ABSTRACT
The spexin-based GALR2 agonist (NS200) is a novel drug, which has shown antidepressant and anxiolytic action in a recent experimental study. In this study, we investigated the effects of NS200 on renal injury in an animal model of type 2 diabetes. Eight-week-old diabetic db/db mice were administered NS200 for 12 weeks. NS200 was intraperitoneally administered at a dose of 1.0 mg/kg/day. Metabolic parameters and structural and molecular changes in the kidneys were compared among the three groups: non-diabetic db/m control, db/db mice, and NS200-treated db/db mice. In db/db mice, NS200 administration did not impact the body weight, food and water intake, urinary volume, fasting blood glucose level, or HbA1c levels. Insulin and glucose tolerance were also unaffected by NS200 treatment. However, NS200 improved urinary albumin excretion and glomerulosclerosis in diabetic kidneys. Activation of TGFβ1 and insulin signaling pathways, such as PI3 K /AKT/ERK, were inhibited by NS200. In conclusion, a spexin-based GALR2 agonist attenuated diabetic nephropathy by alleviating renal fibrosis in mice with type 2 diabetes. Spexin-based GALR2 agonists have considerable potential as novel treatment agents in diabetic nephropathy.
Introduction
Diabetic nephropathy is the most common cause of end-stage renal disease worldwide. Antihypertensive drugs targeting suppression of the renin-angiotensin-aldosterone (RAAS) system have been widely used to prevent organ damage in diabetes (Mistry and Bakris Citation2023). Recent clinical trials have shown that a new class of antidiabetic drugs, including sodium-glucose cotransporter 2 inhibitors and dipeptidyl peptidase 4 inhibitors in addition to RAAS blockade, are effective against diabetic nephropathy and reduce cardiovascular-related events in patients with diabetes (Zinman et al. Citation2015; Garg et al. Citation2017; Neal et al. Citation2017; Mahaffey et al. Citation2018; Furtado et al. Citation2019; Wiviott et al. Citation2019). However, the progression of diabetic nephropathy is not completely controlled. Patients with hyperglycemia develop diabetic complications, including diabetic nephropathy, even after the normalization of blood glucose (Kato and Natarajan Citation2019). Therefore, although current treatments can slow the decline in renal function, the search for novel drugs for diabetic nephropathy has continued (Kim et al. Citation2022).
Recent studies have reported that multidisciplinary management, including blood glucose control, blood pressure and lipid control, and lifestyle modifications (appropriate weight control, diet restriction, and smoking cessation) are important for reducing cardiovascular risk (Alkhatib et al. Citation2023). However, many patients with metabolic syndrome have behavioral risk factors, such as poor diet, sleep, and physical activity, and suffer from depression (Liu et al. Citation2017). Both depression and metabolic syndrome are risk factors for type 2 diabetes and cardiovascular complications, with evidence showing a bidirectional association between them (Rosengren et al. Citation2004). Although depression is commonly associated with eating disorders, regulatory mechanisms underlying feeding and satiety remain unclear.
Spexin, galanin, and kisspeptin are three related bioactive peptides that have gained attention for their roles in regulating energy metabolism, mood, and behavior (Mills et al. Citation2021). These peptides have been classified as members of the same family, but the specific interactions that link energy homeostasis, mood, and behavior remain unknown. Spexin is a novel 14-amino acid neuropeptide involved in energy homeostasis that plays an important role in metabolic diseases (Behrooz et al. Citation2020). Spexin lowers blood glucose levels by increasing blood glucose uptake into skeletal muscle. This study suggests that spexin has beneficial effects in obesity-related disorders. However, to date, no spexin-specific receptors have been identified. Spexin shares receptors with galanin in various organs, especially the brain, to control anxiety and depression (Yun et al. Citation2019; Mills et al. Citation2021). Galanin is a 30-amino acid peptide derived from a 123-amino acid polypeptide precursor in humans. Natural galanin binds to the galanin receptor (GALR) 1 and GALR2 with high affinity but shows relatively low affinity for GALR3. GALRs induce different G protein signaling pathways, with GALR1 and GALR3 activating Gi-coupled inhibitory signaling, and GALR2 activating Gq-coupled stimulatory signaling. Spexin can stimulate GALR2 and GALR3 with high potency but does not stimulate GALR1 (Kim et al. Citation2014). GALRs perform a wide range of physiological functions through subtype-specific signaling pathways.
Owing to the cross-reactivity between spexin and galanin as well as the complexity of the GALR-mediated signaling pathway, a more stable and selective GALR agonist was developed. Thus, the synthetic spexin-based GALR2 agonist has selectivity for GALR2 compared to GALR1 and GALR3 (Reyes-Alcaraz et al. Citation2016) and produces anxiolytic and antidepressant effects in animal experiments. Moreover, it has potential for clinical applications in GALR2-mediated disorders such as obesity, anxiety, and depression (Jeong et al. Citation2019; Yun et al. Citation2019).
In this study, we investigated the effects of a spexin-based GALR2 agonist treatment on kidney injury and obesity-related metabolic syndrome in mice with type 2 diabetes.
Materials and methods
Animal experiments
Eight-week-old male diabetic db/db mice (C57BLKS/J-leprdb/leprdb) and male non-diabetic db/m mice (C57BLKS/J-leprdb/+) were purchased from Central Lab Animal Inc. (Seoul, South Korea). Mice were divided into three experimental groups (n = 10 in each group): db/m mice injected with vehicle, db/db mice injected with vehicle, and db/db mice treated with a spexin-based GALR2 agonist (NS200). The mice received an intraperitoneal injection of NS200 diluted with PBS to a final dose of 1 mg/kg as described in the previous study (Yun et al. Citation2019). The control mice received the same volume of PBS. NS200 was administered five times per week for 12 weeks at the targeted dose. All mice were provided with standard chow and water and were maintained at constant temperature (23 ± 2°C) and humidity (55 ± 5%) with an artificial light cycle. Daily food and water intake was monitored at regular intervals. Body weight, food and water intake, urine volume, fasting blood glucose concentration, and HbA1c levels were measured at baseline and at 4, 8, and 12 weeks. Blood glucose levels were measured using the glucose oxidase method (OneTouch Ultra, Johnson & Johnson Co., CA, USA), and HbA1c levels were calculated using the IN2IT system (Bio-Rad Laboratories, Hercules, CA, USA). To determine urinary albumin excretion, the mice were caged individually, and 24-h urine samples were collected at the indicated times. Urinary albumin concentration was determined using a competitive enzyme-linked immunosorbent assay kit (ALPCO, Westlake, OH, USA). We performed an insulin tolerance test (ITT) and a glucose tolerance test (GTT) to determine the insulin resistance and glucose intolerance state, respectively, of each group at the end of the study. ITT was conducted by intraperitoneal injection of 0.75 units/kg regular insulin in fasted mice and by measuring blood glucose levels at 0, 30, 60, 90, and 120 min thereafter. The GTT was performed by intraperitoneal injection of 2 g dextrose per kilogram of body weight after an 8-h fasting period, and blood samples were collected from the tail vein. Mice were sacrificed under anesthesia with intraperitoneal injections of tribromoethanol (Avertin®; 50 mg/kg), and tissues were weighed and snap-frozen in liquid nitrogen. At the end of the study period, systolic blood pressure was measured using tail-cuff plethysmography (LE 5001-Pressure Meter, Letica SA, Barcelona, Spain). All experiments were conducted in accordance with NIH guidelines and with the approval of the Korea University Institutional Animal Care and Use Committee (KOREA-2018-0154).
Histological and immunohistochemical analyses
The kidney samples were fixed in 4% paraformaldehyde and embedded in paraffin. Tissues were cut into 4-µm-thick slices and stained with hematoxylin and eosin (H&E) and periodic acid- Schiff (PAS). For immunohistochemical staining, sections were transferred to 10 mM/L citrate buffer solution adjusted to a pH of 6.0 and then microwaved for 10–20 min to retrieve antigens for TGFβ1 and F4/80 staining. Alternatively, the sections were transferred to Biogenex Retrieval buffer (pH 8.0; InnoGenex, San Ramon, CA, USA) and treated with trypsin (Sigma, St. Louis, MO, USA) for 30 min at 37°C to detect type IV collagen. To block endogenous peroxidase activity, 3.0% H2O2 in methanol was applied to the tissue sections for 20 min. Samples were incubated at room temperature for 60 min in 3% BSA/3% normal goat serum (type IV collagen), followed by a 30-min incubation in 20% normal sheep serum (TGFβ1). Slides were incubated overnight at 4°C with rabbit polyclonal anti-TGFβ1 antibody (1:200; Santa Cruz Biotechnology Inc.), rabbit polyclonal anti-type IV collagen antibodies (1:150; BioDesign International, Sarco, ME, USA), and rabbit polyclonal anti-spexin antibody (1:100; Phoenix Pharmaceuticals, Burlingame, CA, USA). After overnight incubation, slides were incubated with secondary antibodies for 30 min. For coloration, slides were incubated at room temperature with a mixture of 0.05% 3,3′-diaminobenzidine containing 0.01% H2O2 and then counterstained with Mayer’s hematoxylin. Negative control sections were stained under identical conditions using buffer solution substituted for the primary antibody. Glomerular mesangial expansion was scored semi-quantitatively, and the percentage of the mesangial matrix occupying each glomerulus was rated from 0 to 4 as follows: 0, 0%; 1, < 25%; 2, 25–50%; 3, 50–75%; and 4, > 75%. The degree of glomerulosclerosis was scored as described previously (Kang et al. Citation2009; Kang et al. Citation2010). To evaluate immunohistochemical staining for TGFβ1 and type IV collagen, glomerular fields were graded semi-quantitatively under a high-power field containing 50–60 glomeruli, and an average score was calculated as described in a previous study (Kang et al. Citation2010).
Protein extraction and western blot analysis
Proteins were extracted from the kidney cortex tissues using a commercial extraction kit according to the manufacturer’s instructions (Active Motif, Carlsbad, CA, USA). The protein concentration was determined using the bicinchoninic acid method (Pierce Pharmaceuticals, Rockford, IL, USA). For western blotting, 40 μg of protein per sample was electrophoresed using 10% sodium dodecyl-sulfate polyacrylamide gel electrophoresis minigels. The proteins were transferred onto polyvinylidene difluoride membranes. The membranes were hybridized in blocking buffer overnight at 4°C with rabbit polyclonal anti-TGFβ1 antibody (1:1,000; Novus, MO, USA), rabbit polyclonal anti-type IV collagen antibody (1:1,000; Abcam Inc., Cambridge, MA, USA), mouse monoclonal anti-ED1 (macrophage/monocyte marker) antibody (1:1,000; Abcam Inc.), mouse monoclonal anti-phosphoinositide 3 kinase (PI3 K) antibody (1:500; Abcam Inc.), rabbit polyclonal anti-p-AKT (1:500; Cell Signaling Technology, Beverly, MA, USA), rabbit polyclonal anti-AKT (1:500; Cell Signaling Technology), rabbit polyclonal anti-phosphorylated extracellular signal-regulated kinase (p-ERK) (1:500; Cell Signaling Technology), rabbit polyclonal anti-ERK (1:500; Cell Signaling Technology), rabbit polyclonal anti-p-p38 (1:500; Cell Signaling Technology), rabbit polyclonal anti-p38 (1:500; Cell Signaling Technology), and mouse monoclonal anti-β-actin antibody (1:2,000, Sigma-Aldrich, St. Louis, MO, USA). Membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:1,000 dilution for 60 min at room temperature). The signals were detected using enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
Analysis of gene expression by real-time quantitative polymerase chain reaction
Total RNA was extracted from renal cortical tissues using TRIzol reagent and further purified using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Primers were designed from the respective gene sequences using Primer 3 software (Whitehead Institute, Cambridge, MA, USA), and the secondary structures of the templates were examined and excluded using mfold software (Rensselaer Polytechnic Institute, Troy, NY, USA). The nucleotide sequences of all primers used in this study are presented in . Quantitative gene expression was performed using SYBR Green technology on a LightCycler 1.5 system (Roche Diagnostics Corporation, Indianapolis, IN, USA). Thermocycling conditions consisted of 22–30 cycles of denaturation for 10 s at 95°C, followed by annealing and extension for 30 s at 60°C. Expression of each gene relative to β-actin (relative gene expression) was calculated by subtracting the threshold cycle number of the target gene from that of β-actin and calculating 2 to the power of that number. The specificity of each PCR product was evaluated by melting curve analysis, followed by agarose gel electrophoresis.
Table 1. Primers sequences for real-time quantitative PCR.
Statistical analyses
Non-parametric analyses were performed because of the relatively small number of samples. Results were expressed as mean ± SEM. Comparisons were performed using the Wilcoxon rank-sum test and the Bonferroni correction. The Kruskal-Wallis test was used to compare more than two groups, followed by the Mann-Whitney U test. Values of p < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS for Windows (version 20.0; IBM Corporation, Armonk, NY, USA).
Results
Physical and biochemical parameters of experimental animals
The physical and biochemical parameters of each experimental group are listed in . As expected, diabetic db/db mice showed more significant increases in body weight, food and water intake, and urinary volume than non-diabetic db/m controls over 3 months. Fasting blood glucose and HbA1c levels were also significantly higher in db/db mice than in db/m mice throughout the study period. However, NS200 had no effect on the body weight, food and water intake, or urinary volume in mice with type 2 diabetes. Fasting blood glucose and HbA1c levels did not change after NS200 treatment of db/db mice. To determine the effect of NS200 treatment on insulin resistance parameters, we performed an ITT and GTT after 12 weeks of NS200 treatment. Diabetic db/db mice showed significantly higher ITT and GTT values than those of the db/m controls. However, no changes were observed in either the ITT or GTT in response to NS200 in db/db mice (A and B). These results indicated that spexin-based GALR2 agonists do not improve metabolic syndrome in animals with type 2 diabetes.
Figure 1. Diabetic db/db mice show significant differences in ITT and GTT compared to non-diabetic db/m mice (A, B). However, there are no changes in ITT and GTT between vehicle-injected db/db mice and NS200-treated db/db mice (A, B). Values are expressed as mean ± SEM. *p < 0.05 db/m mice with vehicle vs db/db mice with vehicle, #p < 0.05 db/db mice with vehicle vs db/db mice with NS200
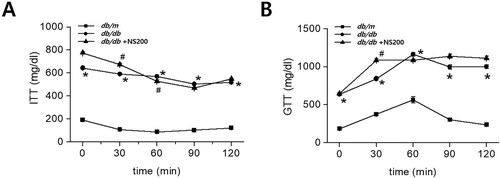
Table 2. Basic physical and biochemical parameters
Effects of spexin-based GALR2 agonist treatment on diabetic kidney disease
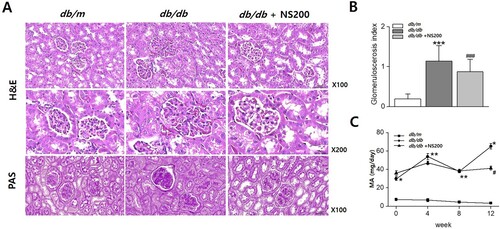
To evaluate the effects of spexin-based GALR2 agonist treatment on renal injury in type 2 diabetic mice, we examined the histological changes to determine the effect of spexin-based GALR2 agonist treatment on diabetic kidney disease. H&E and PAS staining were performed on the kidney tissue of the three groups. A shows representative histological findings in experimental mouse kidneys at the end of the study period. Diabetic db/db mouse kidneys exhibited more severe glomerulosclerosis and greater mesangial expansion than those of non-diabetic db/m controls. NS200 significantly improved glomerulosclerosis in db/db mice, as shown in (A and B). Next, we measured 24 h-urine albumin excretion, which was higher in db/db mice than in db/m controls. Consistent with the renal histological changes, NS200 significantly decreased urinary albumin excretion after 12 weeks (C). These results suggest that spexin-based GALR2 agonists may protect renal injury in diabetic kidney disease.
Figure 2. The kidneys of diabetic db/db mice exhibit more severe glomerulosclerosis than in non-diabetic db/m mice (A, B). NS200 improves glomerulosclerosis in diabetic db/db mice (A, B). Urinary albumin excretion is higher in diabetic db/db mice than in non-diabetic db/m. NS200 decreases urinary albumin excretion after 12 weeks of treatment (C). H&E, Hematoxylin and eosin; PAS, Periodic acid- Schiff. Values are expressed as mean ± SEM. *p < 0.05 db/m mice with vehicle vs db/db mice with vehicle, **p < 0.01 db/m mice with vehicle vs db/db mice with vehicle,***p < 0.001 db/m mice with vehicle vs db/db mice with vehicle, #p < 0.05 db/db mice with vehicle vs db/db mice with NS200, ### p < 0.001 db/db mice with vehicle vs db/db mice with NS200
Role of spexin-based GALR2 agonist in molecular changes of diabetic kidney disease
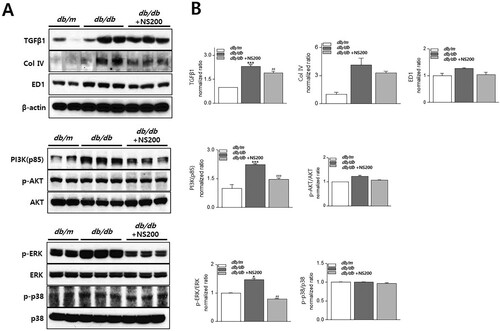
Subsequently, we investigated changes in profibrotic and proinflammatory molecules in diabetic kidney disease. When TGFβ1, type IV collagen, and ED1 protein syntheses were compared, we found an increase in their expression in diabetic db/db mouse kidneys, but these changes were decreased by NS200, as shown in . We also assessed the expression of proteins associated with insulin signaling, including PI3 K, AKT, ERK, and p38. PI3 K and p-ERK were significantly activated in db/db mouse kidneys, and these changes were inhibited by NS200 (). p-AKT activation showed a tendency to increase in db/db mouse kidneys and decrease following NS200 treatment. However, p-p38 and p38 protein expression levels did not change in any of the three groups. Our results suggest that the protective mechanism of a spexin-based GALR2 agonist in diabetic nephropathy are associated with the renal PI3 K/AKT/ERK and TGFβ1 pathway.
Figure 3. The expression of TGFβ1, type IV collagen, and ED1 increases in diabetic db/db mice, and NS200 suppresses their expression (A, B). The expression of PI3 K (p85), p-AKT, and p-ERK is activated in diabetic db/db mice, and this change is inhibited by NS200. However, p-p38 protein expression does not show any difference among the three groups (A, B). Col 4, type IV collagen. p-AKT/AKT means the ratio of p-AKT to total AKT expression. p-ERK/ERK means the ratio of p-ERK to total ERK expression. p-p38/p38 means the ratio of p-p38 to total p38 expression. Values are expressed as ± SEM. *p < 0.05 db/m mice with vehicle vs db/db mice with vehicle, ***p < 0.001 db/m mice with vehicle vs db/db mice with vehicle, ##p < 0.01 db/db mice with vehicle vs db/db mice with NS200, ### p < 0.001 db/db mice with vehicle vs db/db mice with NS200
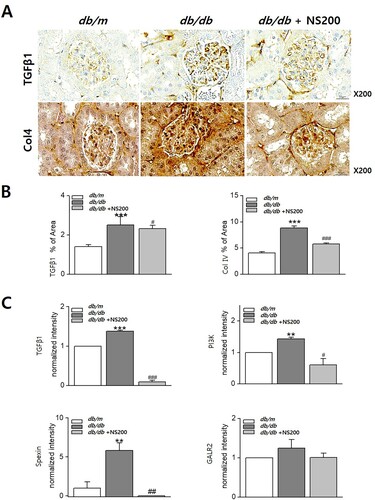
To confirm the role of spexin-based GALR2 agonists in diabetic kidney disease, immunohistochemistry was performed on the kidneys of db/m, db/db, and NS200-treated db/db mice. Expression of TGFβ1 and type IV collagen were increased in diabetic db/db mouse kidneys. However, NS200 inhibited the expression of TGFβ1 and type IV collagen in diabetic db/db mouse kidneys. The scoring indices for immunohistochemistry are shown in . We also compared mRNA expression in diabetic nephropathy. The mRNA expression of TGFβ1 and PI3 K was increased in diabetic db/db mouse kidneys and suppressed by NS200, as shown in . Since there have been few reports on the role of spexin and its receptor in kidney diseases, we examined spexin and GALR2 synthesis in diabetic db/db mouse kidneys. Spexin gene expression was increased in diabetic db/db mouse kidneys and was decreased by NS200; however, GALR2 gene expression did not change in the kidneys of db/m, db/db, or NS200-treated db/db mice (). These results demonstrated that the protective action of the spexin-based GALR2 agonist against diabetic nephropathy through an antifibrotic effect.
Figure 4. The expression of TGFβ1 and type IV collagen increases in diabetic db/db mice (A, B). However, NS200 inhibits TGFβ1 and type IV collagen expression in diabetic db/db mice (A, B). TGFβ1 and PI3 K mRNA expression increases in diabetic db/db mice and decreases by NS200 (C). Spexin mRNA expression increases in diabetic db/db mice and decreases by NS200, but expression of galanin receptor (GALR) 2 does not differ among the three mouse groups (C). Col 4, type IV collagen. Values are expressed as mean ± SEM. **p < 0.01 db/m mice with vehicle vs db/db mice with vehicle, ***p < 0.001 db/m mice with vehicle vs db/db mice with vehicle, #p < 0.05 db/db mice with vehicle vs db/db mice with NS200, ##p < 0.01 db/db mice with vehicle vs db/db mice with NS200, ###p < 0.001 db/db mice with vehicle vs db/db mice with NS200
Discussion
Herein, we observed a protective role of a spexin-based GALR2 agonist in diabetic nephropathy. The spexin-based GALR2 agonist improved urinary albumin excretion and histopathological and molecular changes in renal injury without lowering plasma glucose and HbA1c levels in diabetic mice. Additionally, the spexin-based GALR2 agonist did not alter insulin or glucose tolerance in type 2 diabetic animals. Interestingly, the spexin-based GALR2 agonist improved diabetic nephropathy but did not change metabolic parameters including body weight, blood sugar level, HbA1c, or insulin resistance. These findings indicated that spexin-based GALR2 agonists may have a direct protective effect against kidney injury and not a systemic effect on metabolic syndrome.
In this study, we used leptin receptor-deficient mice as an animal model of type 2 diabetes. The leptin receptor gene, which encodes a high-affinity receptor for leptin receptor, was identified in the mouse choroid plexux (Tartaglia et al. Citation1995; Chen et al. Citation1996). Through the leptin receptor, leptin activates signaling pathways, including Janus kinase 2-signal transducer and activator of transcription 3, insulin receptor substrate-PI3 K, ERK, and AMP-activated kinase, and regulates insulin sensitivity (Liu et al. Citation1998; Morton et al. Citation2005). Defects in either leptin or insulin signaling in the brain result in hyperphagia, glucose intolerance, and insulin resistance (Niswender and Schwartz Citation2003; Park et al. Citation2022). In the hypothalamus, two types of neurons, proopiomelanocortin (POMC) and neuropeptide Y(NPY)/agouti-related peptide (AgRP), control feeding and satiety. Leptin-responsive neurons are observed in nearly all the hypothalamic areas. Deletion of leptin receptors, such as POMC and NPY/AgRP, in neurons can cause hyperphagia and obesity in mice. Spexin is involved in the action of hypothalamic leptin on POMC gene expression to control feeding behavior (Jeong et al. Citation2022). Moreover, spexin plays a crucial role in the regulation of leptin secretion and resistance, and crosstalk between spexin and leptin can occur in patients with obesity and diabetes (Yu et al. Citation2022). This crosstalk may explain why the spexin-based GALR2 agonist did not affect the metabolic parameters of the leptin receptor-deficient diabetic animals in our experiment. We did not investigate the interaction between spexin and leptin; however, we demonstrated the beneficial effect of a spexin-based GALR2 agonist on diabetic kidneys. Interestingly, the spexin-based GALR2 agonist acts on diabetic nephropathy through its antifibrotic action. Indeed, we demonstrated that, in diabetic nephropathy, the spexin-based GALR2 agonist significantly inhibited TGFβ1 and PI3 K/AKT/ERK activation. Taken together, these results suggest that the protective effects of spexin-based GALR2 agonists occur through an antifibrotic pathway related to the renal insulin signaling pathway.
Spexins are newly identified neuropeptides that regulate energy homeostasis. Spexins alter the hypothalamic feeding circuits, leading to anorexia. Spexin increases the hypothalamic expression of POMC, leptin receptors, and melanocortin receptors (Wong et al. Citation2013; Walewski et al. Citation2014). In human studies, spexin levels are lower in patients with type 1 or 2 diabetes and obesity than in controls (Gu et al. Citation2015; Karaca et al. Citation2018; Kolodziejski et al. Citation2018). Microarray studies on fat biopsies have revealed that spexin is the most frequently down-regulated gene in the fat of patients with obesity (Kumar et al. Citation2016). In contrast, pregnant women with diabetes present high plasma spexin levels. In our preliminary study, plasma spexin levels were higher in diabetic mice than non-diabetic controls, even when compared to other renal disease models, such as unilateral ureteral obstruction mice (not shown). In this study, spexin gene expression was also increased in diabetic kidneys. However, it is unclear whether the elevated spexin expression is a compensatory response or an epiphenomenon. This study had some limitations regarding the role of spexins in diabetic nephropathy. We did not observe spexin protein expression in diabetic nephropathy. Spexin is primarily expressed in the brain; however, its role in the kidneys remains unknown. In addition, spexin gene expression in the diabetic kidneys was suppressed by NS200. Interestingly, despite being a selective GALR2 agonist, NS200 did not induce any significant changes in GALR2 gene expression. This suggest that NS200 influence spexin gene expression through an alternative pathway.
Galanin is widely distributed throughout the central nervous system and in peripheral tissues. GALR2 genes is highly expressed in the heart, kidney, liver, hypothalamus, small intestine, and hippocampus but not in the cerebral cortex, adrenal gland, lung, lymph node, pituitary, spleen, and stomach (Borowsky et al. Citation1998). A recent study reported that the expression of GALR2 was inhibited in the mesenteric arteries of spontaneously hypertensive and stroke-prone spontaneously hypertensive rats (Ikawa et al. Citation2019).
In our study, GALR2 gene expression did not differ among control, diabetic, and spexin-based GALR2 agonist-treated diabetic mice. These results suggest that the effect of the spexin-based GALR2 agonist on diabetic nephropathy acts independent of the GALR2 and G-protein coupling signaling pathways. Furthermore, the spexin-based GALR2 agonist inhibited renal insulin signaling pathways, such as PI3 K/AKT/ERK, although the mechanism remains poorly understood. There are limited data regarding the effect of GALR2 on insulin resistance in various organs, such as the heart, liver, skeletal muscle, and adipose tissues, in obesity and diabetes (Kim and Park Citation2010; Fang et al. Citation2018). One study reported that GALR2 activation attenuates insulin resistance in the skeletal muscles of obese mice (Fang et al. Citation2018). That activation of GALR2 alleviated insulin resistance through the p38 mitogen-activated protein kinase/PGC-1α/GLUT4 and AKT/AKT substrate 160/GLU4 T pathways in the skeletal muscle of mice. However, there are no reports on the effect of GALR2 activation on insulin signaling in kidney injury. We demonstrated the effect of a GALR2 agonist on insulin resistance in diabetic kidneys despite the paucity of data on the systemic role of this new drug. Further studies on the specific mechanism of GALR2 activation to ameliorate diabetic nephropathy regarding the renal insulin signaling pathway are necessary.
In conclusion, spexin-based GALR2 agonists improved diabetic nephropathy without altering metabolic syndrome parameters. Our results suggest that, in addition to its antidepressant and anxiolytic effects, the spexin-based GALR2 agonist can provide a new class of therapeutic targets in diabetic nephropathy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alkhatib L, Velez Diaz LA, Varma S, Chowdhary A, Bapat P, Pan H, Kukreja G, Palabindela P, Selvam SA, Kalra K. 2023. Lifestyle modifications and nutritional and therapeutic interventions in delaying the progression of chronic kidney disease: a review. Cureus. 15(2):e34572.
- Behrooz M, Vaghef-Mehrabany E, Maleki V, Pourmoradian S, Fathifar Z, Ostadrahimi A. 2020. Spexin status in relation to obesity and its related comorbidities: a systematic review. J Diabetes Metab Disord. 19(2):1943–1957. doi:10.1007/s40200-020-00636-8.
- Borowsky B, Walker MW, Huang LY, Jones KA, Smith KE, Bard J, Branchek TA, Gerald C. 1998. Cloning and characterization of the human galanin GALR2 receptor. Peptides. 19(10):1771–1781. doi:10.1016/S0196-9781(98)00133-8.
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE et al. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 84(3):491–495. doi:10.1016/S0092-8674(00)81294-5.
- Fang P, Zhang L, Yu M, Sheng Z, Shi M, Zhu Y, Zhang Z, Bo P. 2018. Activiated galanin receptor 2 attenuates insulin resistance in skeletal muscle of obese mice. Peptides. 99:92–98. doi:10.1016/j.peptides.2017.11.018.
- Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, et al. 2019. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 139(22):2516–2527. doi:10.1161/CIRCULATIONAHA.119.039996.
- Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, Pozzilli P, Gesty-Palmer D, Lapuerta P, Simo R, et al. 2017. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 377(24):2337–2348. doi:10.1056/NEJMoa1708337.
- Gu L, Ma Y, Gu M, Zhang Y, Yan S, Li N, Wang Y, Ding X, Yin J, Fan N, et al. 2015. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides. 71:232–239. doi:10.1016/j.peptides.2015.07.018.
- Ikawa T, Watanabe Y, Okuzaki D, Goto N, Okamura N, Yamanishi K, Higashino T, Yamanishi H, Okamura H, Higashino H. 2019. A new approach to identifying hypertension-associated genes in the mesenteric artery of spontaneously hypertensive rats and stroke-prone spontaneously hypertensive rats. J Hypertens. 37(8):1644–1656. doi:10.1097/HJH.0000000000002083.
- Jeong B, Kim KK, Lee TH, Kim HR, Park BS, Park JW, Jeong JK, Seong JY, Lee BJ. 2022. Spexin regulates hypothalamic leptin action on feeding behavior. Biomolecules. 12(2). doi:10.3390/biom12020236.
- Jeong I, Kim E, Seong JY, Park HC. 2019. Overexpression of spexin 1 in the dorsal habenula reduces anxiety in zebrafish. Front Neural Circuits. 13:53. doi:10.3389/fncir.2019.00053.
- Kang YS, Ko GJ, Lee MH, Song HK, Han SY, Han KH, Kim HK, Han JY, Cha DR. 2008. Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol Dial Transplant. 24(1):73–84. doi:10.1093/ndt/gfn448.
- Kang YS, Lee MH, Song HK, Ko GJ, Kwon OS, Lim TK, Kim SH, Han SY, Han KH, Lee JE, et al. 2010. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 78(9):883–894. doi:10.1038/ki.2010.263.
- Karaca A, Bakar-Ates F, Ersoz-Gulcelik N. 2019. Decreased spexin levels in patients with type 1 and type 2 diabetes. Med Princ Pract. 27(6):549–554. doi:10.1159/000493482.
- Kato M, Natarajan R. 2019. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 15(6):327–345. doi:10.1038/s41581-019-0135-6.
- Kim A, Park T. 2010. Diet-induced obesity regulates the galanin-mediated signaling cascade in the adipose tissue of mice. Mol Nutr Food Res. 54(9):1361–1370. doi:10.1002/mnfr.200900317.
- Kim DK, Yun S, Son GH, Hwang JI, Park CR, Kim JI, Kim K, Vaudry H, Seong JY. 2014. Coevolution of the spexin/galanin/kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology. 155(5):1864–1873. doi:10.1210/en.2013-2106.
- Kim S, Lim SW, Choi J. 2022. Drug discovery inspired by bioactive small molecules from nature. Anim Cells Syst (Seoul). 26(6):254–265. doi:10.1080/19768354.2022.2157480.
- Kolodziejski PA, Pruszynska-Oszmalek E, Korek E, Sassek M, Szczepankiewicz D, Kaczmarek P, Nogowski L, Mackowiak P, Nowak KW, Krauss H, et al. 2018. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res. 67(1):45–56. doi:10.33549/physiolres.933467.
- Kumar S, Hossain J, Nader N, Aguirre R, Sriram S, Balagopal PB. 2016. Decreased circulating levels of spexin in obese children. J Clin Endocrinol Metab. 101(7):2931–2936. doi:10.1210/jc.2016-1177.
- Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, Rossetti L. 1998. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 273(47):31160–31167. doi:10.1074/jbc.273.47.31160.
- Liu Y, Ozodiegwu ID, Yu Y, Hess R, Bie R. 2017. An association of health behaviors with depression and metabolic risks: Data from 2007 to 2014 U.S. national health and nutrition examination survey. J Affect Disord. 217:190–196.
- Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, et al. 2018. Canagliflozin for primary and secondary prevention of cardiovascular events. Circulation. 137(4):323–334. doi:10.1161/CIRCULATIONAHA.117.032038.
- Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS. 2021. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol. 17(2):97–113. doi:10.1038/s41574-020-00438-1.
- Mistry N, Bakris GL. 2023. The changing trajectory of diabetic kidney disease. Curr Opin Nephrol Hypertens. 32(1):98–102. doi:10.1097/MNH.0000000000000844.
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. 2005. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2(6):411–420. doi:10.1016/j.cmet.2005.10.009.
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. 2017. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 377(7):644–657. doi:10.1056/NEJMoa1611925.
- Niswender KD, Schwartz MW. 2003. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 24(1):1–10. doi:10.1016/S0091-3022(02)00105-X.
- Park S, Williams KW, Sohn JW. 2022. Leptin-inhibited neurons in the lateral parabrachial nucleus do not alter food intake or glucose balance. Anim Cells Syst (Seoul). 26(3):92–98. doi:10.1080/19768354.2022.2084159.
- Reyes-Alcaraz A, Lee YN, Son GH, Kim NH, Kim DK, Yun S, Kim DH, Hwang JI, Seong JY. 2016. Development of spexin-based human galanin receptor type II-specific agonists with increased stability in serum and anxiolytic effect in mice. Sci Rep. 6:21453. doi:10.1038/srep21453.
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S, et al. 2004. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 364(9438):953–962. doi:10.1016/S0140-6736(04)17019-0.
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al. 1995. Identification and expression cloning of a leptin receptor, OB-R. Cell. 83(7):1263–1271. doi:10.1016/0092-8674(95)90151-5.
- Walewski JL, Ge F, Lobdell H, Levin N, Schwartz GJ, Vasselli JR, Pomp A, Dakin G, Berk PD. 2014. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity (Silver Spring). 22(7):1643–1652. doi:10.1002/oby.20725.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. 2019. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 380(4):347–357. doi:10.1056/NEJMoa1812389.
- Wong MK, Sze KH, Chen T, Cho CK, Law HC, Chu IK, Wong AO. 2013. Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am J Physiol Endocrinol Metab. 305(3):E348–366. doi:10.1152/ajpendo.00141.2013.
- Yu M, Ju M, Fang P, Zhang Z. 2022. Emerging central and peripheral actions of spexin in feeding behavior, leptin resistance and obesity. Biochem Pharmacol. 202:115121. doi:10.1016/j.bcp.2022.115121.
- Yun S, Reyes-Alcaraz A, Lee YN, Yong HJ, Choi J, Ham BJ, Sohn JW, Kim DH, Son GH, Kim H, et al. 2019. Spexin-based galanin receptor type 2 agonist for comorbid mood disorders and abnormal body weight. Front Neurosci. 13:391. doi:10.3389/fnins.2019.00391.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. 2015. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 373(22):2117–2128. doi:10.1056/NEJMoa1504720.
