ABSTRACT
Prolonged exposure to high temperatures is linked to a range of physiological responses in broiler chickens including reduced disease resistance, low growth rate, and high mortality rate. In this study, we investigated the effect of heat stress on gene expression levels in 4-week-old and 6-week-old chickens each exposed to environments conditioned at thermoneutral (21 °C) and high (32 °C) temperatures. The analysis of differentially expressed genes (DEGs) using microarray revealed that genes underlying reactive oxygen species (ROS) production, cell nutrient intake, glucose metabolism, and circadian rhythm were differentially regulated in association with heat stress. We also found that the deviation in expression levels across the transcriptome in response to heat stress was significantly stronger (P< 2.2×10−16) in 6-week-olds compared to younger chickens. We finally observed a significant trend (r = 0.78, P< 2.2×10−16) that genes with a higher estimate of expression in the microarray were more likely to have a higher expression level in RNA-sequencing. Together, our findings provide comprehensive insights into the physiology involved in stress responses at varying developmental stages, which may facilitate chicken breeding to maximize their productivity under adverse conditions.
Introduction
Chicken (Gallus gallus domestica) provide an irreplaceable source of food, but a major constraint on their productivity is heat stress, which leads to stunted growth, increased rate of mortality, decreased reproduction, and reduced meat quality (Irshad et al. Citation2013). Especially, a rapid growth rate of broiler chickens causes more extensive and irreversible molecular and physiological changes under harsh environmental stressors including high and low-temperature extremes (Giloh et al. Citation2012; Nascimento et al. Citation2017).
Heat exposure alters the metabolic signatures and stimulates oxidative damage to broiler skeletal muscles in avian species by upregulating the levels of enzymes that govern β-oxidation, TCA cycle, and fatty acid transport (Mujahid et al. Citation2007). It is also known to increase the level of malondialdehyde (MDA) which is an indicator of lipid peroxidation (Wang et al. Citation2009). The cytotoxicity induced by heat, excessive inflammation, and inability to increase the expression of heat shock proteins (HSPs) altogether lead to the pathogenesis of heat injury and associated tissue damage (Rowell L Citation1983). The heat exposure also causes ferroptosis-like death in mammary gland tissues of goat and affects breastfeeding and productivity (Liu et al. Citation2021). To fully understand the molecular anatomy of heat-associated morbidity and mortality, several studies have attempted the analyses at the transcriptome-wide level (Jung et al. Citation2020). Srikanth et al. recently attempted to investigate transcriptomic responses in low and highland chickens experienced thermal challenges (Srikanth et al. Citation2019). Jastrebski et al. integrated transcriptome and metabolome data to examine the hepatic responses of chickens under chronic heat stress (Jastrebski et al. Citation2017). Nevertheless, it is still unclear how thermoregulatory mechanisms may vary across different developmental stages especially in a rapidly growing broiler chicken. Here, we generated the whole genome-wide expression levels in breast muscle of 4-week-old and 7-week-old broiler chickens exposed at the environments conditioned at moderate (21 °C) and high (32 °C) temperatures.
Materials and Methods
Ethics approval
The animal study was approved by the Animal Care and Use Committee of Gyeongsang National University (GAR-110818-X0035).
Heat stress challenge experiment
In summary, this study involved chickens from two developmental stages; 4 and 6-week-old broiler chickens (4W and 6W, respectively), each comprised a treatment group exposed to the environments conditioned at 32 °C (4WH and 6WH, n = 3 each) and a control group at 21 °C (4WC and 6WC, n = 3 each). The temperatures under thermoneutral and hyperthermic conditions were derived from previous studies (Lin et al. Citation2006; Yoon et al. Citation2014).
A total of 240 one-day-old broiler chicks were procured and raised under a light cycle of 23 h of light and 1 h of dark. Birds were provided ad libitum access to water and fed the same diet. The temperature was decreased by 1 ∼ 2°C every two days thereafter until the temperature reached 21 ± 1°C on the day 21 post-hatch. The 4-week (n = 120) and 6-week-old (n = 120) chicks were each further raised in two separate houses: thermoneutral (21 °C, control group) and heat-stressed (32 °C, treatment group) conditions. For the treatment group, the temperature was gradually increased by 2 °C per hour to reach 32 °C. After one day of high and moderate temperature exposure, three birds from each experimental group (4WC, 4WH, 6WC, and 6WH) were randomly selected and euthanized. A total of twelve breast muscle tissue samples were collected and stored at −80 °C for analysis. Microarray analysis was performed using Affymetrix GeneChip chicken genome arrays containing 38,535 probes (Affymetrix, Santa Clara, CA).
Analysis of differentially expressed genes
R (ver.4.1.0) packages (Team Citation2013) ‘limma’ and ‘affy’ were used to analyze the gene expression profile data by quantifying the microarray fluorescence intensities. The probes with fold change ≥1.5 and FDR < 0.05, were considered significant. After filtering the cases where multiple probes matched with one gene and one probe matched with multiple genes, the differentially expressed genes (DEG) were defined for each heat stress challenge experiment.
Gene set enrichment analysis
DAVID version 6.8 (http://david.abcc.ncifcrf.gov/) (Huang et al. Citation2009) tool was used to identify significant over-representation of genes with particular functional categories such as Gene Ontology (Ashburner et al. Citation2000) and KEGG Pathway (Kanehisa and Goto Citation2000). The P-value of 0.05 was used as the criterion for the statistical significance.
RNA-sequencing data and expression
The 17 RNA-sequencing (RNA-seq) data for the breast muscle and 86 RNA-seq data for various tissues of the chicken were obtained from NCBI (National Center for Biotechnology Information) (https://www.ncbi.nlm.nih.gov/) (Wheeler et al. Citation2007) (Supplementary Table S1).
The RNA-seq data was treated by Trimmomatic (ver. 0.39) (Bolger et al. Citation2014) to filter low-quality reads and then RSEM (ver. 1.3.1) (Li and Dewey Citation2011) software to quantify and estimate the expression level of each gene (transcript per million, TPM) with Galgal 4.85 used as the reference genome. The correlation coefficient was estimated between the microarray and the TPM of RNA-seq data The gene expression levels of both platforms were log-transformed before comparison.
Figure visualization and statistical analyses
Boxplots and heatmap plots were generated using ‘ggpubr,’ ‘gplot2,’ ‘gplot’ and 'wesanderson’ packages of R. We conducted a principal component analysis between each sample and created a plot using the R package ‘ggfortify’
Results & discussion
Experimental design and overall expression patterns
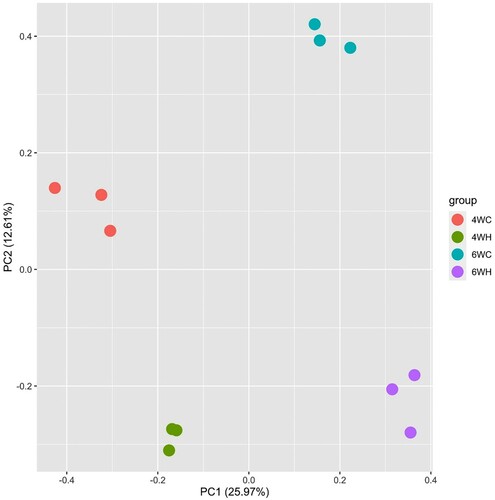
We generated the microarray-based transcriptome data of breast muscle from 4-week and 6-week-old chickens at moderate and high-temperature groups (4WH, 4WC, 6WH, and 6WC) (Materials and Methods). The 4W and 6W represent two broiler growth phases of different growth rates, and the bodyweight of the later period was significantly higher (Roush and Wideman Jr Citation2000; Mountzouris et al. Citation2010). We performed principal component analysis (PCA) of the genome-wide expression profiles (). The analysis ignored group membership but revealed clear experiment distinction as samples from the same experimental group clustered together. The first PC (25.97%) separated developmental stages (4W versus 6W) and PC2 (12.61%) further differentiated expression profiles in the treatment and control group (high and moderate temperatures).
Figure 1. Principal component analysis showing PC1 against PC2 of all twelve samples comprising 4 experimental groups: 4-week-old control (4WC, n = 3), 4-week-old high temperature (4WH, n = 3), 6-week-old control (6WC, n = 3), and 6-week-old high temperature (6WH, n = 3).
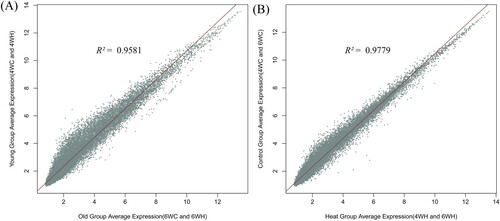
To further understand the similarity in genome-wide expression levels across experimental groups, we estimated the correlation coefficient of average expressions between different developmental stages (4WH and 4WC versus 6WH and 6WC, . A.) and temperature levels (4WH and 6WH versus 4WC and 6WC, . B.). The analysis suggested that the developmental stages caused slightly stronger fluctuations to the expression (R2 = 0.96) than did the heat stress (R2 = 0.98). The principal component and expression level correlation analyses together sufficiently indicated that the cellular and molecular response of breast muscle to developmental transition is more dynamic than to the hyperthermia condition.
Figure 2. Correlation of average expression between experimental groups (A) Average expression comparison of the 4-week-old group (4WC and 4WH combined) against the 6-week-old group (6WC and 6WH combined). (B) Average expression comparison of the thermoneutral control group (4WC and 6WC combined) against high-temperature group (4WH and 6WH combined).
Comparison with RNA-sequencing data
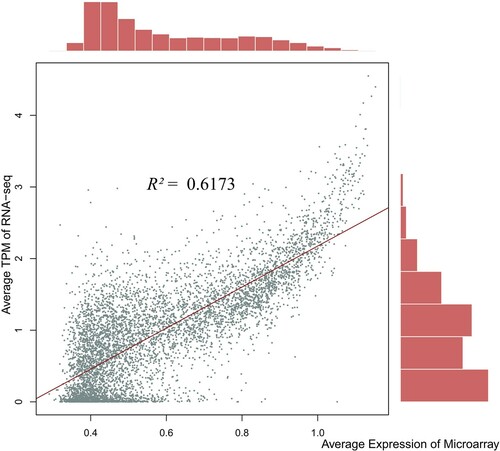
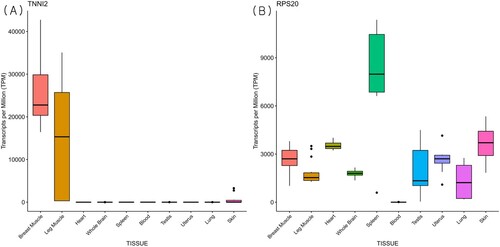
To increase the reliability of our microarray data, the 17 publicly available RNA-seq datasets of the same breast muscle tissue were collected. The comparison with the RNA sequences of the different samples and conditions was made to estimate the overall cross-platform concordance of the genome-wide expression levels. We observed that the results obtained by RNA-seq and microarrays were highly reproducible (R2 = 0.78). The result indicates that genes that show high expression from microarray, in general, tend to have high estimates in the common tissue of breast muscle (). For example, the ten genes with the highest expressions across four experimental groups from microarray showed notably high TPM (transcripts per million) estimates in breast muscle, which is even more prominent when compared to other tissues (, Supplementary Figure S1). The predated hybridization-based method such as microarray is criticized for detecting a limited range of expression and the lack of repeatability (Ioannidis et al. Citation2009; Byron et al. Citation2016). A high concordance estimate with a more recent platform implies the validity of quantitative measurement of expression in microarrays. It also indicates that specific treatment conditions may result in the differential expression on a subset of all available genes, however, in our study, the majority of expressions remained the same.
The landscape of differential expression
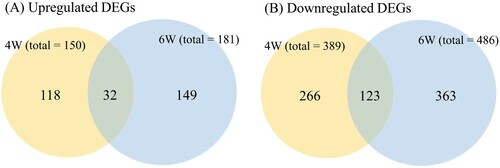
The transcriptome response of breast muscle was analyzed by measuring pairwise global gene expression changes between treatment and control groups at each developmental stage (4WH versus 4WC and 6WH versus 6WC). The ambient heat stress resulted in 539 and 667 DEGs in 4W and 6W respectively (, Supplementary Table S2). A total of 115 DEGs were commonly differentially expressed in two developmental stages. In both growth phases, we noted that the transcriptome tended to be inhibitory rather than enhanced in response to heat (389 downregulated versus 150 upregulated DEGs and 486 downregulated versus 181 upregulated DEGs in 4W and 6W respectively). It is worth noting that we observed a higher number of DEGs in the later developmental stage (6W). The result is also supported by the significantly stronger overall absolute fold changes across the transcriptome in 6W compared to 4W (P< 2.2×10−16; Mann–Whitney u test).
Significantly enriched pathways during hyperthermia
Analysis of Gene Ontology (GO) and KEGG pathway enrichment for DEGs of 4W indicated a significant overrepresentation for the categories associated with an immune response such as ‘antigen processing and presentation (GO: 0019882)’ and ‘antigen processing and presentation of peptide antigen via MHC class I (GO: 0002474)’ (, Supplementary Table S3). Cytokines and Toll-like receptors (TLRs) are essential markers for immunity, and TLRs especially have distinct roles of recognizing pathogen-associated molecular patterns and antigen presentation (Temperley et al. Citation2008). Myeloid cells are crucial effectors of the innate immune response and important regulators of adaptive immunity (Álvarez-Errico et al. Citation2015), and a corresponding biological process ‘negative regulation of myeloid cell differentiation (GO: 0045638)’ was significantly over-represented in 4W. The ubiquitination is a post-translational modification process ‘positive regulation of ubiquitin-protein transferase activity (GO: 0051443)’ that is crucial in the orchestration of an immune response by ensuring the proper functioning of key proteins within receptor signaling complexes that constitute the innate and adaptive immune systems (Pickart and Fushman Citation2004; Zinngrebe et al. Citation2014). During heat stress, the weight of lymphoid organs and thymus is known to decrease, which subsequently leads to a drastic dysfunction of T and B lymphocytes and ultimately reduces the antibody production (Zulkifli et al. Citation2000; Ghazi et al. Citation2012). These pathways together may explain the defense mechanisms, particularly the immune response under a high-temperature environment, which is governed by altered gene expression and regulation.
Table 1. Gene Set Enrichment Analysis of 4-week-chickens. The significant GO terms and KEGG pathways enriched from DEGs of the 4-week experiment. A list of genes in each term is provided in Supplementary Table S3.
Heat stress apparently causes damage to cells by generating reactive oxygen species (ROS) (Lin et al. Citation2006; Goel et al. Citation2021), and associated pathways were significantly enriched in 6W (, Supplementary Table S3), the ‘reactive oxygen species metabolic process (GO: 0072593).’ The excessive production of ROS causes oxidative injury in the mitochondria of muscle cells which in turn decreases body weight gain (Quinteiro-Filho et al. Citation2010). Of the DEGs from both 4W and 6W, PDK4 gene showed one of the highest fold changes, whose altered expression is responsible for abnormal mitochondria, ROS generation and cell death (Qian et al. Citation2004; Xu et al. Citation2018). As a defensive mechanism stimulated to protect the cell of damaged tissues and as an effort to restore the integrity of damaged tissues, genes associated with ‘positive response to wound healing (GO: 0090303)’ were differentially expressed under extremely high-temperature stress. One of the spontaneous responses to acute heat stress is increased expression of heat shock protein (HSP) genes that encode molecular chaperones associated with securing protein structures at high temperatures (Feder and Hofmann Citation1999), and although the corresponding biological processes were not significantly overrepresented, HSP25 and HSPB2 genes were downregulated in both growth phases. The concomitant decrease in expression may suggest an attenuation of the heat shock response from chronic heat stress.
Table 2. Gene Set Enrichment Analysis of 6-week-chickens. The significant GO terms and KEGG pathways enriched from DEGs of the 6-week experiment. A list of genes in each term is provided in Supplementary Table S3.
It is also noteworthy to mention that CCK gene was one of the most upregulated genes (P = 4.54E-13, fold change = 15.09, Supplementary Table S2) in the 4W experiment. Although the tissue-specific expression is generally expected, preserved co-expression relationships between different tissue types have been reported; for example, genes expressed in brain tissue leave a transcriptional footprint in peripheral tissues (Cai et al. Citation2010). CCK is known to respond to heat stress by sending a signal to reduce the feed intake to the hypothalamus, resulting in dietary impairment in chickens (Richards Citation2003; Lei et al. Citation2013). In addition, it has been previously demonstrated that acute heat stress shifts the circadian clock (Buhr et al. Citation2010), by synchronizing clock genes to the heat stress cycle to adapt to cyclic heat stress and modulate the cell cycle and metabolism changes. The differential expression of CCK gene and circadian rhythm-related pathways ‘entrainment of circadian clock by photoperiod (GO: 0043153)’ and ‘circadian regulation of gene expression (GO: 0032922)’ in breast muscle may therefore reflect the surrogate for gene expression in the hypothalamus in response to thermal stress. Although the study intends to find the differential gene expression in breast muscle, the apparent alteration of expression in the brain may correlate the pattern in breast muscle, supported by the findings of moderately or highly correlated expression of brain and non-brain tissues pattern between in humans and mice (Davies et al. Citation2009; Aguet and Muñoz Aguirre Citation2017).
During thermal stress, as a response that helps minimize the production of metabolic heat, feed intake decreases, which can influence nutrient availability (Habashy et al. Citation2017). A strong ambient temperature negatively affects cell nutrient intake, and pathways putatively involved in the absorption of macronutrients in the intestine (Goel et al. Citation2021), ‘insulin response (GO: 0032868),’ ‘insulin resistance (hsa04931),’ and ‘fatty acid metabolism (hsa00071)’ were significantly enriched in 6W. Insulin increases glucose uptake in the muscle and fat, serving as the primary regulator of blood glucose concentration (Saltiel and Kahn Citation2001), and therefore insulin signaling and the regulation of glucose may be the key to nutrient uptake during heat stress and severe nutrient deficiency.
Conclusion
Cells subjected to thermal stress respond by rewiring the transcriptome, including the differential expression of genes that govern the shift of rapid cell proliferation, growth processes, and metabolic adaptation (López-Maury et al. Citation2008). The significantly associated pathways in each 4W and 6W suggest that the transcriptomic responses of the rapidly growing broiler chickens to heat indicated differential cellular stress responses at different developmental stages. Changes in the transcriptome in 4W mainly indicated immune function, whereas chickens with higher body weight in the later stage (6W) responded to heat by altering the expression of genes encoding proteins that function in more diverse stress-associated pathways, including ROS clearance, cell nutrient intake, and shift in circadian rhythm. Our study is unique in assessing the transcriptional activity upon heat treatment at two contrasting growth phases in broiler chicken. The disproportionate ranges of enriched pathways at different ages may indicate that as the genome-wide expressions undergo precise and coordinated changes at defined stages of development, the transitions contribute to age-specific transcriptomic alteration in response to heat stress. These findings reveal that the chicken transcriptional response to heat is prompt, dynamic, and extensive, and may underlie the health outcomes observed during the thermal challenge.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aguet F, Muñoz Aguirre M. 2017. Genetic effects on gene expression across human tissues. Nat. 550:204–213.
- Álvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. 2015. Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol 15(1):7–17.
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. 2000. Gene ontology: tool for the unification of biology. Nat. Genet. 25(1):25–29.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Buhr ED, Yoo S-H, Takahashi JS. 2010. Temperature as a universal resetting cue for mammalian circadian oscillators. Sci. 330(6002):379–385.
- Byron SA, Van Keuren-Jensen KR, Engelthaler DM, Carpten JD, Craig DW. 2016. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat. Rev. Genet. 17(5):257–271.
- Cai C, Langfelder P, Fuller TF, Oldham MC, Luo R, van den Berg LH, Ophoff RA, Horvath S. 2010. Adaptation of hansenula polymorpha to methanol: a transcriptome analysis BMC Genomics. 11(1):1–15.
- Davies MN, Lawn S, Whatley S, Fernandes C, Williams RW, Schalkwyk LC. 2009. To what extent is blood a reasonable surrogate for brain in gene expression studies: estimation from mouse hippocampus and spleen. Front Neurosci. 3:2.
- Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61(1):243–282.
- Ghazi S, Habibian M, Moeini M, Abdolmohammadi A. 2012. Effects of different levels of organic and inorganic chromium on growth performance and immunocompetence of broilers under heat stress. Biol. Trace Elem. Res. 146(3):309–317.
- Giloh M, Shinder D, Yahav S. 2012. Skin surface temperature of broiler chickens is correlated to body core temperature and is indicative of their thermoregulatory status. Poult. Sci. 91(1):175–188.
- Goel A, Ncho CM, Choi Y-H. 2021. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 12(1):1–13.
- Habashy WS, Milfort MC, Fuller AL, Attia YA, Rekaya R, Aggrey SE. 2017. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 61(12):2111–2118.
- Huang DW, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4(1):44–57.
- Ioannidis JP, Allison DB, Ball CA, Coulibaly I, Cui X, Culhane AC, Falchi M, Furlanello C, Game L, Jurman G. 2009. Repeatability of published microarray gene expression analyses. Nat. Genet. 41(2):149–155.
- Irshad A, Kandeepan G, Kumar S, Ashish K, Vishnuraj M, Shukla V. 2013. Factors influencing carcass composition of livestock: A review. J. Anim. Prod. Adv. 3(5):177–186.
- Jastrebski SF, Lamont SJ, Schmidt CJ. 2017. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLOS One. 12(7):e0181900.
- Jung GT, Kim K-P, Kim K. 2020. How to interpret and integrate multi-omics data at systems level. Anim. Cells Syst. 24(1):1–7.
- Kanehisa M, Goto S. 2000. Kegg: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1):27–30.
- Lei L, Hepeng L, Xianlei L, Hongchao J, Hai L, Sheikhahmadi A, Yufeng W, Zhigang S. 2013. Effects of acute heat stress on gene expression of brain–gut neuropeptides in broiler chickens (gallus gallus domesticus)1. J. Anim. Sci. 91(11):5194–5201.
- Li B, Dewey CN. 2011. MTML-msBayes: Approximate Bayesian comparative phylogeographic inference from multiple taxa and multiple loci with rate heterogeneity. BMC Bioinform. 12(1):1–16.
- Lin H, Decuypere E, Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. COMP BIOCHEM PHYS A. 144(1):11–17.
- Liu L, Wang M, Gong N, Tian P, Deng H. 2021. Se improves GPX4 expression and SOD activity to alleviate heat-stress-induced ferroptosis-like death in goat mammary epithelial cells. Anim. Cells Syst. 25(5):283–295.
- López-Maury L, Marguerat S, Bähler J. 2008. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat. Rev. Genet. 9(8):583–593.
- Mountzouris K, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K. 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 89(1):58–67.
- Mujahid A, Akiba Y, Warden CH, Toyomizu M. 2007. Sequential changes in superoxide production, anion carriers and substrate oxidation in skeletal muscle mitochondria of heat-stressed chickens. FEBS Lett. 581(18):3461–3467.
- Nascimento ST, Maia AS, Gebremedhin KG, Nascimento CC. 2017. Metabolic heat production and evaporation of poultry. Poult. Sci. 96(8):2691–2698.
- Pickart CM, Fushman D. 2004. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8(6):610–616.
- Qian L, Song X, Ren H, Gong J, Cheng S. 2004. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 9(3):281.
- Quinteiro-Filho W, Ribeiro A, Ferraz-de-Paula V, Pinheiro M, Sakai M, Sá L, Ferreira A, Palermo-Neto J. 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 89(9):1905–1914.
- Richards M. 2003. Genetic regulation of feed intake and energy balance in poultry. Poult. Sci. 82(6):907–916.
- Roush W, Wideman Jr R. 2000. Evaluation of broiler growth velocity and acceleration in relation to pulmonary hypertension syndrome. Poult. Sci. 79(2):180–191.
- Rowell L LBR. 1983. Cardiovascular aspects of human thermoregulation.
- Saltiel AR, Kahn CR. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nat. 414(6865):799–806.
- Srikanth K, Kumar H, Park W, Byun M, Lim D, Kemp S, Te Pas MF, Kim J-M, Park J-E. 2019. Cardiac and skeletal muscle transcriptome response to heat stress in Kenyan chicken ecotypes adapted to low and high altitudes reveal differences in thermal tolerance and stress response. Front Genet. 10:993.
- Team RC.. 2013. R: A language and environment for statistical computing.
- Temperley ND, Berlin S, Paton IR, Griffin DK, Burt DW. 2008. Evolution of the chicken toll-like receptor gene family: a story of gene gain and gene loss. BMC Genom. 9(1):1–12.
- Wang R, Pan X, Peng Z. 2009. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 88(5):1078–1084.
- Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S. 2007. Database resources of the national center for biotechnology information. Nucleic Acids Res. 36(suppl_1):D13–D21.
- Xu W, Li L, Sun J, Zhu S, Yan Z, Gao L, Gao C, Cui Y, Mao C. 2018. Putrescine delays postovulatory aging of mouse oocytes by upregulating PDK4 expression and improving mitochondrial activity. Aging. 10(12):4093.
- Yoon H, Hwangbo J, Yang Y-R, Kim J, Kim Y-H, Park B, Choi Y-H. 2014. Effects of early heat conditioning on performance in broilers exposed to heat stress. Korean J. Poult. Sci. 41(4):297–303.
- Zinngrebe J, Montinaro A, Peltzer N, Walczak H. 2014. Ubiquitin in the immune system. EMBO Rep. 15(1):28–45.
- Zulkifli I, Abdullah N, Azrin NM, Ho Y. 2000. Growth performance and immune response of two commercial broiler strains fed diets containing lactobacillus cultures and oxytetracycline under heat stress conditions. Br Poult Sci. 41(5):593–597.