?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Black garlic (BG) is a newly explored food stuff obtained via fermentation of raw, healthy garlic, especially in Asian countries. Interstitial cells of Cajal (ICC) are the pacemaker cells of gastrointestinal (GI) motility. The purpose of this study was to investigate the effects of BG extract on the pacemaker potentials of the ICC in the small intestines of mice and the possibility of controlling GI motility. The antioxidant activity of BG extract was also investigated. The whole-cell electrophysiological method was used to measure pacemaker potentials of the ICC in vitro, whereas GI motility was measured using the intestinal transit rate (ITR) in vivo. BG extract depolarized the pacemaker potentials of the ICC. Y25130 and RS39604 5-HT receptor antagonists could not inhibit the effect of BG extract on the pacemaker potentials of the ICC, whereas the 5-HT receptor antagonist SB269970 could. Pre-treatment with external Na+ (5 mM) or Ca2+-free solution inhibited the BG extract-induced depolarization of the ICC. With SB203580, PD98059, or c-jun NH2-terminal kinase II inhibitor pre-treatment, BG extract did not induce pacemaker potential depolarization. Moreover, the ITR values were increased by BG extract. Elevation of the ITR due to BG extract was related with increased protein expression of the 5-HT7 receptors. In addition, BG extract showed antioxidant activity. Collectively, these results highlight the ability of BG extract to regulate GI motility and the possibility of using it to develop GI motility modulators in the future. Moreover, BG showed immense potential as an antioxidant.
Introduction
Garlic (Allium sativum L.) has been used as a spice and traditional medicine for eons. Several studies have shown that garlic has beneficial effects on human health, such as anti-inflammatory, anti-cancer, lipid lowering, maintenance of blood pressure, and blood glucose regulation (Kimura et al. Citation2017). However, unprocessed, raw garlic has a characteristic odor and spicy taste, which can limit its use because of gastrointestinal (GI) problems when consumed (Kodera et al. Citation2002). Black garlic (BG) is obtained after garlic has been fermented for a certain duration under high humidity and temperature conditions (Kimura et al. Citation2017). BG does not produce a strong off-flavor caused by the reduction of allicin, which converts it to an antioxidant during processing (Yuan et al. Citation2016). BG extract has demonstrated several bioactivities, including anti-oxidative, anti-allergic, anti-diabetes, anti-inflammation, anti-carcinogenic, and GI emptying effects (Jeong et al. Citation2016; Chen et al. Citation2018).
Interstitial cells of Cajal (ICC) are essential pacemaker cells that regulate the GI motility (Huizinga et al. Citation1995; Ward et al. Citation2000; Kim et al. Citation2005; Hwang et al. Citation2020. Kim et al, Citation2021). Thus, research on ICC plays a very important role in understanding GI motility regulation. However, little is reported on the effects of BG extract on ICC and GI motility. Thus, we investigated the effects of BG extract on the pacemaker potentials of ICC and GI motility. In addition, we assessed the antioxidant activity of BG extract.
Materials and methods
Preparation of the BG extract
BG was purchased from Taewoo Food Co. (Daejeon, Korea). A total of 2 kg of BG was extracted in 70% ethyl alcohol (20 L) for 6 h at 80°C and filtered through a Whatman No. 4 filter paper. After concentrating the solvents using rotary evaporation at 50°C, the yield was approximately 19.8% on a dry weight (w/w) basis.
Preparation of ICC
Small intestines of mice were excised and after removing the mucous membrane, cut it into pieces. The cells were dispersed in a solution containing various enzymes, including collagenase, and were cultured in smooth muscle growth medium (Clonetics, San Diego, CA, U.S.A.) supplemented with a murine stem cell factor (Sigma-Aldrich, St. Louis, MO, U.S.A.) in a 95% O2 incubator.
Electrophysiological experiments
A physiological salt solution (KCl 5, NaCl 135, CaCl2 2, glucose 10, MgCl2 1.2, and HEPES 10) was used to culture clusters of ICC. To examine the pacemaker activity, a solution comprising KCl 140, MgCl2 5, K2ATP 2.7, NaGTP 0.1, creatine phosphate disodium 2.5, HEPES 5, and EGTA 0.1 was added to the cultured clusters of the ICC using a pipette. Whole-cell configuration was performed using an Axopatch 200B amplifier (Axon Instruments, Foster, CA, U.S.A.).
Intestinal transit rate (ITR)
Evans blue (5%, w/v) was administered to healthy ICR mice after administration of BG extract into the stomach. After 30 min of Evans blue administration, the ITR of the mice was checked.
GI motility dysfunction (GMD) model mice
We generated the GMD mouse models by using the acetic acid (AA, 0.6%, w/v, in saline)-induced peritoneal stimulation, as previously described (Lyu and Lee Citation2013). AA was injected intraperitoneally and the research process was carried out as described previously (Kim et al. Citation2013; Wu et al. Citation2013).
Western blotting
After the proteins were separated, they were transferred on a polyvinylidene difluoride (PVDF) membrane. After 1 h of incubation with a blocking buffer, PVDF membranes were probed with anti-5-HT3 receptor (Abcam, Cambridge, UK), anti-5-HT4 receptor (Abcam, Cambridge, UK), anti-5-HT7 receptor (Mybiosource, San Diego, CA, U.S.A.), and anti-β-actin (Santa Cruz Biotechnology, Dallas, TX, U.S.A.) antibodies. Other experiments are the same as in previous studies (Han et al. Citation2021).
Animals
Forty-nine mice (24 male and 25 female; 3–8-d-old) from ICR were used for the ICC experiments. In addition, 39 mice (male; 5–6-week-old) were used for the ITR experiments on heathy mice and mice with GI motility disease, whereas 10 mice (male; 5–6-week-old) were used for the protein expression experiments. The ICC and ITR experiments were completed within 12 h of culturing, respectively. We experimented according to The Institutional Animal Care and Use Committee at Pusan National University approved (approval no. PNU-2019-2462). Also, animals were handled according to the Guide for the Care and Use of Laboratory Animals.
Reactive oxygen species (ROS) scavenging activity
A 0.2-mM 1,1-diphenyl-2-picrylhydrazyl (DPPH) solution was prepared by dissolving DPPH reagent in EtOH. The prepared solution and BG extract were mixed in a 1:1 ratio and incubated for 30 min in a dark room. Absorbance at 517 nm was measured and DPPH radical scavenging activity was calculated using the following formula:
where A is the absorbance value of the sample solution and B is the absorbance value of the control solution.
Drugs
PD98059 and SB203580 were purchased from Tocris Bioscience (United Kingdom), whereas c-jun NH2-terminal kinase (JNK) II inhibitor was purchased from Calbiochem (San Diego, CA, U.S.A.). All other agents were purchased from Sigma-Aldrich.
Statistical analyses
Data are expressed as the mean ± standard error of the mean. Significant differences were evaluated using one-way analysis of variance (ANOVA) or the Student’s t-test. P values < 0.05 were considered as significant.
Results
Effects of BG extract on the pacemaker potentials of ICC
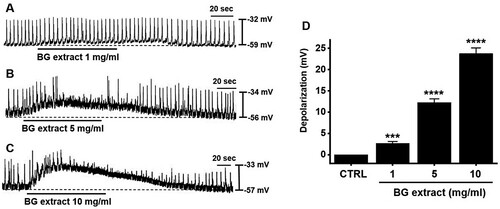
The whole-cell techniques showed that ICC spontaneously induced pacemaker potentials with an average resting membrane potential of –56.7 ± 1.4 mV (n = 164; ). BG extract (1–10 mg/mL) depolarized the pacemaker potentials (A–1C). The average depolarization were 2.7 ± 0.4 mV (n = 11; P < 0.001) at 1 mg/mL, 12.3 ± 0.9 mV (n = 10; P < 0.0001) at 5 mg/mL, and 23.8 ± 1.4 mV (n = 12; P < 0.0001) at 10 mg/mL (D). These results indicated that BG extract depolarized the pacemaker potentials of ICC.
Figure 1. Effects of BG extract on the ICC pacemaker potentials from murine small intestines. (A–C) BG extract depolarized the ICC pacemaker potentials in a dose-dependently. (D) The changes of pacemaker potential depolarization caused by BG extract are summarized. Mean ± SEs. **P < 0.01. BG: Black garlic. CTRL: Control.
Importance of the 5-HT7 receptors in BG extract-induced pacemaker potential depolarization of ICC
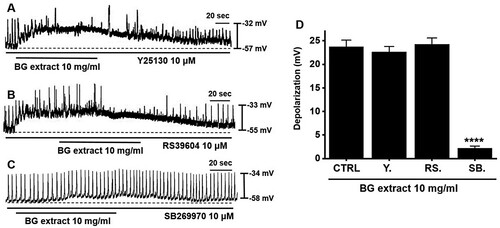
Previous studies have shown that only three receptors (5-HT3, 4, and 7) were identified in the murine small intestinal ICC (Liu et al. Citation2011; Shahi et al. Citation2011). To check the involvement of 5-HT receptors, we exposed ICC to 5-HT receptor antagonists, namely, Y25130 (a 5-HT3 receptor antagonist), RS39604 (a 5-HT4 receptor antagonist), and SB269970 (a 5-HT7 receptor antagonist). Y25130 and RS39604 did not inhibit BG extract-induced responses (A and 2B). However, SB269970 inhibited BG extract-induced responses (C). The average depolarization were 22.6 ± 1.4 mV (n = 12) with Y25130, 24.3 ± 1.5 mV (n = 10) with RS39604, and 2.2 ± 0.5 mV (n = 12; P < 0.0001) with SB269970 treatment (D). These results indicated that BG extract affected the pacemaker potentials in the ICC via the 5-HT7 receptors.
Figure 2. Effects of 5-HT receptor antagonists on BG extract-induced ICC pacemaker potential depolarization. (A and C) Pre-treatment with Y25130 or SB269970 and BG extract depolarized the ICC pacemaker potentials. (B) After pre-treatment with RS39604, BG extract did not depolarize. (D) Responses to BG extract are summarized. Mean ± SEs. **P < 0.01. BG: Black garlic. CTRL: Control. Y: Y25130. RS: RS39604. SB: SB269970.
Importance of extracellular Na+ and Ca2+ in BG extract-induced pacemaker potential depolarization of ICC
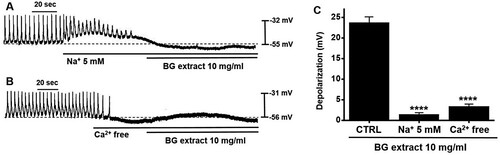
Both external Na+ and Ca2+ play a key role in regulating GI motility (Ward et al. Citation2000). To investigate the importance of external Na+ or Ca2+ in the BG extract-induced responses, we used external Na+ (5 mM) or Ca2+-free conditions. Pre-treatment with the external Na+ or Ca2+-free solution suppressed the pacemaker potentials and inhibited BG extract-induced responses (A and 3B). The average degrees of depolarization were 1.4 ± 0.5 mV (n = 9; P < 0.0001) with Na+ solution and 3.4 ± 0.6 mV (n = 13; P < 0.0001) with Ca2+-free solution (C). These results indicated that the BG extract-induced response was controlled by external Na+ or Ca2+.
Figure 3. Effects of external Na+ (5 mM) or Ca2+-free solution on BG extract-induced ICC pacemaker potential depolarization. (A and B) In case of external Na+ or Ca2+-free solution, BG extract did not depolarize the pacemaker potential. (C) Responses to BG extract are summarized. Mean ± SEs. **P < 0.01. BG: Black garlic. CTRL: Control.
Importance of mitogen-activated protein kinase (MAPK) in BG extract-induced pacemaker potential depolarization of ICC
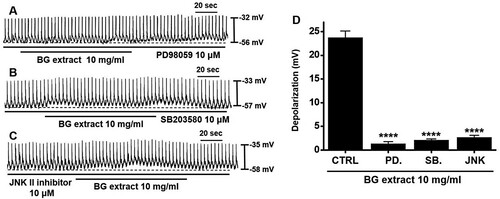
It has been reported that MAPK regulates the proliferation and differentiation of the GI tract (Jeong et al. Citation2012). Therefore, we assessed whether MAPK signaling affects the efficacy of BG extract on pacemaker potentials by treatment with PD98059 (a p42/44 inhibitor), SB203580 (a p38 inhibitor), and JNK II inhibitor. With PD98059 (n = 11), SB203580 (n = 10), or JNK II (n = 10) inhibitor treatment, BG extract did not induce the pacemaker potential depolarization (). These results indicated that the BG extract-induced response was dependent on MAPK signaling.
Figure 4. Effects of MAPK inhibitors on BG extract-induced ICC pacemaker potential depolarization. After pre-treatment with (A) PD98059, (B) SB203580, and (C) JNK II inhibitors, BG extract did not depolarize. (D) Responses to BG extract are summarized. Mean ± SEs. **P < 0.01. BG: Black garlic. CTRL: Control. PD: PD98059. SB: SB203580. JNK: JNK II inhibitor.
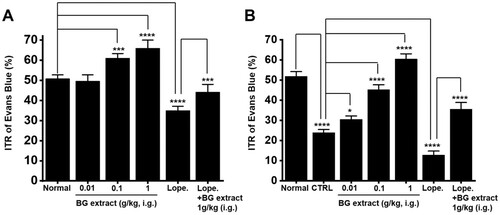
Effects of BG extract on the ITR
In mice, the ITR was 50.8 ± 1.9% (n = 10; A). BG extract increased the ITR to 49.6 ± 3.1% (n = 10) at 0.01 g/kg, 61.1 ± 2.3% (n = 10; p < 0.0001) at 0.1 g/kg, and 66.0 ± 3.9% (n = 10; p < 0.0001) at 1 g/kg (A). Loperamide reduced the ITR, and BG extract recovered this loperamide-induced ITR reduction. For loperamide, the ITR was 35.1 ± 2.1% (n = 10; p < 0.0001), whereas for loperamide and BG extract, the ITR was 44.2 ± 3.8% (n = 10; p < 0.01), A. These results indicated that BG extract increased the ITR.
Effects of BG extract on the ITR in mice with GMD
We generated the GMD mouse model. AA decreased the ITR (23.9 ± 1.5% (n = 10) vs. 51.9% ± 2.3% (n = 10) in normal mice; P < 0.0001; B). However, BG extract at 0.01, 0.1, and 1 g/kg restored this response to 30.5 ± 1.7% (n = 10; P < 0.05), 45.3 ± 2.4% (n = 13; P < 0.0001), and 60.5 ± 2.6% (n = 10; P < 0.0001), respectively (B). Loperamide reduced the ITR in GMD mice to 12.8 ± 2.0% (n = 12; P < 0.0001) and BG extract recovered this value to 35.6 ± 3.2% (n = 11; P < 0.0001) (B). These results indicated that BG extract recovered the ITR in GMD mice.
Regulation of BG extract-induced small intestinal 5-HT receptor expression
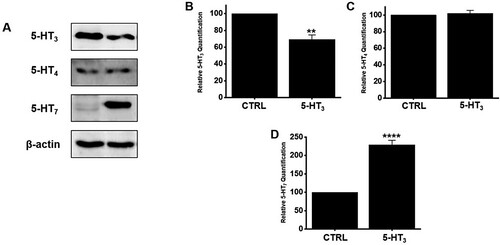
5-HT is mainly present in the GI tract, and an increase or decrease in the expression of 5-HT directly affects GI motility (Camilleri Citation2009). Moreover, 5-HT3, 4, and 7 receptors were found in ICC (Liu et al. Citation2011; Shahi et al. Citation2011). When BG extract was used, the expression of 5-HT3 receptors decreased significantly, the expression of 5-HT4 receptors did not change, and the expression of 5-HT7 receptors increased significantly (A). The expression of 5-HT3 receptors decreased by 69.3 ± 5.4% (n = 5; P < 0.01) whereas that of 5-HT7 receptors increased by 228.9 ± 12.3% (n = 7; P < 0.0001) after BG extract treatment (B and 6D). However, the expression of 5-HT4 receptors was unchanged (n = 6; C). These results suggested that the ITR increase by BG extract was done by an increase in the expression of the 5-HT7 receptors.
Figure 6. Effects of BG extract on the protein expression of 5-HT3, 4, and 7 receptors in mice. (A) 5-HT7 receptor expression increased considerably, but 5-HT4 receptors was unchanged. However, the expression of 5-HT3 receptors decreased. (B-D) Band density is showed relative to CTRL. Mean ± SEs. **P < 0.01. ****P < 0.0001. BG: Black garlic. CTRL: Control. β-Actin was the loading control.
DPPH radical scavenging activity of BG extract
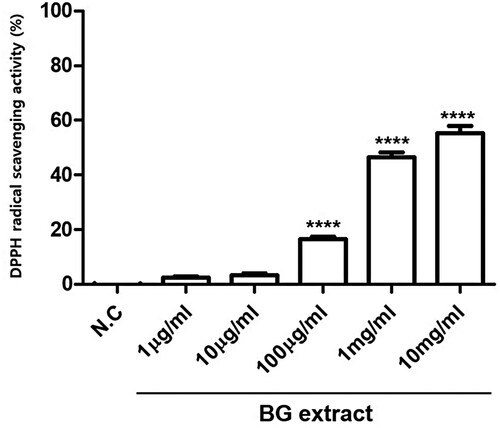
To investigate the antioxidant activity of BG extract, ROS scavenging activity was measured using the DPPH assay. BG extract significantly exhibited ROS scavenging activity (16.6 ± 4.6% (n = 7; P < 0.001) at 0.1 mg/mL, 46.4 ± 9.5% (n = 7; P < 0.001) at 1 mg/mL, and 55. 4 ± 11.4% (n = 7; P < 0.001) at 10 mg/mL; ). These results suggested that BG extract has significant antioxidative effects.
Figure 7. ROS scavenging activity was measured using the DPPH reagent. BG extract was treated with 0.001, 0.01, 0.1, 1, and 10 mg/mL DPPH reagent, and 100% EtOH was used as a negative control (N.C). The results are from three independent experiments. Mean ± SEs. ****P < 0.0001. BG: Black garlic. DPPH: 1,1-diphenyl-2-picrylhydrazyl.
Discussion
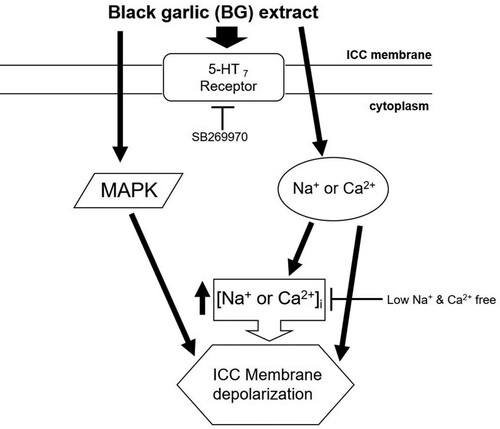
Garlic has been used as a medicinal ingredient for a long time (Jeong et al. Citation2012). BG is a processed food, where fresh garlic is fermented at high humidity and high temperatures for 60–90 d (Kim et al. Citation2012; Yang et al. Citation2019). Although BG has various activities, including influencing GI motility (Jeong et al. Citation2016; Chen et al. Citation2018), its effect on the regulation of ICC function has not been reported yet. The ingredients of BG extract may change for various reasons, such as the type of solvent used for extraction. However, in this experiment, instead of experimenting with extracts of several batches, the experiment was conducted with the extract of one batch. Although no analysis of the components of the BG extract was conducted, the results of previous studies showed that, as compared to regular garlic, BG contained large amounts of diallyl trisulfide and allyl methyl trisulfide and a small amount of epicatechin (Martínez-Casas et al. Citation2017). Furthermore, it has been reported that lactic acid is a major organic acid component of BG extract (Lu et al. Citation2017). Another study also showed that BG contains S-allyl-L-cysteine, S-allylmercaptocysteine, pyruvate, and amino acids (Kim et al. Citation2015). In this study, we checked the efficacy of BG extract but not the efficacy of the individual ingredients. We plan to conduct a study to reveal the effective ingredients of BG in the future. We found that BG extract modulated the ICC pacemaker potentials. BG extract depolarized the ICC pacemaker potentials (). External Na+ (5 mM) or Ca2+-free solution inhibited BG extract-induced pacemaker depolarization of ICC (). BG extract increased the ITR. It also recovered the loperamide-induced decrease in ITR in vivo (A). Moreover, BG extract recovered the ITR in AA-induced GMD in mice (B). Therefore, it is thought that BG may control GI motility through the adjustment of the pacemaker potential of the ICC.
ICC generate spontaneously active pacemaker potentials (Huizinga et al. Citation1995). 5-HT is secreted from enterochromaffin cells present mostly in the gut. Liu et al. (Citation2011) showed that the ICC pacemaker activity was controlled through 5-HT3 receptors, but Shahi et al. (Citation2011) suggested that it was controlled through 5-HT3, 4, and 7 receptors. In addition, Wouters et al. (Citation2007) stated that 5-HT2B receptors regulate the growth of ICC. However, in this study, the 5-HT7 receptor antagonist SB269970 inhibited BG extract-induced responses, whereas the 5-HT3 and 5-HT4 receptor antagonists Y25130 and RS39604, respectively, did not. This shows that BG extract modulates pacemaker potentials due to the 5-HT7 receptors (). In addition, BG extract-induced ITR increase was mediated by 5-HT7 receptors (). Therefore, we hypothesize that 5-HT7 receptors play a vital role in the regulation of GI motility by BG extract. 5-HT7 receptors are present in lymphoid tissues, smooth muscle cells, ICC, and neurons within the gut (Tonini et al. Citation2005; Kim and Khan Citation2014). Various studies have demonstrated the relevance of 5-HT7 receptors in GI motility regulation (Tonini et al. Citation2005). In the future, we will study the mechanisms and roles of 5-HT7 receptors in GI motility and ICC in detail. In addition, MAPK signaling is also a major target for new treatments with GI motility disease (Ihara et al. Citation2011). In this study, MAPK inhibitors suppressed the effects of BG extract. It was observed that p38, p42/44, and JNK signaling is involved in the BG extract-mediated control of pacemaker potentials.
The human body has a variety of complex antioxidant defense mechanisms to counter the harmful effects of free radicals or other oxidants (Alam et al. Citation2013). Antioxidants are known to be very effective in preventing degenerative diseases and improving the quality of life (Alam et al. Citation2013). Compared to garlic, BG has approximately 10-fold stronger superoxide dismutase-like activity and antioxidant effects against hydrogen peroxide (Sato et al. Citation2006). In this study, we showed that the potent antioxidative effects of BG by using the DPPH radical scavenging assay ().
Recently, natural herbal medicine has been attracting increasing attention as an alternative medicine with few side effects (Ekor Citation2014). Since many people have benefited from natural herbal medicine, we hope that research on the development of new treatments for GI diseases will be more active in the future.
Collectively, the results from the present study showed that BG extract depolarized the pacemaker potentials of the ICC via the 5-HT7 receptors, extracellular Na+ and Ca2+ concentration regulation, and MAPK pathways (). Furthermore, BG extract increased the ITR in normal and GMD model mice. BG extract-induced ITR increase was mediated through the 5-HT7 receptors. In addition, BG extract showed significant antioxidative effects. Therefore, BG might be a prokinetic agent that can cure or prevent GMD, and herbal medicine may become a very important strategy for the treatment of GI tract disorders.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alam MN, Bristi NJ, Rafiquzzaman M. 2013. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 21(2):143–152.
- Camilleri M. 2009. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 16(1):53–59.
- Chen YA, Tsai JC, Cheng KC, Liu KF, Chang CK, Hsieh CW. 2018. Extracts of black garlic exhibits gastrointestinal motility effect. Food Res Int. 107:102–109.
- Ekor M. 2014. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 4:177.
- Han DH, Kim JN, Kwon MJ, Han T, Kim YT, Kim BJ. 2021. Salvia miltiorrhiza induces depolarization of pacemaker potentials in murine small intestinal interstitial cells of Cajal via extracellular Ca 2+ and Na + influx. Mol Med Rep. 23(5):348.
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. 1995. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 373(6512):347–349.
- Hwang Minwoo, Kim Jeong Nam, Kim Byung Joo. 2020. Hesperidin depolarizes the pacemaker potentials through 5-HT 4 receptor in murine small intestinal interstitial cells of Cajal. Animal Cells and Systems. 24(2):84–90. https://doi.org/https://doi.org/10.1080/19768354.2020.1746398.
- Ihara E, Akiho H, Kazuhiko N, Turner SR, MacDonald JA. 2011. MAPKs represent novel therapeutic targets for gastrointestinal motility disorders. World J Gastrointest Pathophysiol. 2(2):19–25.
- Jeong YY, Park HJ, Cho YW, Kim EJ, Kim GT, Mun YJ, Lee JD, Shin JH, Sung NJ, Kang D, et al. 2012. Aged red garlic extract reduces cigarette smoke extract-induced cell death in human bronchial smooth muscle cells by increasing intracellular glutathione levels. Phytother Res. 26(1):18–25.
- Jeong YY, Ryu JH, Shin JH, Kang MJ, Kang JR, Han J, Kang D. 2016. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules. 21(4):430.
- Kim BJ, Kim HW, Lee GS, Choi S, Jun JY, So I, Kim SJ. 2013. Poncirus trifoliate fruit modulates pacemaker activity in interstitial cells of Cajal from the murine small intestine. J Ethnopharmacol. 149(3):668–675.
- Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. 2005. Melastatin-Type transient receptor potential channel 7 Is required for intestinal Pacemaking activity. Gastroenterology. 129(5):1504–1517.
- Kim D, Kim KH, Yook HS. 2015. Analysis of active components of Giant Black Garlic. J Korean Soc Food Sci Nutr. 44(11):1672–1681.
- Kim Dongwoo, Koun Soonil, Kim Seung Young, Ha Young Ran, Choe Jung Wan, Jung Sung Woo, Hyun Jong Jin, Jung Young Kul, Koo Ja Seol, Yim Hyung Joon, Lee Sang Woo. 2021. Prokinetic effects of diatrizoate meglumine (Gastrografin®) in a zebrafish for opioid-induced constipation model. Animal Cells and Systems. 25(5):264–271. https://doi.org/https://doi.org/10.1080/19768354.2021.1991472.
- Kim JH, Nam SH, Rico CW, Kang M. 2012. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int J Food Sci Technol. 47(6):1176–1182.
- Kim JJ, Khan WI. 2014. 5-HT7 receptor signaling: improved therapeutic strategy in gut disorders. Front Behav Neurosci. 8:396.
- Kimura S, Tung YC, Pan MH, Su NW, Lai YJ, Cheng KC. 2017. Black garlic: a critical review of its production, bioactivity, and application. J Food Drug Anal. 25(1):62–70.
- Kodera Y, Suzuki A, Imads O, Kasuga S, Sumioka I, Kanezawa A, Taru N, Fujikawa M, Nagae S, Masamoto K, et al. 2002. Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J Agric Food Chem. 50(3):622–632.
- Liu HN, Ohya S, Nishizawa Y, Sawamura K, Iino S, Syed MM, Goto K, Imaizumi Y, Nakayama S. 2011. Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS One. 6(9):e24928.
- Lu X, Li N, Qiao X, Qiu Z, Liu P. 2017. Composition analysis and antioxidant properties of black garlic extract. J Food Drug Anal. 25(2):340–349.
- Lyu JH, Lee HT. 2013. Effects of dried citrus unshiu peels on gastrointestinal motility in rodents. Arch Pharm Res. 36(5):641–648.
- Martínez-Casas L, Lage-Yusty M, López-Hernández J. 2017. Changes in the aromatic profile, sugars, and bioactive Compounds When purple garlic Is transformed into Black garlic. J Agric Food Chem. 65(49):10804–10811.
- Sato E, Kohno M, Hamano H, Niwano Y. 2006. Increased antioxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum Nutr. 61(4):157–160.
- Shahi PK, Choi S, Zuo DC, Yeum CH, Yoon PJ, Lee J, Kim YD, Park CG, Kim MY, Shin HR, et al. 2011. 5-hydroxytryptamine generates tonic inward currents on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 15(3):129–135.
- Tonini M, Vicini R, Cervio E, De Ponti F, De Giorgio R, Barbara G, Stanghellini V, Dellabianca A, Sternini C. 2005. 5-HT7 receptors modulate peristalsis and accommodation in the Guinea pig ileum. Gastroenterology. 129(5):1557–1566.
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. 2000. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 525 Pt 2(Pt 2):355–361.
- Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. 2007. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 133(3):897–906.
- Wu YS, Lu HL, Huang X, Liu DH, Meng XM, Guo X, Kim YC, Xu WX. 2013. Diabetes-induced loss of gastric ICC accompanied by up-regulation of natriuretic peptide signaling pathways in STZ-induced diabetic mice. Peptides. 40:104–111.
- Yang P, Song H, Wang L, Jing H. 2019. Characterization of Key Aroma-Active Compounds in Black Garlic by Sensory-Directed Flavor Analysis. J Agric Food Chem. 67(28):7926–7934.
- Yuan H, Sun L, Chen M, Wang J. 2016. The comparison of the contents of sugar, Amadori, and Heyns compounds in fresh and black garlic. J Food Sci. 81(7):C1662–C1668.