C) Myc-SUMO1 or Myc-SUMO1-ΔGG expression vectors were introduced into 293 T cells together with Flag-KDM4A-WT or Flag-KDM4A mutants in which K was mutated to R (K463R, K471R, K549R, K999R, and K1036R). Total lysates were used for immunoprecipitation with FLAG agarose beads, and followed by immunoblotting. (D) Lysates of 293 T cells transiently cotransfected with Flag-KDM4A-WT, Flag-K471R, or Flag-K3R (K463R/K471R/K549R) with Myc-SUMO1 or Myc-SUMO1-ΔGG were immunoprecipitated with FLAG beads followed by immunoblotting. (E) The 293 T cells were transiently transfected with Flag-KDM4A-WT or Flag-KDM4A K3R. Total cell extracts were subjected to immunoprecipitation by anti-FLAG agarose beads, followed by immunoblot analysis using an anti-SUMO antibody. Asterisks indicate SUMO-modified KDM4A proteins. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
SUMO modification does not alter the histone demethylase activity, subcellular localization, or stability of KDM4A
KDM4A serves as a histone demethylase of H3K9 and H3K36 (Klose et al. Citation2006). In some cases, sumoylation alters the subcellular localization of proteins and often impairs protein degradation (Cubeñas-Potts and Matunis Citation2013). Thus, we questioned if sumoylation of KDM4A is involved in control of subcellular localization, protein stability, or enzymatic activity as a histone demethylase. We sought to determine whether sumoylation of KDM4A affected the H3K9 methylation pattern or subcellular localization of KDM4A. For this purpose, 293 T cells were transfected with vectors expressing Flag-KDM4A-WT, Flag-KDM4A (K471R), or Flag-KDM4A (K3R) followed by analysis of H3K9 methylation levels by indirect immunofluorescence. Consistent with a previous report (Klose et al. Citation2006), we observed loss of H3K9me3 and little change in H3K9me1 methylation in cells expressing KDM4A-WT. In addition, H3K9me3 levels were only slightly changed in KDM4A (K471R)-expressing cells ((A) and 3(B)). Thus, these data suggest that sumoylation does not affect the H3K9 histone demethylation activity of KDM4A.
Figure 3. Sumoylation does not affect the histone demethylase activity, subcellular localization, or protein stability of KDM4A in 293 T cells. (A) (B) Flag-KDM4A-WT and Flag-KDM4A (K471R) proteins were expressed in NIH3T3 cells. After 2 days, cells were subjected to immunostaining assays with anti-H3K9Me1 (A), anti-H3K9Me3 (B), and anti-Flag. The loss of H3K9Me3 is indicated with an arrow. The 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining indicates the location of nuclei in each field. (C) Flag-KDM4A-WT and Flag-KDM4A (K3R) (MT) proteins were expressed in NIH3T3 cells. After 2 days, cells were subjected to immunofluorescence assays with anti-Flag antibody. (D) 293 T cells expressing Flag-KDM4A, Flag-KDM4A (K471R), and Flag-KDM4A (K3R) were treated with cycloheximide (CHX). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Cells were harvested at the indicated times and analyzed by immunoblotting.
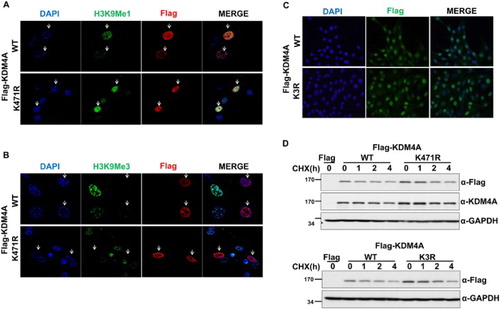
Next, we monitored the subcellular localization of KDM4A-WT and KDM4A mutants (K471R, K3R) by immunostaining analysis of transfected cells. Consistent with previous observations (Tan et al. Citation2011), we found that KDM4A-WT protein was uniformly distributed in the nucleus ((A)). All KDM4A mutants, including K471R and K3R, were also observed in the nucleus ((A–C)), suggesting that sumoylation did not alter the nuclear localization of KDM4A.
Finally, we examined the role of sumoylation on KDM4A protein stability. Cell lines (293 T) stably expressing Flag-KDM4A-WT, Flag-KDM4A (K471R), or Flag-KDM4A (K3R) were treated with cycloheximide and the protein levels analyzed by immunoblot analysis. These data showed that Flag-KDM4A-WT had a half-life of 2 h ((D)). The half-lives of Flag-KDM4A (K471R) and Flag-KDM4A (K3R) were similar to that of Flag-KDM4A-WT, suggesting that sumoylation does not influence the protein stability of KDM4A ((D)).
Sumoylation facilitates the interaction between KDM4A and p53
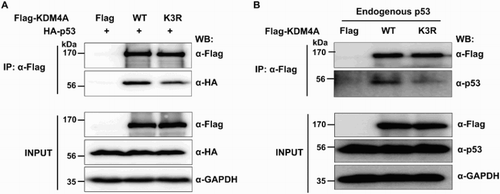
KDM4A interacts with tumor suppressor protein p53, and KDM4A amino acids 301–703 are responsible for the interaction (Kim et al. Citation2012). Intriguingly, the SUMO-conjugation site, K471, of KDM4A is included within the interaction domain (301–703aa), thereby raising the possibility that sumoylation may influence the interaction between p53 and KDM4A. In order to determine if sumoylation may be required for the interaction of KDM4A with p53, we investigated the interaction of p53 with Flag-KDM4A-WT or Flag-KDM4A (K3R) mutant proteins using co-immunoprecipitation (co-IP). Flag-KDM4A-WT or Flag-KDM4A (K3R) expression vectors were co-transfected with HA-tagged p53 vector into 293 T cells and immunoprecipitation assays were performed. Consistent with a previous report (Kim et al. Citation2012), Flag-KDM4A strongly interacted with HA-tagged p53. In contrast, mutation of sumoylation sites within KDM4A reduced the interaction between p53 and KDM4A ((A)). These data suggest that sumoylation is required for the interaction between p53 and KDM4A, at least under ectopic expression conditions. Next, we repeated the co-IP assay using anti-p53 antibody to determine the effect of sumoylation on the interaction between Flag-KDM4A and endogenous p53, and found that endogenous p53 interacted with overexpressed Flag-KDM4A-WT but not with Flag-KDM4A (K3R) ((B)). Therefore, these results indicate that sumoylated KDM4A preferentially binds p53, whereas unmodified KDM4A has a relatively low ability to bind p53.
Figure 4. Sumoylation of KDM4A is required for association of p53 with KDM4A. (A) Flag-KDM4A or Flag-KDM4A-K3R were ectopically co-expressed with HA-tagged p53 in 293 T cells. Total lysates were immunoprecipitated with FLAG-agarose beads and the interaction between KDM4A and p53 was analyzed by immunoblot analysis using anti-HA antibodies. Bottom panel shows the expression of Flag-KDM4A, Flag-KDM4A-K3R, and HA-p53 in the input. (B) Flag-KDM4A or Flag-KDM4A (K3R) were transiently expressed in 293 T cells. The proteins were immunoprecipitated with anti-FLAG antibody and then immunoblotted with anti-p53 antibodies. 10% of input was used in the immunoprecipitation step in (A) and (B). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. IP, immunoprecipitate; WB, western blot
Discussion
KDM4A-deficient cells exhibit upregulation of p53 target gene (p21, bcl2) expression and lead to reduced colon cancer cell proliferation (Kim et al. Citation2012). Here, we demonstrated that KDM4A was modified by SUMO1, and that this sumoylation was involved in the binding between KDM4A and p53. Furthermore, our data suggest that KDM4A may act as a negative regulator of p53; sumoylation was required for enhanced association of p53 with KDM4A.
What is the biological significance of sumoylation on the interaction between p53 and KDM4A? The enhanced association between sumoylated KDM4A and p53 may be required for negative modulation of p53 functions, including interfering with targeting of p53 to its target gene promoters or downregulating its protein stability. Ectopic expression of Flag-KDM4A (K3R) did not affect either mRNA or protein levels of p53, nor expression of p21, a target of p53, regardless of the presence of the endogenous KDM4A gene (data not shown). This data may suggest that sumoylation of KDM4A is not involved in regulation of p53 expression in undamaged cells. As KDM4A is recruited to the p21 promoter upon exposure to adriamycin (Kim et al. Citation2012), sumoylation of KDM4A may be required for damage-induced expression of the p53-p21 axis via efficient targeting of the sumoylated KDM4A-p53 complex to the p21 promoter. However these findings cannot rule out the possibility that other post-translational modifications such as ubiquitination, methylation, and acetylation can affect the mutant phenotypes observed in KDM4A-K471A or K3R mutants. So the function of sumoylation of the KDM4A in colon cancer cells still requires further investigation.
Methylation of p53 stabilizes the protein (West and Gozani Citation2011), and various studies identify cross-talk between SUMO and other post-translational modifications (Lamoliatte et al. Citation2017). Moreover, the ubiquitin-E3-ligase, murine double minute 2 (MDM2), is a critical negative regulator of p53 in order to keep p53 tightly in normal cells (Lamoliatte et al. Citation2017). Several studies also suggest that post-translational modification of both p53 and MDM2 regulate their interaction and the ability of MDM2 to target p53 for degradation (Sherr and Weber Citation2000; Shi and Gu Citation2012). Thus, it is possible that sumoylated KDM4A may negatively control p53 levels through enhancing MDM2-mediated p53 degradation. SCFFBXO22 ubiquitin ligase-KDM4A complexes regulate degradation of methylated p53, leading to control of senescence (Johmura et al. Citation2016), which suggests a role for sumoylated KDM4A in SCFFBXO22-mediated p53 regulation. Very recently, another study reported that KDM4A is sumoylated at K471 by SUMO2/3, which in turn enhances the transcriptional activity and replication of viral genes (Yang et al. Citation2017). Kaposi's sarcoma-associated herpesvirus (KSHV) basic domain leucine-zipper (K-bZIP) acts as a viral SUMO2/3-specific E3 ligase for sumoylation of KDM4A, and this sumoylation is associated with the histone demethylase activity of KDM4A (Yang et al. Citation2017). In the present study, as KDM4A is modified via SUMO1, the sumoylation may not affect the demethylase activity of KDM4A, implying differential regulation of KDM4A function depending on the type of SUMO molecules involved.
Here, we demonstrated that sumoylation of KDM4A was required for binding to p53. Sumoylation of KDM4A may enhance the association between p53 and KDM4A, and sumoylated KDM4A may act as a gatekeeper for damage-induced efficient targeting of p53 to its target promoter. Collectively, we propose for the first time that sumoylation of KDM4A may be a regulatory switch for p53.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anand R, Marmorstein R. 2007. Structure and mechanism of lysine-specific demethylase enzymes. J Biol Chem. 282:35425–35429. doi: 10.1074/jbc.R700027200
- Baba A, Ohtake F, Okuno Y, Yokota K, Okada M, Imai Y, Ni M, et al. 2011. PKA-Dependent Regulation of the histone lysine demethylase complex PHF2-ARID5B. Nat Cell Biol. 13:668–675. doi: 10.1038/ncb2228
- Bueno MTD, Richard S. 2013. SUMOylation negatively modulates target gene occupancy of the KDM5B, a histone lysine demethylase. Epigenetics. 8:1162–1175. doi: 10.4161/epi.26112
- Cheng M-b, Zhang Y, Cao C-y, Zhang W-l, Zhang Y, Shen Y-f. 2014. Specific phosphorylation of histone demethylase KDM3A determines target gene expression in response to heat shock. PLoS Biol. 12. doi: 10.1371/journal.pbio.1002026
- Cloos PAC, Christensen J, Agger K, Helin K. 2008. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev 22:1115–1140. doi: 10.1101/gad.1652908
- Cubeñas-Potts C, Matunis MJ. 2013. SUMO: a multifaceted modifier of chromatin structure and function. Dev Cell. 24:1–12. doi: 10.1016/j.devcel.2012.11.020
- Guerra-Calderas L, González-Barrios R, Herrera LA, de León DC, Soto-Reyes E. 2015. The role of the histone demethylase KDM4A in cancer. Cancer Genet. 208:215–224. doi: 10.1016/j.cancergen.2014.11.001
- Hamamoto R, Saloura V, Nakamura Y. 2015. Critical roles of Non-histone protein lysine methylation in human tumorigenesis. Nat Rev Cancer. 15:110–124. doi: 10.1038/nrc3884
- Johmura Y, Sun J, Kitagawa K, Nakanishi K, Kuno T, Naiki-Ito A, Sawada Y, et al. 2016. SCFFbxo22-KDM4A targets methylated p53 for degradation and regulates senescence. Nat Commun. 7:10574. doi: 10.1038/ncomms10574
- Jürgen Dohmen R. 2004. SUMO protein modification. Biochim Biophys Acta. 1695:113–131. doi: 10.1016/j.bbamcr.2004.09.021
- Kim TD, Shin S, Berry WL, Oh S, Janknecht R. 2012. The JMJD2A demethylase regulates apoptosis and proliferation in colon cancer cells. J Cell Biochem. 113:1368–1376. doi: 10.1002/jcb.24009
- Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. 2006. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine[thinsp]9 and lysine[thinsp]36. Nature. 442:312–316. doi: 10.1038/nature04853
- Lamoliatte F, McManus FP, Maarifi G, Chelbi-Alix MK, Thibault P. 2017. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat Commun. 8:14109. doi: 10.1038/ncomms14109
- Lee SW, Lee MH, Park JH, Kang SH, Yoo HM, Ka SH, Oh YM, Jeon YJ, Chung CH. 2012. SUMOylation of hnRNP-K Is required for p53-mediated cell-cycle arrest in response to DNA damage. EMBO J. 31:4441–4452. doi: 10.1038/emboj.2012.293
- Park SH, Yu SE, Chai YG, Jang YK. 2014. CDK2-Dependent phosphorylation of Suv39H1 Is involved in control of heterochromatin replication during cell cycle progression. Nucleic Acids Res. 42:6196–6207. doi: 10.1093/nar/gku263
- Rotili D, Mai A. 2011. Targeting histone demethylases: A new avenue for the fight against cancer. Genes Cancer. 2:663–679. doi: 10.1177/1947601911417976
- Salifou K, Ray S, Verrier L, Aguirrebengoa M, Trouche D, Panov KI, Vandromme M. 2016. The histone demethylase JMJD2A/KDM4A links ribosomal RNA transcription to nutrients and growth factors availability. Nat Commun. 7:10174. doi: 10.1038/ncomms10174
- Sherr CJ, Weber JD. 2000. The ARF/p53 pathway. Curr Opin Genet Dev. 10:94–99. doi: 10.1016/S0959-437X(99)00038-6
- Shi D, Gu W. 2012. Dual roles of MDM2 in the regulation of p53: ubiquitination dependent and ubiquitination independent mechanisms of MDM2 repression of p53 activity. Genes Cancer. 3:240–248. doi: 10.1177/1947601912455199
- Tan M-KM, Lim H-J, Harper JW. 2011. SCFFBXO22 regulates histone H3 lysine 9 and 36 methylation levels by targeting histone demethylase KDM4A for ubiquitin-mediated proteasomal degradation. Mol Cell B. 31:3687–3699. doi: 10.1128/MCB.05746-11
- Toffolo E, Rusconi F, Paganini L, Tortorici M, Pilotto S, Heise C, Verpelli C, et al. 2014. Phosphorylation of neuronal lysine-specific demethylase 1LSD1/KDM1A impairs transcriptional repression by regulating interaction with CoREST and histone deacetylases HDAC1/2. J Neurochem. 128:603–616. doi: 10.1111/jnc.12457
- Wagner T, Uhlig KM, Knauer SK, Stauber RH. 2012. Dynamically regulated sumoylation of HDAC 2 controls P 53 deacetylation and restricts apoptosis following genotoxic stress. Mol Cell Biol. 1:284–293.
- West LE, Gozani dO. 2011. Regulation of p53 function by lysine methylation. Epigenomics. 3:361–369. doi: 10.2217/epi.11.21
- Wilkinson KA, Henley JM. 2012. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 428:133–145. doi: 10.1042/BJ20100158
- Yang WS, Campbell M, Chang PC. 2017. SUMO modification of a heterochromatin histone demethylase JMJD2A enables viral gene transactivation and viral replication. PLoS Pathog. 13:1–23.
