ABSTRACT
Clinical observations have revealed that non-resolving low-grade inflammation is linked to the pathogenesis of chronic inflammatory diseases, for example arthritis, atherosclerosis, Alzheimer’s disease, diabetes, and chronic kidney disease. Interestingly, low levels of circulating lipopolysaccharides (LPS) derived from the outer membrane of gram-negative bacteria appear to be one of the primary causes of persistent low-grade inflammation. The inner surface of the blood vessels is lined with endothelial cells; therefore, even low levels of circulating LPS can directly activate these cells and elicit specific cellular responses, such as an increase in the expression levels of cell adhesion molecules and proinflammatory mediators. In endothelial cells, LPS exposure results in an inflammatory response through activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases. Cynarin, a phytochemical found in artichokes, has several pharmacological properties against endothelial inflammation. In the present study, we discovered that cynarin suppressed the LPS-induced increase in the expression levels of vascular cell adhesion molecule-1 and proinflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and interleukin-1β in EA.hy926 cells. Further, cynarin inhibited the activation of p38 and NF-κB pathways by inducing the negative regulator mitogen-activated protein kinase phosphatase 3 (MKP-3) in LPS-stimulated EA.hy926 cells. In conclusion, cynarin alleviates inflammation by upregulating MKP-3, a negative regulator of p38 and NF-κB, and it may be a therapeutic option for treating endothelial inflammation-related diseases.
Introduction
The term ‘low-grade endotoxemia’ was recently coined to describe subclinically elevated circulating blood endotoxin levels (Terawaki et al. Citation2010). It is closely correlated with chronic inflammatory diseases such as type 2 diabetes, chronic kidney disease, non-alcoholic fatty liver disease, and cardiovascular disease (Minihane et al. Citation2015). In addition to localized or persistent infections, leaky intestinal barriers are potential sources of blood endotoxins. Low-dose endotoxemia is more likely to develop in people with unhealthy lifestyle choices, such as smoking (Glaros et al. Citation2007), drinking (Rao Citation2009), high-fat diets (Mohammad and Thiemermann Citation2020), as well as in people with HIV infection (Palmer et al. Citation2016) or liver cirrhosis (Raparelli et al. Citation2017); ageing is also a predictor for low-grade endotoxemia (Goto et al. Citation1994; Jin et al. Citation2020).
Endothelial cells form the interior surface of blood vessels (Dauphinee and Karsan Citation2006). Gram-negative bacterial endotoxins such as lipopolysaccharides (LPS) cause inflammation in endothelial cells by directly stimulating the vascular endothelium and inducing several cellular responses, such as elevating the expression levels of cell adhesion molecules and proinflammatory cytokine/chemokines (Bierhaus et al. Citation2000). Such endotoxins also increase endothelial permeability and recruit monocytes and macrophages, enhancing inflammation (Cohen Citation2002).
Mitogen-activated protein kinases (MAPKs) are a family of serine/threonine kinases that react to a variety of stimuli, including mitogens, proinflammatory cytokines, and environmental stress (Kyriakis and Avruch Citation2001). While extracellular signal-related kinases (Erk) and big MAPK-1 are predominantly involved in growth and cytoprotection, Jun amino-terminal kinases (JNK) and p38 proteins play critical roles in the inflammatory and stress response (Hoefen and Berk Citation2002). In addition to MAPK activation, the nuclear factor-kappa B (NF-κB) pathway is vital for endothelial inflammation (Oates Citation2015). Cytoplasmic NF-κB activation is controlled by inhibitors of kappa B (IκB). Extracellular proinflammatory stimuli cause IκB to be phosphorylated and degraded; as a result, free NF-κB is translocated to the nucleus, stimulating the expression of downstream genes (Karin and Ben-Neriah Citation2000). Importantly, we recently discovered that endotoxin-induced activation of p38 and NF-B, both of which play important roles in endothelial inflammation, is suppressed by MKP-3 (Unenkhuu et al. Citation2021).
Cynarin, also known as 1,3-O-dicaffeoylquinic acid, is a phytochemical found in artichokes, which has antioxidant (Topal et al. Citation2016), antihypertensive (Hakkou et al. Citation2017), anti-atherosclerotic (Li et al. Citation2004), anti-HIV (McDougall et al. Citation1998), anti-carcinogenic (Zhou et al. Citation2020), and cholesterol-lowering properties (Chen et al. Citation2018). Despite its anti-inflammatory effects, little is known about how cynarin affects the inflammatory responses in endotoxin-stimulated endothelial cells.
Therefore, in this study, we explored the effects of cynarin on the inflammatory response in EA.hy926 human endothelial cells treated with LPS. Furthermore, we examined the role of the p38/NF-κB pathway and cynarin-regulated MKP-3 expression in endothelial cells. This study sheds light on the molecular processes underlying the anti-inflammatory properties of cynarin, which will aid the development of drugs to prevent and treat low-grade endotoxemia-induced endothelial inflammation.
Materials and methods
Reagents
LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA). Cynarin was obtained from MedChemExpress (Monmouth Junction, NJ, USA).
Cell culture
Human endothelial cells (EA.hy926; ATCC CRL-2922™) were cultured in DMEM (HyClone Laboratories, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories) and 1% penicillin/streptomycin (WELGENE, Gyeongsan, Republic of Korea) at 37 °C in humidified 5% CO2 atmosphere.
Cell viability assay
EA.hy926 cells were seeded at a density of 1 × 104 cells/well in 96-well plates. A Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville, MD, USA) assay was used to assess cell viability 24 h after incubation with cynarin at various concentrations (0–10 μM), following the manufacturer’s instructions. The relative viability of cynarin-treated cells was measured at 450 nm using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific).
Western blotting
Western blotting was performed as previously described (Unenkhuu et al. Citation2021). The primary antibodies used in the experiments were as follows: vascular cell adhesion molecule-1 (VCAM-1; diluted at 1:1,000, Cell Signaling Technology, Danvers, MA, USA), phospho-NF-κB p65 (serine 536 [Ser536], diluted at 1:1,000, Cell Signaling Technology), NF-κB p65 (diluted at 1:1,000, Cell Signaling Technology), phospho-p38 MAPK (diluted at 1:1,000, Cell Signaling Technology), p38 MAPK (diluted at 1:1,000, Cell Signaling Technology), β-actin (diluted 1:2,000, Santa Cruz Biotechnology, Dallas, TX, USA), and MKP-3 (diluted 1:1,000, Abcam, Cambridge, UK). The HRP-conjugated secondary antibodies (1:5,000 dilution) obtained from Santa Cruz Biotechnology were used for 1 h at room temperature. Signals were detected via enhanced chemiluminescence using a ChemiDoc imaging system (Bio-Rad, Hercules, CA, USA).
Rna isolation, reverse transcription, and real-time qPCR
Total RNA extraction from EA.hy926 cells was performed using an AccuPrep Universal RNA Extraction Kit (Bioneer, Daejeon, Republic of Korea) according to the manufacturer’s instructions. AccuPower CycleScript RT PreMix (Bioneer) was used to conduct reverse transcription after RNA isolation and quantitation. Gene expression at the mRNA level was determined via real-time quantitative polymerase chain reaction (RT-qPCR) using an AccuPower 2X GreenStar qPCR Master Mix (Bioneer) and a CFX Connect Real-Time PCR detection system (Bio-Rad). The expression of 18S rRNA was used as an internal control. Primer sequences used for RT-qPCR are listed in .
Table 1. Primer sequences for real-time quantitative PCR.
Immunofluorescence staining
After treatment with cynarin and LPS, the EA.hy926 cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min, permeabilized with 0.3% Triton X-100, and blocked with 2% bovine serum albumin in PBS for 1 h. Following incubation with rabbit anti-NF-κB p65 at 4 °C overnight, the cells were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific) for 1 h at room temperature. Nuclei were stained with Hoechst 33258. Images were acquired using a fluorescence microscope (EVOS FL Cell Imaging System).
Statistics
All data are presented as the mean value and standard error of the mean (SEM) of n measurements. Statistical comparisons of the results were performed using one-way analysis of variance. Differences were considered statistically significant when the p-value was less than 0.05.
Results
Cynarin decreases the expression levels of VCAM-1 and inflammatory cytokines in LPS-stimulated endothelial cells
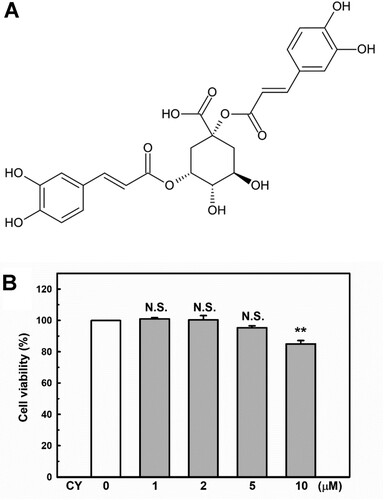
The chemical structure of cynarin is shown in A. The CCK-8 assay was conducted to explore the cytotoxicity of cynarin on EA.hy926 cells. As shown in B, cynarin is safe for use in EA.hy926 cells at doses of 1–5 μM.
Figure 1. Chemical structure and cytotoxicity induced by cynarin. (A) Chemical structure of cynarin. (B) Cell viability of EA.hy926 cells after treatment with different cynarin concentrations as determined using the cell counting kit-8 assay. The results are presented as the mean ± standard error of the mean (SEM) of three independent experiments. **P < 0.01, compared to the vehicle control group.
Circulating monocytes are the first cells to elicit a proinflammatory response during endotoxemia. These cells respond to extremely low endotoxin (pg/mL) levels (Stoll et al. Citation2004), while VCAM-1 expression initiates events that lead to monocyte accumulation on the vessel wall (Li et al. Citation1993). Therefore, the effect of cynarin on VCAM-1 protein and mRNA expression levels in LPS-stimulated EA.hy926 cells was investigated. As shown in A + B, LPS exposure significantly increased VCAM-1 expression levels, which were inhibited by cynarin in a dose-dependent manner. To determine whether cynarin could inhibit the expression of proinflammatory cytokines, including monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor (TNF)-α, and interleukin (IL)−1β, the mRNA levels of proinflammatory cytokines in LPS-treated EA.hy926 cells were examined. Compared with LPS-only treatment, cynarin pretreatment significantly decreased the transcription levels of MCP-1, TNF-α, and IL-1β (C–E).
Figure 2. Effects of cynarin on lipopolysaccharide (LPS)-induced proinflammatory molecule expression in EA.hy926 cells. EA.hy926 cells were treated with cynarin for 4 h before adding LPS (1 μg/mL) for another 24 h. (A + B) VCAM-1 expression level was measured via western blotting. β-actin was considered as a loading control. Data are presented as the mean ± SEM (n = 3). **P < 0.01, compared to the LPS-only group. (C–E) The mRNA expression levels of inflammatory genes [monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor (TNF-α), and interleukin (IL)−1β] as detected via real-time PCR. 18S rRNA was used as an internal control. Data are presented as the mean ± SEM (n = 3). Asterisks indicate significant differences (P < 0.01).
![Figure 2. Effects of cynarin on lipopolysaccharide (LPS)-induced proinflammatory molecule expression in EA.hy926 cells. EA.hy926 cells were treated with cynarin for 4 h before adding LPS (1 μg/mL) for another 24 h. (A + B) VCAM-1 expression level was measured via western blotting. β-actin was considered as a loading control. Data are presented as the mean ± SEM (n = 3). **P < 0.01, compared to the LPS-only group. (C–E) The mRNA expression levels of inflammatory genes [monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor (TNF-α), and interleukin (IL)−1β] as detected via real-time PCR. 18S rRNA was used as an internal control. Data are presented as the mean ± SEM (n = 3). Asterisks indicate significant differences (P < 0.01).](/cms/asset/e4d44a64-d5d5-45c5-8313-74f92f5018f8/tacs_a_2077438_f0002_ob.jpg)
Cynarin inhibits the LPS-induced activation of p38 MAPK and NF-κB in LPS-stimulated endothelial cells
MAPKs are essential regulators of endothelial inflammation (Hoefen and Berk Citation2002). The stimulation of p38 appears to be particularly important (Schumann et al. Citation1996; Tamura et al. Citation1998). Additionally, p38 controls the expression of VCAM-1 (Pietersma et al. Citation1997) and MCP-1 (Goebeler et al. Citation1999) after cytokine activation. Therefore, we examined whether cynarin inhibited p38 phosphorylation in LPS-treated EA.hy926 cells. As shown in A, p38 phosphorylation was significantly increased in LPS-treated cells; however, cynarin pretreatment (1–5 µM) inhibited this effect.
Figure 3. Effects of cynarin on LPS-stimulated phosphorylation of p38 and NF-κB p65 in EA.hy926 cells. (A + B) The cells were treated with cynarin for 1 h before adding LPS (1 μg/mL) for another 30 min. Relative protein expressions were analyzed via western blotting. The data show the mean ± SEM of three independent experiments. *P < 0.05 and **P < 0.01, compared to the vehicle control group. ##P < 0.01, compared to the LPS-only group.
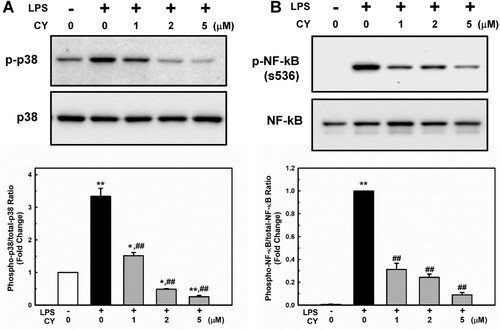
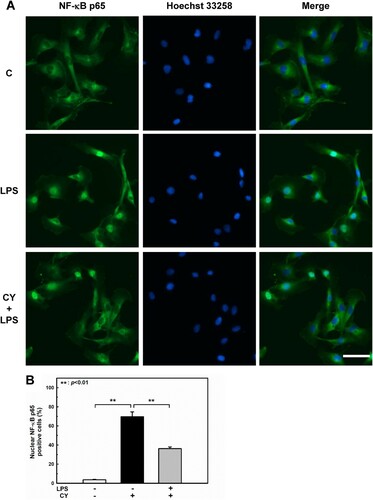
Activation of NF-κB also regulates gene expression during endothelial activation (Chen et al. Citation2017). The phosphorylation of NF-κB at Ser536 promotes its nuclear translocation (Sakurai et al. Citation1999) and facilitates binding to specific promoter sequences (Buss et al. Citation2012). The influence of cynarin on LPS-induced NF-κB activation in EA.hy926 cells was studied. In LPS-treated cells, phosphorylation of NF-κB p65 was significantly increased; however, pretreatment with cynarin suppressed this effect (A). Inhibition of LPS-induced NF-κB p65 activation by cynarin was consistent with its nuclear translocation (). These findings suggest that cynarin suppresses the LPS-induced activation of p38 and NF-κB.
Figure 4. Effects of cynarin on nuclear translocation of NF-κB p65 in LPS-stimulated EA.hy926 cells. The cells were treated with cynarin (5 µM) for 1 h before adding LPS (1 μg/mL) for another 30 min. (A) Representative immunofluorescence staining images showing NF-κB p65 (green) and the nucleus (blue). Scale bars: 50 µm. (B) Quantitation of NF-κB p65 nuclear translocation in the indicated groups. Results are shown as the mean ± SEM (n = 3). Asterisks indicate significant differences (P < 0.01).
Cynarin inhibits the LPS-induced activation of p38 MAPK and NF-κB in LPS-stimulated endothelial cells
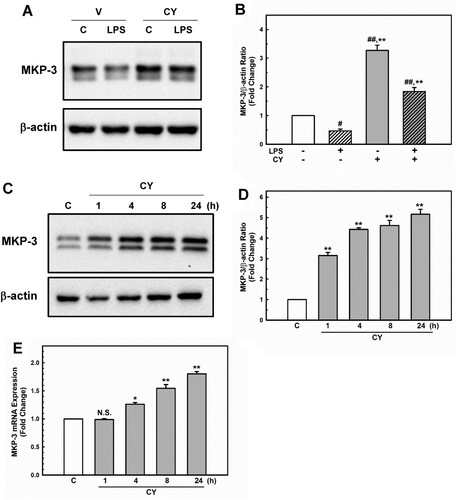
MKP-3, a member of the MAPK phosphatase family, has been shown to attenuate endothelial inflammatory responses via MAPK dephosphorylation and inactivation (Whetzel et al. Citation2009; Leng et al. Citation2018). Recently, we showed that MKP-3 inhibits the p38/NF-κB pathway, which protects against LPS-induced endothelial inflammation (Unenkhuu et al. Citation2021). Cynarin (Puangpraphant et al. Citation2011; Liang and Kitts Citation2018) and MKP-3 (Unenkhuu et al. Citation2021) have been shown to inhibit p38/NF-κB activation. Therefore, we aimed to determine whether cynarin upregulates MKP-3 expression. Interestingly, EA.hy926 cells pre-incubated with cynarin exhibited a higher level of MKP-3 than the control cells (A + B, cynarin only). Moreover, cynarin markedly increased MKP-3 protein (C + D) and mRNA (E) levels in a time-dependent manner. These findings implied that cynarin positively controls MKP-3 expression, leading to reduced p38 and NF-κB activation.
Figure 5. Effects of cynarin on MKP-3 expression in EA.hy926 cells. (A + B) The cells were treated with cynarin (5 µM) for 1 h before adding LPS (1 μg/mL) for another 30 min. MKP-3 expression was measured via western blotting analysis. β-actin was considered as an internal control. Data are presented as the mean ± SEM (n = 3). **P < 0.01, compared to the LPS-only group. #P < 0.05 and ##P < 0.01, compared to the vehicle control group. The cells were treated with cynarin (5 µM) for 1, 4, 8, or 24 h. Protein expression of MKP-3 was determined using western blotting analysis (C + D). MKP-3 mRNA expression was analyzed using real-time PCR (E). The data show the mean ± SEM of three independent experiments. *P < 0.05 and **P < 0.01, compared to the vehicle control group.
Discussion
Low levels of endotoxins induce a mild but persistent induction of proinflammatory mediators, in contrast to high levels of endotoxins, which can elicit a robust and transient expression of proinflammatory mediators (Glaros et al. Citation2013; Lee et al. Citation2021). Even after a single meal, a high-fat diet increases LPS leakage from the gut, and individuals with diabetes have been observed to have higher plasma LPS levels (Harte et al. Citation2012; Gomes et al. Citation2017). Endothelial cells are the first cells that respond to endotoxemia because they form an interface between the blood flow and the vessel wall (Dauphinee and Karsan Citation2006). Persistent low-grade endotoxemia has been linked to the development of chronic vascular inflammatory diseases such as atherosclerosis (Wiedermann et al. Citation1999) and dysfunctional microcirculation (Czabanka et al. Citation2007). LPS has been hypothesized to play a role in cardiovascular diseases by promoting atherosclerotic plaque formation upon the activation of proinflammatory pathways (Lehr et al. Citation2001; Vink et al. Citation2002; Kallio et al. Citation2008). Additionally, even in low-risk individuals who do not have traditional vascular risk factors, prolonged infections caused by gram-negative bacteria increase the risk of developing atherosclerosis (Wiedermann et al. Citation1999).
Cynarin is a biologically active functional food component and is the major dicaffeoylquinic acid derivative found in artichokes. It possesses various pharmacological properties, such as free radical scavenging and antioxidant activity (Jimenez-Escrig et al. Citation2003). In endothelial cells, artichoke extracts reduced oxidative stress induced by inflammatory mediators and oxidized low-density lipoprotein (Zapolska-Downar et al. Citation2002) and enhanced endothelial nitric oxide synthase (eNOS) expression and NO generation (Li et al. Citation2004). However, they failed to induce eNOS expression (Li et al. Citation2004). Xia et al. reported that cynarin exerts an inhibitory effect on inducible NOS (iNOS) expression induced by a cytokine mixture containing interferon (IFN)- γ, IL-1β, and TNF-α in human coronary artery smooth muscle cells (Xia et al. Citation2014). However, the mechanism by which cynarin inhibits iNOS expression has not been elucidated. Therefore, this study was designed to investigate the anti-inflammatory effects of cynarin in LPS-stimulated endothelial cells.
In the present study, cynarin exhibited anti-inflammatory activity by decreasing VCAM-1 expression level, mediating monocyte recruitment (Gerszten et al. Citation1998), and lowering proinflammatory cytokine levels. Additionally, cynarin pretreatment markedly abolished p38 activation, which is necessary for VCAM-1 (Pietersma et al. Citation1997) and MCP-1 (Goebeler et al. Citation1999) induction in LPS-stimulated endothelial cells. Moreover, cynarin significantly inhibited phosphorylation at Ser536 of NF-κB and the nuclear translocation of NF-κB p65, which is another critical regulator of LPS-induced inflammatory responses. Phosphorylation at Ser536 in the transactivation domain of NF-κB p65 by multiple kinases, including p38 (Rahman et al. Citation2004; Schmeck et al. Citation2004), promotes its nuclear translocation (Sakurai et al. Citation1999) and facilitates inflammatory gene expression (Buss et al. Citation2012). Artichoke extracts inhibited the activity of NF-κB in acute alcohol-induced liver injury (Tang et al. Citation2017) and LPS-stimulated THP-1 human leukemic cells (Milackova et al. Citation2017); therefore, our current results might be in accordance with these previous findings.
MKP-3 inhibits endothelial inflammation (Whetzel et al. Citation2009; Leng et al. Citation2018; Unenkhuu et al. Citation2021); therefore, we examined whether cynarin upregulated MKP-3 expression. As expected, cynarin markedly increased MKP-3 expression level. Interestingly, the decrease in MKP-3 protein levels after LPS treatment was completely recovered by cynarin pretreatment (A + B, LPS vs. CY + LPS). This might be due to the antioxidant activity of cynarin, because LPS induces oxidative stress (Zhang et al. Citation2017) and subsequently induces MKP-3 degradation (Chan et al. Citation2008). P53 may also mediate MKP-3 induction by cynarin because cynarin-containing artichoke extracts activate p53 (Simsek and Uysal Citation2013), which has been identified as a transcriptional factor of MKP-3 (Piya et al. Citation2012). Although endothelial cells also express MKP-1, which regulates the inflammatory response (Furst et al. Citation2007), it did not affect MKP-1 levels (data not shown), suggesting that it acts through MKP-3 to inhibit LPS-induced inflammation in endothelial cells.
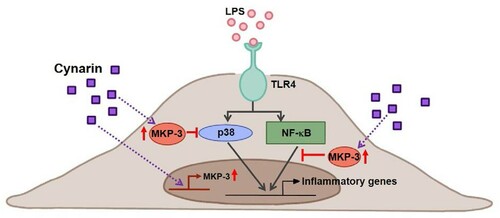
Overall, the data from the present study suggest that cynarin inhibits endothelial inflammation by inhibiting the p38 and NF-κB pathways by inducing the expression of the negative regulator MKP-3 (). Hence, cynarin may be a promising treatment option for various disorders related to endothelial inflammation.
Acknowledgments
This study was supported by a research grant from Inha University (INHA-66268) awarded to Hong Seok Kim. The authors would like to thank Siyoon Kim, Cheongna Dalton School, Incheon 22742, Republic of Korea, for her assistance with the graphics shown in .
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bierhaus A, Chen J, Liliensiek B, Nawroth PP. 2000. LPS and cytokine-activated endothelium. Semin Thromb Hemost. 26(5):571–588.
- Buss H, Handschick K, Jurrmann N, Pekkonen P, Beuerlein K, Muller H, Wait R, Saklatvala J, Ojala PM, Schmitz ML, et al. 2012. Cyclin-dependent kinase 6 phosphorylates NF-κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS One. 7(12):e51847.
- Chan DW, Liu VW, Tsao GS, Yao KM, Furukawa T, Chan KK, Ngan HY. 2008. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis. 29(9):1742–1750.
- Chen X, Xiu M, Xing J, Yu S, Min D, Guo F. 2017. Lanthanum chloride inhibits LPS mediated expressions of pro-inflammatory cytokines and adhesion molecules in HUVECs: Involvement of NF-κB-Jmjd3 signaling. Cell Physiol Biochem. 42(5):1713–1724.
- Chen Y, Chen X, Luo G, Zhang X, Lu F, Qiao L, He W, Li G, Zhang Y. 2018. Discovery of potential inhibitors of squalene synthase from traditional Chinese medicine based on virtual screening and in vitro evaluation of lipid-lowering effect. Molecules. 23(5):1040.
- Cohen J. 2002. The immunopathogenesis of sepsis. Nature. 420(6917):885–891.
- Czabanka M, Peter C, Martin E, Walther A. 2007. Microcirculatory endothelial dysfunction during endotoxemia - insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol. 5(4):266–275.
- Dauphinee SM, Karsan A. 2006. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 86(1):9–22.
- Furst R, Schroeder T, Eilken HM, Bubik MF, Kiemer AK, Zahler S, Vollmar AM. 2007. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB J. 21(1):74–80.
- Gerszten RE, Lim YC, Ding HT, Snapp K, Kansas G, Dichek DA, Cabanas C, Sanchez-Madrid F, Gimbrone MA, Rosenzweig A, et al. 1998. Adhesion of monocytes to vascular cell adhesion molecule-1–transduced human endothelial cells. Circ Res. 82(8):871–878.
- Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, Stocker R, Jessup W, Garner B. 2007. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol. 73(9):1340–1346.
- Glaros TG, Chang S, Gilliam EA, Maitra U, Deng H, Li L. 2013. Causes and consequences of low grade endotoxemia and inflammatory diseases. Front Biosci. S5:754–765.
- Goebeler M, Kilian K, Gillitzer R, Kunz M, Yoshimura T, Brocker EB, Rapp UR, Ludwig S. 1999. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor-–induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood. 93(3):857–865.
- Gomes JMG, Costa JA, Alfenas RCG. 2017. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism. 68:133–144.
- Goto T, Eden S, Nordenstam G, Sundh V, Svanborg-Eden C, Mattsby-Baltzer I. 1994. Endotoxin levels in sera of elderly individuals. Clinical Diagnostic Laboratory Immunology. 1(6):684–688.
- Hakkou Z, Maciuk A, Leblais V, Bouanani NE, Mekhfi H, Bnouham M, Aziz M, Ziyyat A, Rauf A, Hadda TB, et al. 2017. Antihypertensive and vasodilator effects of methanolic extract of inula viscosa: biological evaluation and POM analysis of cynarin, chlorogenic acid as potential hypertensive. Biomed Pharmacother. 93:62–69.
- Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, Sabico S, O'Hare JP, Ceriello A, Saravanan P, et al. 2012. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 35(2):375–382.
- Hoefen RJ, Berk BC. 2002. The role of MAP kinases in endothelial activation. Vasc Pharmacol. 38(5):271–273.
- Jimenez-Escrig A, Dragsted LO, Daneshvar B, Pulido R, Saura-Calixto F. 2003. In vitro antioxidant activities of edible artichoke (cynara scolymusL.) and effect on biomarkers of antioxidants in rats. J Agric Food Chem. 51(18):5540–5545.
- Jin CJ, Baumann A, Brandt A, Engstler AJ, Nier A, Hege M, Schmeer C, Kehm R, Hohn A, Grune T, et al. 2020. Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. American Journal of Physiology-Gastrointestinal and Liver Physiology. 318(4):G736–G747.
- Kallio KA, Buhlin K, Jauhiainen M, Keva R, Tuomainen AM, Klinge B, Gustafsson A, Pussinen PJ. 2008. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immun. 14(4):247–253.
- Karin M, Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu Rev Immunol. 18:621–663.
- Kyriakis JM, Avruch J. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 81(2):807–869.
- Lee H, Jang JH, Kim SJ. 2021. Malonic acid suppresses lipopolysaccharide-induced BV2 microglia cell activation by inhibiting the p38 MAPK/NF-κB pathway. Animal Cells Syst (Seoul). 25(2):110–118.
- Lehr HA, Sagban TA, Ihling C, Zahringer U, Hungerer KD, Blumrich M, Reifenberg K, Bhakdi S. 2001. Immunopathogenesis of atherosclerosis. Circulation. 104(8):914–920.
- Leng YP, Ma YS, Li XG, Chen RF, Zeng PY, Li XH, Qiu CF, Li YP, Zhang Z, Chen AF. 2018. L-homocysteine-induced cathepsin V mediates the vascular endothelial inflammation in hyperhomocysteinaemia. Br J Pharmacol. 175(8):1157–1172.
- Li H, Cybulsky MI, GimbroneJr. MA, Libby P. 1993. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arteriosclerosis and Thrombosis: A Journal of Vascular Biology. 13(2):197–204.
- Li H, Xia N, Brausch I, Yao Y, Forstermann U. 2004. Flavonoids from artichoke (cynara scolymus L.) Up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J Pharmacol Exp Ther. 310(3):926–932.
- Liang N, Kitts DD. 2018. Chlorogenic acid (CGA) isomers alleviate interleukin 8 (IL-8) production in caco-2 cells by decreasing phosphorylation of p38 and increasing cell integrity. Int J Mol Sci. 19(12):3873.
- McDougall B, King PJ, Wu BW, Hostomsky Z, Reinecke MG, RobinsonJr. WE. 1998. Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase. Antimicrob Agents Chemother. 42(1):140–146.
- Milackova I, Kapustova K, Mucaji P, Hosek J. 2017. Artichoke leaf extract inhibits AKR1B1 and reduces NF-κB activity in human leukemic cells. Phytother Res. 31(3):488–496.
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, et al. 2015. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 114(7):999–1012.
- Mohammad S, Thiemermann C. 2020. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front Immunol. 11:594150.
- Oates JC. 2015. Endothelial dysfunction in injury and inflammation. Am J Med Sci. 349(1):2.
- Palmer CD, Romero-Tejeda M, Sirignano M, Sharma S, Allen TM, Altfeld M, Jost S. 2016. Naturally occurring subclinical endotoxemia in humans alters adaptive and innate immune functions through reduced MAPK and increased STAT1 phosphorylation. The Journal of Immunology. 196(2):668–677.
- Pietersma A, Tilly BC, Gaestel M, de Jong N, Lee JC, Koster JF, Sluiter W. 1997. P38 mitogen activated protein kinase regulates endothelial VCAM-1 expression at the post-transcriptional level. Biochem Biophys Res Commun. 230(1):44–48.
- Piya S, Kim JY, Bae J, Seol DW, Moon AR, Kim TH. 2012. Dusp6 is a novel transcriptional target of p53 and regulates p53-mediated apoptosis by modulating expression levels of Bcl-2 family proteins. FEBS Lett. 586(23):4233–4240.
- Puangpraphant S, Berhow MA, Vermillion K, Potts G, Gonzalez de Mejia E. 2011. Dicaffeoylquinic acids in yerba mate (ilex paraguariensisSt. hilaire) inhibit NF-κB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and -3 in human colon cancer cells. Mol Nutr Food Res. 55(10):1509–1522.
- Rahman A, Anwar KN, Minhajuddin M, Bijli KM, Javaid K, True AL, Malik AB. 2004. CAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-κB activation and ICAM-1 expression in endothelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 287(5):L1017–L1024.
- Rao R. 2009. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 50(2):638–644.
- Raparelli V, Basili S, Carnevale R, Napoleone L, Del Ben M, Nocella C, Bartimoccia S, Lucidi C, Talerico G, Riggio O, et al. 2017. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology. 65(2):571–581.
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. 1999. Iκb kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 274(43):30353–30356.
- Schmeck B, Zahlten J, Moog K, van Laak V, Huber S, Hocke AC, Opitz B, Hoffmann E, Kracht M, Zerrahn J, et al. 2004. Streptococcus pneumoniae-induced p38 MAPK-dependent phosphorylation of RelA at the interleukin-8 promotor. J Biol Chem. 279(51):53241–53247.
- Schumann RR, Pfeil D, Lamping N, Kirschning C, Scherzinger G, Schlag P, Karawajew L, Herrmann F. 1996. Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood. 87(7):2805–2814.
- Simsek EN, Uysal T. 2013. In vitro investigation of cytotoxic and apoptotic effects of Cynara L. species in colorectal cancer cells. Asian Pac J Cancer Prev. 14(11):6791–6795.
- Stoll LL, Denning GM, Li WG, Rice JB, Harrelson AL, Romig SA, Gunnlaugsson ST, MillerJr. FJ, Weintraub NL. 2004. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. The Journal of Immunology. 173(2):1336–1343.
- Tamura DY, Moore EE, Johnson JL, Zallen G, Aiboshi J, Silliman CC. 1998. P38 mitogen-activated protein kinase inhibition attenuates intercellular adhesion molecule-1 up-regulation on human pulmonary microvascular endothelial cells. Surgery. 124(2):403–408. ; discussion 408.
- Tang X, Wei R, Deng A, Lei T. 2017. Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice. Nutrients. 9(9):1000.
- Terawaki H, Yokoyama K, Yamada Y, Maruyama Y, Iida R, Hanaoka K, Yamamoto H, Obata T, Hosoya T. 2010. Low-grade endotoxemia contributes to chronic inflammation in hemodialysis patients: examination with a novel lipopolysaccharide detection method. Ther Apher Dial. 14(5):477–482.
- Topal M, Gocer H, Topal F, Kalin P, Kose LP, Gulcin I, Cakmak KC, Kucuk M, Durmaz L, Goren AC, et al. 2016. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the illyrian thistle (onopordum illyricumL.). J Enzyme Inhib Med Chem. 31(2):266–275.
- Unenkhuu B, Kim DB, Kim HS. 2021. MKP-3 suppresses LPS-induced inflammatory responses in HUVECs via inhibition of p38 MAPK/NF-κB pathway. Animal Cells Syst (Seoul). 25(4):235–244.
- Vink A, Schoneveld AH, van der Meer JJ, van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G, et al. 2002. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 106(15):1985–1990.
- Whetzel AM, Bolick DT, Hedrick CC. 2009. Sphingosine-1-phosphate inhibits high glucose-mediated ERK1/2 action in endothelium through induction of MAP kinase phosphatase-3. American Journal of Physiology-Cell Physiology. 296(2):C339–C345.
- Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. 1999. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease. J Am Coll Cardiol. 34(7):1975–1981.
- Xia N, Pautz A, Wollscheid U, Reifenberg G, Forstermann U, Li H. 2014. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules. 19(3):3654–3668.
- Zapolska-Downar D, Zapolski-Downar A, Naruszewicz M, Siennicka A, Krasnodebska B, Koldziej B. 2002. Protective properties of artichoke (cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 71(24):2897–2908.
- Zhang M, Pan H, Xu Y, Wang X, Qiu Z, Jiang L. 2017. Allicin decreases lipopolysaccharide-induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2. Cell Physiol Biochem. 41(6):2255–2267.
- Zhou Y, Fu X, Guan Y, Gong M, He K, Huang B. 2020. 1,3-Dicaffeoylquinic acid targeting 14-3-3 tau suppresses human breast cancer cell proliferation and metastasis through IL6/JAK2/PI3K pathway. Biochem Pharmacol. 172:113752.