ABSTRACT
Puromycin treatment can cause glomerular injury to the kidney, leading to proteinuria. However, the pathogenesis of acute kidney injury and subsequent regeneration after puromycin administration in animal models remain unclear. In this work, we examined the characteristics of kidney injury and subsequent regeneration following puromycin treatment in adult zebrafish. We intraperitoneally injected 100 μg of puromycin into zebrafish; sacrificed them at 1, 3, 5, 7, or 14 days post-injection (dpi); and examined the morphological, functional, and molecular changes in the kidney. Puromycin-treated zebrafish presented more rapid clearance of rhodamine dextran than control animals. Morphological changes were observed immediately after the puromycin injection (1–7 dpi) and had recovered by 14 dpi. The mRNA production of lhx1a, a renal progenitor marker, increased during recovery from kidney injury. Levels of NFκB, TNFα, Nampt, and p-ERK increased significantly during nephron injury and regeneration, and Sirt1, FOXO1, pax2, and wt1b showed an increasing tendency. However, TGF-β1 and smad5 production did not show any changes after puromycin treatment. This study provides evidence that puromycin-induced injury in adult zebrafish kidneys is a potential tool for evaluating the mechanism of nephron injury and subsequent regeneration.
Introduction
Acute kidney injury (AKI) is associated with short-term mortality and long-term complications. Despite progress in understanding the pathophysiology of AKI, it is a complicated condition with various etiologies in patients with various underlying diseases (Sato et al. Citation2020). Many drugs can injure the kidneys and cause irreversible damage (Wu and Huang Citation2018; Petejova et al. Citation2019). During the past few decades, animal models have been used as invaluable tools for biomedical research. The zebrafish, Danio rerio, is a useful model that is rapidly gaining attention given its high genetic homology to mammals and easy manipulation, which make it cost-effective in toxicity evaluations of new drugs (Choi et al. Citation2021; Jung et al. Citation2021). In mammals, both the pronephros and the mesonephros are transient, while the metanephros develops into the permanent kidney. In contrast, zebrafish develop pronephros and mesonephros but not metanephros. The zebrafish pronephros is functional during the embryonic and early larval stages and has been the focus of most renal studies in zebrafish. However, the mesonephros has some unique and relevant features and has attracted attention in recent years (Jerman and Sun Citation2017).
Studies of zebrafish kidneys have been formulated to investigate regeneration and provide insights into therapeutic agents (Poureetezadi and Wingert Citation2016). Kidney regeneration paradigms in zebrafish have included administration of nephrotoxins such as gentamicin, a commonly used antibiotic that causes AKI in larval zebrafish (Hentschel et al. Citation2005). Gentamicin causes renal toxicity by inhibiting the lysosomal function of proximal tubular epithelial cells, producing phospholipidosis and tubular degeneration (Vera-Roman et al. Citation1975). Cisplatin is an antineoplastic agent used to treat a variety of solid tumors, although its use in humans is limited by kidney toxicity. Cisplatin also causes histological changes and a decrease in renal function in larval zebrafish (Hentschel et al. Citation2005; Guo et al. Citation2022); (Safirstein et al. Citation1986). Cisplatin exerts its nephrotoxic effect on the S3 segment of the proximal tubule, which is located in the outer stripe of the outer medulla (Dobyan et al. Citation1980). Puromycin has been validated to induce glomerular injury in a larval zebrafish model as an aminonucleoside antibiotic with activity against a broad range of organisms (Hentschel et al. Citation2007; Rider et al. Citation2018). However, puromycin causes necrosis of glomerular podocytes, resulting in severe glomerulopathy (Grond, Weening, et al. Citation1988). Severe proteinuria results from loss of the glomerular barrier against protein filtration, and tubular injury occurs secondary to the formation of proteinaceous casts in the proximal tubules.
Most studies of puromycin toxicity in animal models have focused on the renal glomeruli (Grond, Muller, et al. Citation1988; Hentschel et al. Citation2007; Rider et al. Citation2018; Chen et al. Citation2022). Regeneration of a kidney injured by puromycin has not been studied. Indeed, as a nephrotoxic drug, puromycin induces critical proximal tubular damage, as well as glomerulopathy. A recent study described a puromycin injury model that used pluripotent stem cell–derived kidney organoids. Morphological observations revealed disruption of glomerular and tubular structures within the kidney organoids upon puromycin treatment (Nguyen et al. Citation2022). Therefore, it is interesting to investigate nephron regeneration after puromycin-induced damage to glomerular podocytes and tubular epithelial cells in an experimental animal model.
Furthermore, the mechanisms that mediate renal recovery after human AKI are poorly understood (McCampbell et al. Citation2015). Although many patients with AKI have a high risk of progression to chronic kidney disease (CKD), which can lead to end-stage renal disease (Chevalier Citation2016; Kurzhagen et al. Citation2020), no clear pathogenesis link between AKI and CKD has been reported (Ruiz-Ortega et al. Citation2020). If AKI patients could recover through a process of regeneration before CKD develops, many patients could avoid renal replacement therapy such as hemodialysis and transplantation. In this work, we are the first to examine the morphological and molecular characteristics of changes caused by nephron injury and subsequent regeneration after puromycin-induced AKI in an adult zebrafish model.
Materials and methods
Zebrafish maintenance
Wild-type zebrafish of strain AB were used for this study. The eight-month-old zebrafish were housed at 28.5°C with a standard 14:10 h light: dark cycle. All experimental procedures were approved by the Korea University Institutional Animal Care and Use Committee and carried out in accordance with the Korean National Veterinary Research and Quarantine Service guidelines for animal experiments.
Fluorescence filtration assay
The adult zebrafish were anesthetized with 0.1–0.5% tricaine (ethyl-m-aminobenzoate methanesulfonate, 1% Na2HPO4, pH 7.0) (Sigma-Aldrich, MO, USA) and then injected with 50 μg of rhodamine dextran (10 or 40 kDa, Molecular Probes, OR, USA) and 100 μg of puromycin (Sigma Aldrich, MO, USA) into the peritoneum. The zebrafish were transferred to medium for recovery, harvested, fixed in 4% paraformaldehyde, kept overnight at 4°C, washed with PBS, and dehydrated with increasing concentrations of ethanol (70%, 85%, 95%, and 100%). The ethanol was removed, and the samples were processed for paraffin embedding, as performed previously. The blocks were sectioned and viewed directly using fluorescence microscopy.
Histological examination
Frozen kidney tissues from the adult zebrafish were cut into 10-μm sections and mounted on positively charged/adhesive slides (NovaUltra, MD, USA) for hematoxylin and eosin (H&E) staining. The sections were fixed with 10% formalin for 10 min, dried in air for 30 min, and rinsed in tap water. After staining them in Mayer’s hematoxylin solution for 2 min, rinsing them in running tap water for 2 min, and rinsing them in 95% alcohol for 10 dips or 30 s, the slides were counterstained in eosin solution for 45 s. The slides were rinsed quickly in 95% alcohol (10 dips or less than 30 s), dehydrated through 2 changes of 100% alcohol (5 min each), and cleared in 2 changes of xylene (5 min each). Then they were mounted with xylene-based mounting medium (Shandon, MA, USA).
In situ RNA hybridization
To analyze the expression of lim homobox 1a (lhx1a) by in situ RNA hybridization, we isolated kidneys from adult zebrafish sacrificed 7 and 14 dpi. To synthesize anti-sense RNA probes, pGEM-Teasy vector (Promega, USA) containing lhx1a was transcribed and labeled (New England Biolabigens, USA). Adult zebrafish were fixed in Petri dishes containing 4% formaldehyde in 1 ⅹ PBS overnight at 4°C. The next day, the fish were dehydrated in 100% methanol for 15 min at room temperature and placed at −20°C in 100% methanol for 2 h before use. The fish were rehydrated in successive dilutions of methanol in 1 ⅹ PBS: 5 min in 75% (vol/vol) methanol; 5 min in 50% (vol/vol) methanol; and 5 min in 25% (vol/vol) methanol. Then, the fish were permeabilized, incubated with anti-DIG antibody alkaline phosphatase, stained, mounted, and observed microscopically. Transcription was detected by whole-mount in situ RNA hybridization in a fixed zebrafish kidney as previously described (Thisse and Thisse Citation2008).
Western blot analyses
Kidneys were lysed in protein extraction solution (ELPIS, Daejeon, Korea). The protein concentration was measured using the bicinchoninic acid protein assay (Pierce, IL, USA). The proteins were transferred onto a nitrocellulose membrane (Immobilon-P, MA, USA), which was hybridized with rabbit polyclonal anti-NFκB (1:1000, GeneTex, CA, USA), rabbit polyclonal anti-TNFα (1:1000, GeneTex, CA, USA), rabbit polyclonal anti-Nampt antibody (1:10000, Proteintech, IL, USA), rabbit polyclonal anti-Sirt1 (1:1000, Proteintech, IL, USA), rabbit polyclonal anti-FOXO1 (1:1000, LSBio, WA, USA), rabbit polyclonal anti-pax2a (1:1000, GeneTex, CA, USA), rabbit polyclonal anti-wt1b (1:1000, GeneTex, CA, USA), rabbit polyclonal anti-p-ERK (1:1000, Cell Signaling, MA, USA), rabbit polyclonal anti-ERK (1:1000, Cell Signaling, MA, USA), rabbit polyclonal anti-TGF-β1 (1:1000, Novus Biologicals, CO, USA), rabbit polyclonal anti-smad5 (1:200, GeneTex, CA, USA), and mouse monoclonal anti-β-actin antibody (1:10000, Sigma-Aldrich, MO, USA) in blocking buffer overnight at 4°C. The membrane was then incubated for 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibody diluted 1:5,000. Specific signals were detected using ECL (Amersham, Buckinghamshire, UK).
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Scientists (SPSS) version 13.0 software (SPSS Inc., Chicago, USA). Data were analyzed by Student’s t-test or one-way ANOVA followed by Dennett’s or Tukey’s post-test. P-values less than 0.05 were considered statistically significant.
Results
Puromycin injures glomerular filtration in adult zebrafish
Administration of a single dose of 50 −150 mg/kg puromycin in rats causes severe glomerular changes (Ryan et al. Citation1975). A high dose of 350 mg/kg puromycin leads to glomerular injury and severe pericardial edema in larval zebrafish (Hentschel et al. Citation2007). We chose a low dose of 100 μg of puromycin in adult zebrafish, and approximately 75% of them survived until 14 days after the intraperitoneal injection (). A previous study in a larval zebrafish model of AKI determined the rate of decline in fluorescence intensity at the hearts of gentamicin-injected fish and then quantified the change in renal function (Hentschel et al. Citation2005). However, that method is inadequate to evaluate renal function because drugs such as gentamicin have cardiac toxicity as well as nephrotoxicity in animals. In our experiment, we evaluated functional filtration by analyzing renal fluorescence intensity. Clearance of rhodamine dextran can show different results depending on the molecular size of the dextran, the severity of glomerular and tubular damage, and the circulating blood volume (Hentschel et al. Citation2007). We used both 10 and 40 kDa rhodamine dextran and compared the fluorescence over time. The puromycin-treated group showed higher fluorescence intensity than the controls at 3 dpi and lower fluorescence intensity than the controls at 5, 7, and 14 dpi. The renal filtration assay with 10 or 40 kDa rhodamine dextran through the nephrons of adult zebrafish kidneys showed similar results. Rhodamine dextran washed out from the kidneys of puromycin-treated zebrafish more rapidly than from the controls ().
Figure 1. Survival after puromycin administration. The flowchart shows the schematic timeline of the experimental procedures. Approximately 75% of the injected animals survived on day 0 and showed no mortality until sacrifice. dpi, days post-injection; con, control; puro, puromycin
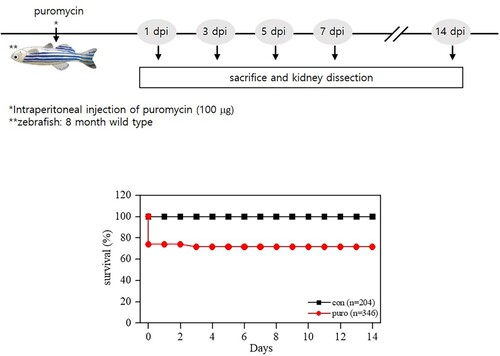
Figure 2. Dextran filtration assays. The puromycin-treated group showed higher fluorescence intensity than the controls at 3 dpi and lower fluorescence intensity than the controls at 5, 7, and 14 dpi. Both 10 and 40 kDa rhodamine dextran washed out of the kidneys of puromycin-treated zebrafish more rapidly than from the controls. *p < 0.5 **p < 0.01 ***p < 0.001 vs. controls
Nephrons regenerate after puromycin injury in adult zebrafish
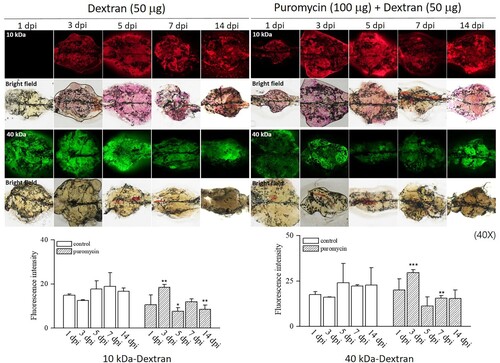
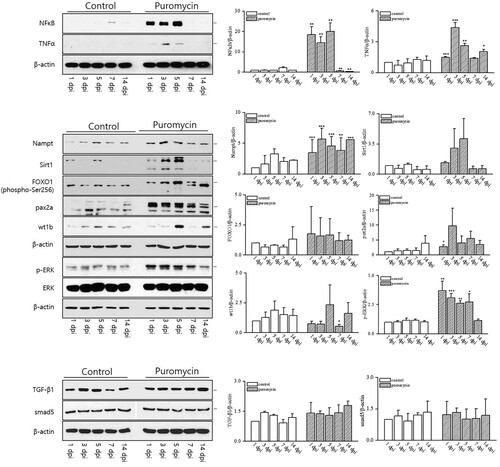
In this study, we used H&E staining to examine morphological changes in the kidneys of adult zebrafish treated with puromycin. Nephron damage began immediately after puromycin injection in adult zebrafish. As shown in , from 1 dpi to 7 dpi, severe tubular necrosis and atrophy were observed, and inflammatory cells infiltrated the interstitium of the kidney. The number of nephrons decreased noticeably at 1, 3, and 5 dpi and then recovered to a level similar to that in the controls at 7 and 14 dpi. We subsequently evaluated the in situ hybridization of lhx1a as a renal progenitor marker. We observed an increase in lhx1a production during recovery from AKI (). The mechanism of nephron regeneration after AKI remains unknown. Therefore, we used western blot analyses to investigate several pathways related to injury and recovery, as shown in . These pathways have been shown to be related to CKD progression in humans and animal models (Sato et al. Citation2020). We found a significant increase in NFκB production from 1 dpi to 5 dpi and then a decrease until 14 dpi. TNFα increased at 1, 3, 5, and 14 dpi. Nampt expression also increased significantly from 1 to 14 dpi. Sirt1, FOXO1, pax2a, and wt1b did not change significantly after puromycin treatment, but they showed an increasing tendency. In addition, p-ERK increased significantly during nephron injury and regeneration. However, TGF-β1 and smad5 production did not change following puromycin treatment. These results demonstrate that a pathway containing NFκB, TNFα, Nampt, and p-ERK is involved in the kidney injury and regeneration processes.
Figure 3. Histological findings after puromycin administration using H&E staining. The puromycin-treated group showed severe renal tubular necrosis (open arrows) and interstitial inflammation (arrowheads), but the nephrons (closed arrows) had regenerated at 7 and 14 dpi. The quantitative analysis of nephrons after puromycin treatment shows lower scores than the controls until 7 and 14 dpi. **p < 0.01 ***p < 0.001 vs. controls
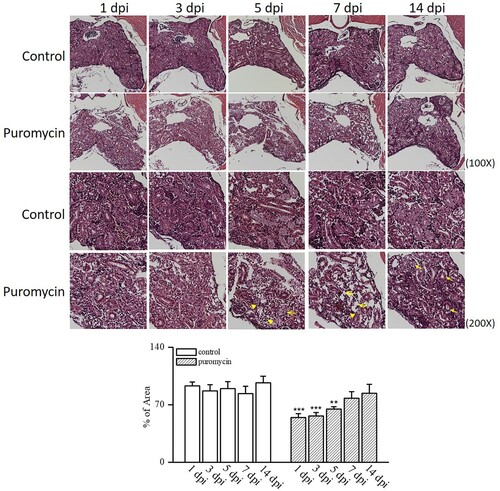
Figure 4. Lim homobox 1a (lhx1a) expression as a renal progenitor marker by in situ hybridization. lhx1a production increased during recovery from acute kidney injury by puromycin administration. *p < 0.5 vs. controls
Figure 5. The renal molecular pathways induced by puromycin administration. NFκB production increased significantly from 1 dpi to 5 dpi and then decreased until 14 dpi. TNFα increased at 1, 3, 5, and 14 dpi. Nampt expression also increased significantly from 1 to 14 dpi. p-ERK increased significantly during nephron injury and regeneration. *p < 0.5 **p < 0.01 ***p < 0.001 vs. controls (SPSS, Student’s t-test)
Discussion
Despite advances in understanding of the pathophysiologic mechanisms of AKI, the condition cannot yet be effectively treated in the clinic. Clinical trials of potential nephroprotective agents have failed due to the complexity of AKI and the clinical heterogeneity of AKI patients (Venkatachalam et al. Citation2015). Several factors, including ischemia/reperfusion, exposure to toxins, and sepsis, have been recognized to cause AKI, which increases the risk of CKD and end-stage renal disease (Jerman and Sun Citation2017; Fu et al. Citation2018). AKI can affect any part of the nephron, including the tubular epithelia, glomerulus, interstitium, and vasculature (Basile et al. Citation2012).
Zebrafish have emerged as an ideal model to study AKI because gentamicin damages the proximal tubules of the kidney (Hentschel et al. Citation2005). Gentamicin-induced AKI models with both larval and adult zebrafish have been used extensively to study regeneration after injury. As a successful model of both adult renal disease and congenital anomalies, manipulating zebrafish for study of kidney diseases is relatively easy (Jerman and Sun Citation2017). The pronephros of a zebrafish embryo consists of a pair of segmented nephrons that share a blood filter, and each nephron contains two proximal and two distal tubule segments (Jerman and Sun Citation2017). The mesonephros is a single, relatively flat organ attached to the dorsal body wall and consists of characteristic bilaterally symmetric regions referred to as the head, trunk, and tail (Zhou et al. Citation2010). The mesonephros begins to form between 12 and 14 days post-fertilization, with progressive addition of nephrons to the existing pronephric pair (Zhou et al. Citation2010).
A previous study in larval zebrafish demonstrated puromycin-induced glomerular injury using a filtration assay and electron microscopy (Rider et al. Citation2018). Because of its accompanying cardiovascular toxicity, adriamycin is unsuitable for inducing glomerular injury. In puromycin-treated larvae, podocyte effacement was confirmed by electron microscopy, and glomerular filtration function was validated using a protein excretion assay. In our study, we revealed that both 10 and 40 kDa dextran are useful for filtration assays in a puromycin-induced zebrafish model. Although puromycin has been widely used to induce renal glomerular injury in animal models, the morphological and molecular changes it causes during nephron injury have rarely been reported. In this study, we examined the changes associated with nephron injury and regeneration in adult zebrafish from 1 dpi to 14 dpi after puromycin administration.
Our puromycin-induced AKI model in adult zebrafish produced significant tubular injury, as well as glomerular injury, followed by nephron regeneration. Various inflammatory and regenerative pathways might be related to puromycin-induced kidney injury and could provide a key mechanism for understanding recovery after AKI. Our results provide evidence of the potential of puromycin as a tool to evaluate nephron injury following regeneration.
Acknowledgements
We are grateful for a support of Zebrafish Translational Medical Research Center in Korea University Ansan Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Basile DP, Anderson MD, Sutton TA. 2012. Pathophysiology of acute kidney injury. Compr Physiol. 2(2):1303–1353.
- Chen B, Alam Z, Ge Y, Dworkin L, Gong R. 2022. Pharmacological melanocortin 5 receptor activation attenuates glomerular injury and proteinuria in rats with puromycin aminonucleoside nephrosis. Front Physiol. 13:887641.
- Chevalier RL. 2016. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 311(1):F145–F161.
- Choi Y, Seo H, Cho M, Kim J, Chung HS, Lee I, Kim MJ. 2021. Rutin inhibits DRP1-mediated mitochondrial fission and prevents ethanol-induced hepatotoxicity in HepG2 cells and zebrafish. Anim Cells Syst (Seoul). 25(1):74–81.
- Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW. 1980. Mechanism of cis-platinum nephrotoxicity: II. morphologic observations. J Pharmacol Exp Ther. 213(3):551–556.
- Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. 2018. Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol. 315(4):F1098–F1106.
- Grond J, Weening JJ, van Goor H, Elema JD. 1988. Application of puromycin aminonucleoside and Adriamycin to induce chronic renal failure in the rat. Contrib Nephrol. 60:83–93.
- Guo X, Xu L, Velazquez H, Chen TM, Williams RM, Heller DA, Burtness B, Safirstein R, Desir GV. 2022. Kidney-targeted renalase agonist prevents cisplatin-induced chronic kidney disease by inhibiting regulated necrosis and inflammation. J Am Soc Nephrol. 33(2):342–356.
- Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M. 2007. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol. 293(5):F1746–F1750.
- Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. 2005. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 288(5):F923–F929.
- Jerman S, Sun ZX. 2017. Using zebrafish to study kidney development and disease. Curr Top Dev Biol. 124:41–79. English.
- Jung J, Kim J, Huh TL, Rhee M. 2021. Trim46 contributes to the midbrain development via Sonic Hedgehog signaling pathway in zebrafish embryos. Anim Cells Syst (Seoul). 25(1):56–64.
- Kurzhagen JT, Dellepiane S, Cantaluppi V, Rabb H. 2020. AKI: an increasingly recognized risk factor for CKD development and progression. J Nephrol. 33(6):1171–1187.
- McCampbell KK, Springer KN, Wingert RA. 2015. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cells Int. 2015:547636.
- Nguyen L, Wruck W, Erichsen L, Graffmann N, Adjaye J. 2022. The nephrotoxin puromycin aminonucleoside induces injury in kidney organoids differentiated from induced pluripotent stem cells. Cells. 11(4):635.
- Petejova N, Martinek A, Zadrazil J, Teplan V. 2019. Acute toxic kidney injury. Ren Fail. 41(1):576–594.
- Poureetezadi SJ, Wingert RA. 2016. Little fish, big catch: zebrafish as a model for kidney disease. Kidney Int. 89(6):1204–1210.
- Rider SA, Bruton FA, Collins RG, Conway BR, Mullins JJ. 2018. The efficacy of puromycin and Adriamycin for induction of glomerular failure in larval zebrafish validated by an assay of glomerular permeability dynamics. Zebrafish. 15(3):234–242.
- Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. 2020. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 16(5):269–288.
- Ryan GB, Rodewald R, Karnovsky MJ. 1975. An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest. 33(5):461–468.
- Safirstein R, Winston J, Goldstein M, Moel D, Dikman S, Guttenplan J. 1986. Cisplatin nephrotoxicity. Am J Kidney Dis. 8(5):356–367.
- Sato Y, Takahashi M, Yanagita M. 2020. Pathophysiology of AKI to CKD progression. Semin Nephrol. 40(2):206–215.
- Thisse C, Thisse B. 2008. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 3(1):59–69.
- Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. 2015. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 26(8):1765–1776.
- Vera-Roman J, Krishnakantha TP, Cuppage FE. 1975. Gentamicin nephrotoxicity in rats. I. Acute biochemical and ultrastructural effects. Lab Invest. 33(4):412–417.
- Wu H, Huang J. 2018. Drug-Induced nephrotoxicity: pathogenic mechanisms, biomarkers and prevention strategies. Curr Drug Metab. 19(7):559–567.
- Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. 2010. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 299(5):F1040–F1047.
