ABSTRACT
Epidermal growth factor-like domain multiple 6 (Egfl6) is a basement membrane protein and plays an important role in hair follicle morphogenesis, angiogenesis, notochord development in vertebrates. Although egfl6 expression in the developing head was observed in zebrafish, its role for craniofacial development and the determination of the pharyngeal region expressing egfl6, have not been reported yet. Here, we report the expression patterns and function of egfl6 in craniofacial development in zebrafish. egfl6 was expressed sequentially in the developing pharyngeal pouches that are key epithelial structures governing the development of the vertebrate head. However, loss-of-function mutations in egfl6 did not cause any craniofacial defects, including the pouches as well as the thymus and facial cartilages whose development is contingent upon appropriate pouch formation. egfl6 was unlikely redundant with egfl7 expressed in a distinct pharyngeal region from that of egfl6 in craniofacial development because reduction of egfl7 with a MO in egfl6 mutants did not affect craniofacial development. In addition, we found that egfl6 carried an endogenous start loss mutation in the wild-type Tübingen strain, implying egfl6 would be a non-functional gene. Taken all together, we suggest that egfl6 expression in the pharyngeal pouches is not required for craniofacial development in zebrafish.
Introduction
In vertebrates, a series of epithelial branches termed pharyngeal pouches arises in the pharyngeal endoderm during craniofacial development (Grevellec and Tucker Citation2010). Zebrafish forms six pouches, with mice and humans forming five, in the embryonic head (Graham Citation2008). The pouches segment the neural crest-derived pharyngeal arches sequentially, then provide signals, such as Sonic Hedgehog and Jagged, for the arch cells to be survived and differentiate into the facial skeletons (Miller et al. Citation2000; Zuniga et al. Citation2010). In addition, a population of pouch cells becomes the rudiments of endocrine glands, such as the thymus and parathyroid (Grevellec and Tucker Citation2010). Consistent with the essential role of pouches in organizing the head, abnormal development of the third and fourth pouches in human causes DiGeorge syndrome (DGS) with features, including facial anomalies, hypoplastic thymus with immune deficit, palatal anomalies, neonatal hypocalcemia, and heart defect (Driscoll et al. Citation1992).
Recent studies carried out in mice and zebrafish are shedding light on the developmental mechanisms underlying pouch formation. Loss-of-function mutations in tbx1 gene in mice and zebrafish show defects almost identical to those of people with DGS, including the loss of or abnormal pouches, facial anomalies, hypoplastic thymus, and heart defects. Accordingly, tbx1 was determined as the DGS gene (Lindsay et al. Citation2001; Piotrowski et al. Citation2003). Genetically, Tbx1 interacts with Fgf3 and Fgf8 for pouch formation in mice and zebrafish (Crump et al. Citation2004a; Herzog et al. Citation2004; Aggarwal et al. Citation2006; Choe and Crump Citation2014). Besides, transcription factors Pax1/9 (Peters et al. Citation1998; Liu et al. Citation2020), Foxi1/3 (Nissen et al. Citation2003; Solomon et al. Citation2003; Edlund et al. Citation2014; Jin S et al. Citation2018), and Nkx2.3 (Li et al. Citation2019) are required for pouch formation in mice and zebrafish. In addition to Fgf, signaling pathways, such as Wnt (Choe et al. Citation2013), ephrin/Eph (Choe and Crump Citation2015), Integrin (Crump et al. Citation2004b), and BMP (Lovely et al. Citation2016; Li et al. Citation2019) have been implicated in pouch development in zebrafish. Interestingly, a single-cell RNA sequencing performed in zebrafish embryos revealed previously unidentified genes expressed in 24 h-post-fertilization (hpf) cells of pharyngeal endoderm (PE) (Wagner et al. Citation2018). These included keratin 8, keratin 18, EGF-like-domain, multiple 6 (egfl6), and nanos1 (Wagner et al. Citation2018). In order to better understand the genetic mechanism underlying the development of pouches in zebrafish, here we analyze the potential role of egfl6 in pouch formation.
Since the first identification of EGFL6 in human fetal tissues (Yeung et al. Citation1999), orthologs of EGFL6, also called MAM and EGF containing gene (MAEG), have been identified in vertebrates, with biological functions being analyzed during embryonic development in mice and zebrafish. In mice, Egfl6 has been shown as a molecular marker for dermatome, with immunohistochemistry showing the distribution of Egfl6 in the basement membrane of developing hair follicles, in which Egfl6 serves as an adhesive ligand for the α8β1 integrin (Buchner et al. Citation2000; Osada et al. Citation2005; Fujiwara et al. Citation2011). In zebrafish embryos, egfl6 is expressed in the developing somites, with the expression being expanded to the whole trunk; immunohistochemistry and knockdown experiments show that Egfl6 expression accumulated in the notochord is required for the normal development of notochord (Wang et al. Citation2015). Egfl6 secreted from the somites is also involved in embryonic angiogenesis (Wang et al. Citation2016). While egfl6 expression is also seen in the developing hindbrain, pharyngeal region, and fin epidermis (Wang et al. Citation2015), the roles of egfl6 in the development of these tissues have not yet been analyzed. We find the pouch-specific expression of zebrafish egfl6 during pouch morphogenesis and no defects in the pouches and their derivatives by loss of egfl6.
Materials and methods
Zebrafish lines
All zebrafish work was approved by Gyeongsang National University Institutional Animal Care and Use Committee. Zebrafish were raised and maintained by the Animal Protection Act (2017), Korea. Tg(∼3.4her5:EGFP) (Tallafuss and Bally-Cuif Citation2003) and Tg(sox:EGFP) (Carney et al. Citation2006) lines used in this study were published. To generate egfl6 mutant lines with CRISPR/Cas9 system, 150 pg of in vitro-synthesized gRNA and 900 pg in vitro-transcribed mRNA encoding a nuclear-localized Cas9 were injected into one-cell stage wild-type Tübingen (TU) embryos. To identify carriers with germline transmission deletions in the egfl6 gene, embryos were raised to adulthood and outbred to wild-type TU zebrafish. The carriers for egfl6 mutant alleles were in-crossed, and the resulting embryos were used for in situ hybridization, immunochemistry, and alcian blue staining. For genotyping of egfl6 mutant alleles, PCR amplicons produced by primers egfl6_F (5′-CAGCCATGCATACACAAA-3′) and egfl6_R (5′-CTGTCAGTATGGGCTGCT-3′) were digested with TaqI; while a wild-type allele had 208 and 252 bp, egfl6 mutant alleles generated 452 bp (egfl6GNU12), 455bp (egfl6GNU13), and 468 bp (egfl6GNU14). egfl6-morpholino (MO) and egfl7-MO published previously (Parker et al. Citation2004; Wang et al. Citation2015) were obtained from Genetools, and 1 nl of a 300-μM solution was injected at the one-cell-stage.
Staining
Fluorescent in situ hybridizations in conjunction with GFP immunohistochemistry (NC9589665, Torrey Pines Biolabs, 1:1000), Alcama/ZN8 immunohistochemistry (AB_531,904, Zebrafish International Resource Center, 1:400), and Alcian Blue staining were performed as described previously (Crump et al. Citation2004a; Zuniga et al. Citation2011; Lee et al. Citation2020; Chowdhury et al. Citation2021). Partial cDNA fragments of egfl6, egfl7, and rag1 were amplified from mixed-stage embryos and cloned into the pGEM®-T easy vector (A1360, Promega). Antisense riboprobes were synthesized with T7 or SP6 RNA polymerase (111,750,25910, Roche Life Sciences) using digoxigenin (DIG)-labeled nucleotides (Roche) from sequence-verified plasmids. See for primers.
Table 1. List of primers used to generate in situ probes.
Imaging
Fluorescent images were acquired on an Olympus FV1000MPE confocal microscope. Approximately 100-μm-thick z-stacks were captured with an Olympus UPLFLN 10X Objective lens and were assembled using Fluoview Advanced Software. Facial cartilages dissected manually were imaged on an Olympus BX50 upright microscope using mosaic V2.1 software.
Results
Expression of egfl6 in pharyngeal pouch morphogenesis
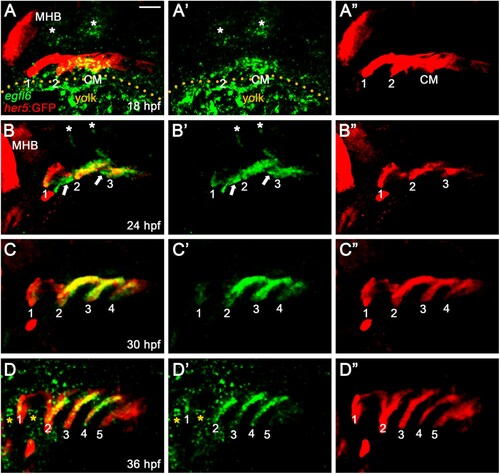
To investigate the potential role of Egfl6 in pouch formation, we first analyzed the expression patterns of egfl6 by in situ hybridization during pouch morphogenesis. In zebrafish, a total of six pouches form sequentially in the pharyngeal endoderm from 18 to 36 hpf, with the first two pouches forming simultaneously at 18 hpf and the sixth pouch hard to see at 36 hpf (Choe et al. Citation2013). We analyzed egfl6 expression at 18, 24, 30, and 36 hpf in wild-type embryos harboring Tg(her5:EGFP) transgene that drives GFP expression in the pharyngeal endoderm and pouches (Tallafuss and Bally-Cuif Citation2003). At 18 hpf, egfl6 was expressed in her5-positive pharyngeal endoderm, including the second pouch and posterior cell mass, with no egfl6 expression being seen in the first pouch ((A)). As previously reported (Wang et al. Citation2015), egfl6 expression was observed in the developing hindbrain (asterisks in (A)). At 24 hpf, egfl6 expression in the second and third pouches was obvious, with a weak egfl6 expression appearing in the first pouch ((B)). In addition, new egfl6 expression was observed apparently in a subpopulation of mesodermal cells between pouches at 24 hpf (arrows in (B)). egfl6 expression in the developing hindbrain continued but was reduced (asterisks in (B)). At 30 hpf, egfl6 expression in the developing pouches continued, whereas the mesodermal expression of egfl6 between pouches disappeared ((C)). In addition, egfl6 expression in the hindbrain was not seen at 30 hpf ((C)). Although egfl6 expression was weak in the fifth pouch, egfl6 was expressed in all pouches at 36 hpf ((D)), with new egfl6 expression being seen in unidentified tissues adjacent to the first and second pouches (asterisks in (D)). While it was suggested previously that the pharyngeal tissue expressing egfl6 at 28 hpf was the pharyngeal arches (Wang et al. Citation2015), our analysis of egfl6 expression in conjunction with a pharyngeal endoderm transgenic reporter indicates that egfl6 is expressed in the pharyngeal pouches from 18 to 36 hpf. Given the importance of pouches in craniofacial development, egfl6 expression in pouches may be required for craniofacial development through pouch development.
Figure 1. Expression of egfl6 in pouch formation. (A-D) Fluorescence in situ hybridization of egfl6 (green) in conjunction with the GFP immunohistochemistry (red) in wild-type Tg(her5:GFP) animals. (A) At 18 hpf, egfl6 expression is seen in the her5-positive second (2) pouch and the posterior cell mass (CM), with no egfl6 expression seen in the first (1) pouch. egfl6 expression is also seen in the developing hindbrain (asterisks). Note of non-specific green staining in the yolks (dotted line). (B) At 24 hpf, egfl6 is expressed in all three her5-positive pouches (1-3), with new egfl6 expression appearing in the mesoderm between pouches (arrows). egfl6 expression is still seen in the developing hindbrain (asterisks). (C) At 30 hpf, egfl6 expression is only observed in all four her5-positive pouches (1-4), with the egfl6 expression in the mesoderm gone. (D) At 36 hpf, egfl6 is expressed in all pouches, with its expression in the fifth (5) pouch being faint. Also, unidentified tissues adjacent to the first (1) and second (2) pouches express egfl6 (asterisks). Note that the sixth pouch is barely seen at the level of tissues. MHB: midbrain−hindbrain boundary. (A′–D′) Green channel only. (A”–D”) Red channel only. Scale bar: 40 μm.
Generation of loss-of-function mutations in egfl6
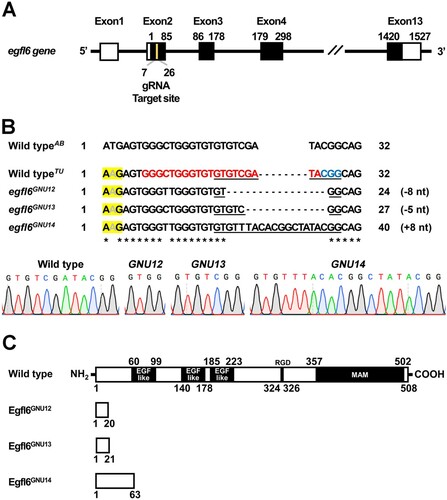
To access the function of egfl6 in craniofacial development, we induced loss-of-function mutations in the egfl6 gene with CRISPR/Cas9 system. egfl6 consists of thirteen exons, which encode the conserved five domains, including three EGF_like domains, RGD (Arg-Gly-Asp) domain, and MAM (meprin/A5-protein/PTPmu) domain (Yeung et al. Citation1999; Wang et al. Citation2015). A gRNA targeting nucleotides 143–162 from the transcription start site of egfl6 was designed with ZiFIT ((A)). We secured three mutant alleles of egfl6 (egfl6GNU12, egfl6GNU13, egfl6GNU14) ((B)). While wild-type egfl6 encodes 508 amino acids, egfl6GNU12, egfl6GNU13, and egfl6GNU14 are predicted to encode 20, 21, and 63 amino acids, respectively, because of premature stop codon induced by the in/del mutation in each mutant allele ((B,C)). Since the conserved five domains of Egfl6 are missing in the three mutant alleles, they are expected to be null alleles ((C)).
Figure 2. Generation of loss-of-function mutations in egfl6 gene. (A) Structure of egfl6 gene. egfl6 gene consists of 13 exons bearing sequences for the protein-coding region (black box) and the 5′ and 3′ untranslated regions (open box). The gRNA target site in the second exon is marked in yellow. (B) Mutant alleles of egfl6 gene. The in/del mutation of each mutant allele is shown in the multiple sequence alignments, with the gRNA target and the PAM sites being marked in red and blue, respectively in the wild-typeTU egfl6 sequence. The electrophoretograms show the lesion in each egfl6 mutant allele that is underlined in the multiple sequence alignments. The start loss mutation in egfl6 gene in the wild-type TU strain is highlighted in yellow. (C) Schematic of the Egfl6 protein encoded by the wild-type and mutant alleles. The conserved five domains marked in the wild-type Egfl6 protein are missing in all mutant Egfl6 proteins.
Craniofacial development is unaffected by the loss of egfl6
To investigate the role of Egfl6 in craniofacial development, we first analyzed pouch formation in egfl6 mutants with Alcama immunohistochemistry. In wild-type animals, five pouches with bilayered structure form at 34 hpf ((A)). In egfl6 mutants, we have not seen any defects in pouch formation in terms of the number and the bilayered structure of pouches at 34 hpf ((B)). Since a subpopulation of cells in the third pouch is further differentiated into thymus rudiments (Piotrowski and Nusslein-Volhard Citation2000), we analyzed whether thymus development was affected by the loss of egfl6 at 4 dpf. In both wild-type and egfl6 mutant animals, we have observed the thymus with in situ hybridization for recombination activating 1 (rag1), a molecular marker for thymus ((E,F)). Normal pouches and thymus seen in egfl6 mutants suggest that the pouch-specific expression of egfl6 is not involved in the development of pouches or their derivatives during craniofacial development.
Figure 3. Normal craniofacial development in egfl6 mutants. (A–D) In both wild-type (A, n = 92) and egfl6 mutant (B, n = 31) embryos at 34 hpf, immunohistochemistry for Alcama (green) shows five pouches (1–5). Reduction of egfl6 with a MO in wild-type (C, n = 64) or egfl6 mutant (D, n = 24) animals display normal five pouches. Sensory ganglia are indicated with asterisks. (E-H) Fluorescent in situ hybridization for rag1 (green) at 4 dpf. In both wild-type (C, n = 74) and egfl6 mutant (D, n = 29) zebrafish, rag1 is expressed normally in the thymus. Reduction of egfl6 in wild-type (G, n = 61) or egfl6 mutant (H, n = 17) animals shows normal thymus. (I-L) Ventral whole-mount views of dissected facial cartilages at 5 dpf. Both wild-type (E, n = 105) and egfl6 mutant (F, n = 37) zebrafish invariantly form a triangled shape of hyomandibular (HM) and five ceratobranchial (CB) cartilages on each side. egfl6-MO-injected animals in wild-type (K, n = 88) or egfl6 mutant (H, n = 15) animals have normal facial cartilages, including the HM and CBs. Scale bar: 40 μm.
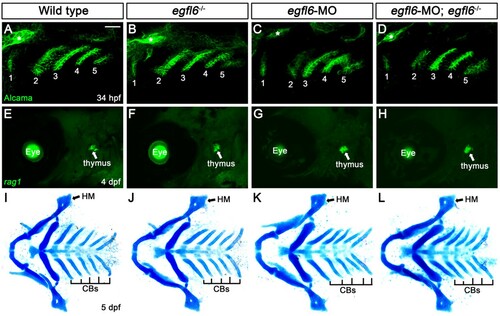
Since pouches are required for the neural crest-derived cells in the pharyngeal arches to differentiate into facial cartilages, including hyomandibular (HM) and ceratobranchial (CB) cartilages (Piotrowski and Nusslein-Volhard Citation2000; Crump et al. Citation2004b), we examined the HM and CB cartilages in egfl6 mutants with Alcian blue staining at 5 dpf. All facial cartilages, including the HM and CB, were normal in wild-type and egfl6 mutant animals ((I,J)), suggesting that egfl6 expression in the pouches is not required for facial cartilage development. So far, we have not seen any defects in the face of egfl6 mutants despite the pouch-specific expression of egfl6 during craniofacial development.
We further analyzed the potential role of egfl6 in craniofacial development by reducing egfl6 with an efficient splice-blocking MO (Supplementary Figure 1 and Supplementary Material). Like egfl6 mutants, reduction of egfl6 with a MO in wild-type or egfl6 mutant animals did not affect the development of pouches, thymus, and facial cartilage, confirming that Egfl6 is not essential for craniofacial development ((C,D,G,H,K,L)).
Distinct expression of egfl7 from that of egfl6 in pharyngeal region
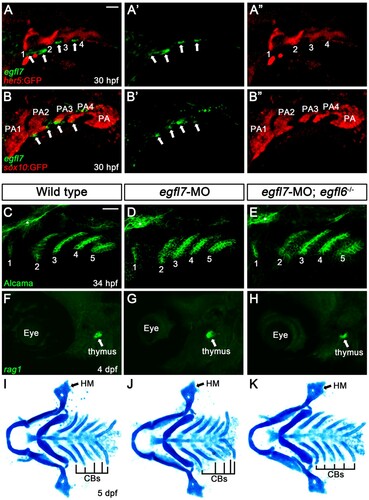
The normal craniofacial development of egfl6 mutants could be due to the genetic redundancy with other Egfl proteins. Previously, it has been shown that Egfl6 has similar structural and functional features with Egfl7 (Kang et al. Citation2020) and that Egfl6 can regulate angiogenesis along with Egfl7 in zebrafish (Wang et al. Citation2016). To examine potential redundancy of Egfl6 with Egfl7 in craniofacial development, we analyzed the expression of egfl7 in the pharyngeal region of wild-type Tg(her5:EGFP) animal, a reporter of pharyngeal pouch, at 30 hpf; egfl7 was expressed segmentally in small patches in pharyngeal region, with the small patches rarely overlapped with pouches (arrows in (A)). To register the region expressing egfl7 at 30 hpf, we also analyzed egfl7 expression in Tg(sox:EGFP) reporter that drives GFP expression in the neural crest-derived pharyngeal arches (Carney et al. Citation2006); the small patches expressing egfl7 were located at the ventral tip of arches but not in the arches (arrows in (B)). Thus, egfl7 was unlikely expressed in the neural crest-derived arches. Considering the pouch-specific expression of egfl6 at 30 hpf, egfl7 expression in the distinct pharyngeal region from that of egfl6 implies that Egfl6 is unlikely redundant with Egfl7 in craniofacial development. Indeed, reduction of egfl7 in wild-type or egfl6 mutant animals with an efficient splice-blocking MO did not affect craniofacial development, including the pouches, thymus, and facial cartilage ((C–K), Supplementary Figure 2, and Supplementary Material). Although we still cannot completely rule out a possibility of genetic redundancy of Egfl6 with other Egfl proteins, we suggest that egfl6 expression in the pouches is dispensable for craniofacial development in zebrafish.
Figure 4. Expression of egfl7 in the pharyngeal region. (A, B) Fluorescence in situ hybridization of egfl7 (green) in conjunction with the GFP immunohistochemistry (red) in wild-type animals at 30 hpf. (A) egfl7 is expressed segmentally in small patches (arrows) adjacent to her5-positive pouches (1-4). (B) egfl7 expressing small patches (arrows) are located at the ventral tip of sox10-positive pharyngeal arches (PA2-4) but rarely overlapped with PAs. (C-E) Alcama immunohistochemistry (green) labels five pouches (1-5) in wild-type (n = 92), egfl7-MO (n = 80), and egfl7-MO-injected egfl6 mutant (n = 21) embryos at 34 hpf. Sensory ganglia are indicated with asterisks. (F-H) At 4 dpf, rag1 expression (green) in the thymus is normal in wild-type (n = 74), egfl7-MO (n = 76), and egfl7-MO-injected egfl6 mutant (n = 14) zebrafish. (I-K) Facial cartilages, including the HM and CBs, are normal in wild-type (n = 105), egfl7-MO (n = 84), and egfl7-MO-injected egfl6 mutant (n = 19) animals at 5 dpf. Scale bar: 40 μm.
Endogenous start loss mutation in egfl6 gene in wild-type Tübingen strain
While we generated loss-of-function mutations in the egfl6 gene in the background of wild-type Tübingen (TU) strain, we identified an endogenous variant of the egfl6 gene at the start codon, resulting in start loss mutation in the wild-type TU strain (yellow highlight in (B)). The endogenous start loss mutation was unexpected as it was previously reported that the start codon was normal in the wild-type AB strain (Wang et al. Citation2016). Currently, we could not verify the presence of endogenous Egfl6 protein in the wild-type TU strain due to the absence of antibodies against Egfl6 and the failure of GFP knock-in at the egfl6 locus. Although we cannot completely rule out a possibility of non-AUG codon usage for normal egfl6 gene expression, the egfl6 gene is likely pseudogenized in the wild-type TU strain, further suggesting that egfl6 is unnecessary for normal craniofacial development.
Discussion
We have analyzed the expression and function of egfl6 in craniofacial development. While egfl6 is expressed in pouches that are key epithelial structures required for normal craniofacial development, loss-of-function mutations in egfl6 resulted in no defects in the head and face, including the pouches, thymus, and facial cartilages. The normal craniofacial development seen in egfl6 mutants is unlikely due to the genetic redundancy of Egfl6 with Egfl7 that shares similar structural and functional features with Egfl6. Although we could not determine precisely the region expressing egfl7 in this study, it is expressed in the non-pouch and non-arch pharyngeal region at 30 hpf. The distinct expression domains of egfl6 and egfl7 in the pharyngeal region suggest that the role of Egfl6 and Egfl7 in craniofacial development would be different from each other. Considering together the anatomy of the pharyngeal tissues consisting of the pharyngeal arches and pouches, the ectodermal clefts, and the lateral plate mesoderm (LPM) (Graham Citation2008) and the well-characterized role of Egfl7 secreted from LPM in trunk angiogenesis (Parker et al. Citation2004), we speculate that a subpopulation of LPM cells expresses egfl7 probably for the development of facial blood vessels or facial muscles. Analysis of egfl7 expression in conjunction with a molecular marker for LPM will determine the pharyngeal tissue expressing egfl7.
Previously it was reported that Egfl6 is required for the development of the notochord and blood vessels in the trunk during zebrafish embryogenesis (Wang et al. Citation2015; Wang et al. Citation2016). However, our study indicates that the egfl6 gene carries an endogenous start loss mutation in the wild-type TU strain. Although it is necessary to verify the presence of Egfl6 proteins in the wild-type TU strain with Egfl6 immunohistochemistry or GFP knock-in at the egfl6 locus, the egfl6 gene appears to be a pseudogene or non-functional gene at least in the wild-type TU strain. Taken together with the normal craniofacial development of egfl6 mutants in spite of the pouch-specific expression of egfl6, egfl6 seems to be dispensable for craniofacial development and probably for the normal development in the wild-type TU strain. Since egfl6 bears the normal AUG start codon in the wild-type AB strain (Wang et al. Citation2016), comparative analyses of the expression and function of egfl6 in both the TU and AB strains would provide better insights into the biological roles of egfl6 in zebrafish development.
Acknowledgements
We thank Sujeong Gim for technical assistance. This work was supported by the Global Ph. D. Fellowship program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019H1A2A1075288) (to S.J), by Gyeongsang National University academic support (to H. N, H. J, J. P), and by a grant from Basic Science Research Program through the NRF funded by the Ministry of Science and ICT (2019R1A2C1004704) (to C.P.C).
Supplemental Material
Download MS Word (15 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aggarwal VS, Liao J, Bondarev A, Schimmang T, Lewandoski M, Locker J, Shanske A, Campione M, Morrow BE. 2006. Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet. 15(21):3219–3228.
- Buchner G, Broccoli V, Bulfone A, Orfanelli U, Gattuso C, Ballabio A, Franco B. 2000. MAEG, an EGF-repeat containing gene, is a new marker associated with dermatome specification and morphogenesis of its derivatives. Mech Dev. 98(1-2):179–182.
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN. 2006. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 133(23):4619–4630.
- Choe CP, Collazo A, Trinh le A, Pan L, Moens CB, Crump JG. 2013. Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev Cell. 24(3):296–309.
- Choe CP, Crump JG. 2014. Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development. 141(18):3583–3593.
- Choe CP, Crump JG. 2015. Eph-Pak2a signaling regulates branching of the pharyngeal endoderm by inhibiting late-stage epithelial dynamics. Development. 142(6):1089–1094.
- Chowdhury MAU, Raslan AA, Lee E, Eum J, Hwang BJ, Kwon SH, Kee Y. 2021. Histopathological assessment of laterality defects in zebrafish development. Anim Cells Syst (Seoul). 25(3):136–145.
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. 2004a. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 131(22):5703–5716.
- Crump JG, Swartz ME, Kimmel CB. 2004b. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2(9):E244.
- Driscoll DA, Budarf ML, Emanuel BS. 1992. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. Am J Hum Genet. 50(5):924–933.
- Edlund RK, Ohyama T, Kantarci H, Riley BB, Groves AK. 2014. Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers. Dev Biol. 390(1):1–13.
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. 2011. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 144(4):577–589.
- Graham A. 2008. Deconstructing the pharyngeal metamere. J Exp Zool\ Part B. 310B(4):336–344.
- Grevellec A, Tucker AS. 2010. The pharyngeal pouches and clefts: development, evolution, structure and derivatives. Semin Cell Dev Biol. 21(3):325–332.
- Herzog W, Sonntag C, von der Hardt S, Roehl HH, Varga ZM, Hammerschmidt M. 2004. Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development. 131(15):3681–3692.
- Jin S OJ, Stellabotte F, Choe CP. 2018. Foxi1 promotes late-stage pharyngeal pouch morphogenesis through ectodermal Wnt4a activation. Dev Biol. 441(1):12–18.
- Kang J, Wang J, Tian J, Shi R, Jia H, Wang Y. 2020. The emerging role of EGFL6 in angiogenesis and tumor progression. Int J Med Sci. 17(10):1320–1326.
- Lee Y, Kim D, Lee CJ. 2020. Suppressive effects of valproic acid on caudal fin regeneration in adult zebrafish. Anim Cells Syst (Seoul). 24(6):349–358.
- Li L, Ning G, Yang S, Yan Y, Cao Y, Wang Q. 2019. BMP signaling is required for nkx2.3-positive pharyngeal pouch progenitor specification in zebrafish. PLoS Genet. 15(2):e1007996.
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, et al. 2001. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 410(6824):97–101.
- Liu YH, Lin TC, Hwang SL. 2020. Zebrafish Pax1a and Pax1b are required for pharyngeal pouch morphogenesis and ceratobranchial cartilage development. Mech Dev. 161:103598.
- Lovely CB, Swartz ME, McCarthy N, Norrie JL, Eberhart JK. 2016. Bmp signaling mediates endoderm pouch morphogenesis by regulating Fgf signaling in zebrafish. Development. 143(11):2000–2011.
- Miller CT, Schilling TF, Lee K, Parker J, Kimmel CB. 2000. Sucker encodes a zebrafish endothelin-1 required for ventral pharyngeal arch development. Development. 127(17):3815–3828.
- Nissen RM, Yan J, Amsterdam A, Hopkins N, Burgess SM. 2003. Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development. 130(11):2543–2554.
- Osada A, Kiyozumi D, Tsutsui K, Ono Y, Weber CN, Sugimoto N, Imai T, Okada A, Sekiguchi K. 2005. Expression of MAEG, a novel basement membrane protein, in mouse hair follicle morphogenesis. Exp Cell Res. 303(1):148–159.
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, et al. 2004. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 428(6984):754–758.
- Peters H, Neubuser A, Kratochwil K, Balling R. 1998. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12(17):2735–2747.
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, et al. 2003. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 130(20):5043–5052.
- Piotrowski T, Nusslein-Volhard C. 2000. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (danio rerio). Dev Biol. 225(2):339–356.
- Solomon KS, Kudoh T, Dawid IB, Fritz A. 2003. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 130(5):929–940.
- Tallafuss A, Bally-Cuif L. 2003. Tracing of her5 progeny in zebrafish transgenics reveals the dynamics of midbrain-hindbrain neurogenesis and maintenance. Development. 130(18):4307–4323.
- Wagner DE, Weinreb C, Collins ZM, Briggs JA, Megason SG, Klein AM. 2018. Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science. 360(6392):981–987.
- Wang X, Wang X, Yuan W, Chai R, Liu D. 2015. Egfl6 is involved in zebrafish notochord development. Fish Physiol Biochem. 41(4):961–969.
- Wang X, Yuan W, Wang X, Qi J, Qin Y, Shi Y, Zhang J, Gong J, Dong Z, Liu X, et al. 2016. The somite-secreted factor Maeg promotes zebrafish embryonic angiogenesis. Oncotarget. 7(47):77749–77763.
- Yeung G, Mulero JJ, Berntsen RP, Loeb DB, Drmanac R, Ford JE. 1999. Cloning of a novel epidermal growth factor repeat containing gene EGFL6: expressed in tumor and fetal tissues. Genomics. 62(2):304–307.
- Zuniga E, Rippen M, Alexander C, Schilling TF, Crump JG. 2011. Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development. 138(23):5147–5156.
- Zuniga E, Stellabotte F, Crump JG. 2010. Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development. 137(11):1843–1852.
