ABSTRACT
Parental care strategies, ranging from biparental to uniparental, evolve based on factors affecting sexual conflict over care. Plasticity in how parents respond to reduction in each other’s care effort is thus proposed to be important in the evolution of parental care behaviors. Models predict that ‘obligate’ biparental care is stable when a parent responds to reduced partner effort with ‘partial’ compensation, trading-off current and future reproduction. A meta-analysis of experimental studies on biparental birds also revealed partial compensation, supporting coevolution of parental care type and plasticity pattern. However, few studies have addressed this issue across different taxa and different parental care types. In laboratory mice, a female-biased ‘facultative’ biparental species, fathers paired with a competent mother rarely provide care. We show that, when mated with a pup-neglecting mutant mother, fathers increased care effort to ‘fully’ compensate for the lost maternal care in both pup survival rate and total care amount. Pup retrieval latency was significantly shorter, and neural activity in relevant brain regions twice as high, suggesting enhanced motivation. This study with mice not only opens a road to explore the neural correlates of paternal plasticity but will also help understand how behavioral plasticity contributes to adaptive evolution of parental care behaviors.
Introduction
Parental care strategy (mode, pattern, system, type) refers to the extent of parental cooperation or the degree of asymmetry in the relative contribution of the sexes to parental care. It ranges from uniparental (male or female) to facultative biparental (male-biased or female-biased) to obligate biparental care. In obligate biparental species, for example, the two parents care for their young in approximately equal shares (e.g. many birds, prairie voles, California mice). Parental care strategies are evolutionary outcome of conflict of interest between the male and female partners (sexual conflict) over parental investment (Olson et al. Citation2008; McNamara and Wolf Citation2015; Remes et al. Citation2015). To maximize lifetime reproductive success, parents trade-off between current and future reproduction. Then there is the question of behavioral plasticity in how much a parent should increase care when its partner reduces care effort (e.g. injury, disease, desertion, death, etc.) to compensate the loss. In this vein, game-theoretical models predicted that obligate biparental care is evolutionarily stable when parents only ‘partially’ compensate for reduced care by their partner (McNamara et al. Citation1999; McNamara et al. Citation2003; Houston et al. Citation2005). A meta-analysis of experimental studies on biparental birds using mate removal and/or handicapping showed that the compensatory increase in parental care behavior of one parent in response to a reduction in the partner’s parental contribution was ‘partial’ compensation, consistent with the prediction by the models (Harrison et al. Citation2009). It is proposed that selection may favor particular combinations of parental care type and plasticity pattern, leading to coadaptation (Royle et al. Citation2014). Although studies on biparental wild birds provided important insights into the coevolution of parental care type and plasticity pattern, few studies tested the hypothesis across a broad range of taxa or across a continuum of parental cooperation. Evidence of the evolution of different types of plasticity (e.g. zero, partial or full compensation) in different parental care strategies may help to vindicate the coevolution, which encourages comparative analyses of empirical data from diverse systems.
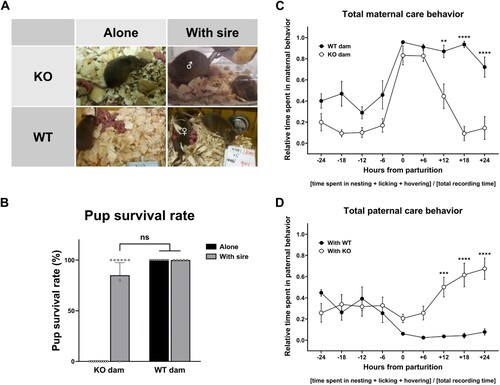
In laboratory mice, which is a female-biased, facultative biparental species, fathers’ relative contribution to offspring care is considered meager and uninfluential under most ordinary conditions (Priestnall and Young Citation1978). Mouse fathers appear to have direct or indirect effects on pup survival (Barnett and Dickson Citation1985; Wright and Brown Citation2000), as well as on their partner’s maternal care behavior, indirectly affecting the physical and behavioral development of the litter (Mashoodh et al. Citation2012; Korgan et al. Citation2016; Korgan et al. Citation2018). However, mouse mothers are generally self-sufficient to rear their young without partner contribution (Kim et al. Citation2022) ((A,B)). To investigate the paternal response to reduced partner care, the present study used mutant female mice as pup-neglecting mothers. Phospholipase Cβ1 (PLCβ1) knockout (KO) dams care for their pups as well as wild-type (WT) dams during the maternal onset phase right after parturition. However, they neglect pups from about 18 h postpartum (PPH 18); thus, if left alone as a single parent without their mate, they lose all pups to death in 2–3 days after parturition (Kim et al. Citation2022). We found that sires mated with a KO dam increase care effort to compensate for the lost maternal care in terms of both pup survival rate and total care to a comparable level to controls. This result demonstrates paternal plasticity in laboratory mice where fathers increase care in response to reduced care by the mother. It provides an example of ‘full’ compensation by the increased contribution of parental care from the less-caring sex, which responds to the reduction of care from the main caregiver in the female-biased facultative biparental care system. This mouse study aims to provide the basis for a future exploration of the neural correlates of paternal plasticity, and will help understand the contribution of behavioral plasticity to the adaptive evolution of parental care behaviors.
Figure 1. Wild-type sires mated with a pup-neglecting PLCβ1-KO dam (KO) increased care effort to compensate for reduced dam care and avoid total brood loss. (A) Snapshots at about PPH 24 of: KO dam alone neglecting pups that are left scattered all over the floor (top left); wild-type sire (♂) hovering over pups while the KO dam sleeps away (top right); wild-type dam (WT) alone licking pups that are grouped (bottom left); WT dam (♀) nursing pups while the sire does not do much work (bottom right). (B) Pup survival rates for a KO or WT dam alone (black), and either dam with its wild-type male partner (sire) remaining in the mating cage (gray). (C, D) Total maternal and paternal care behavior in ‘sire with WT dam’ pairs (With WT, black circle) and ‘sire with KO dam’ pairs (With KO, white circle), measured every 6 h during the 48-h peripartum period. All values are Mean ± SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001; ns indicates ‘not significant’.
Materials and methods
Animals
PLCβ1 WT and KO female and WT male mice (Mus musculus) were obtained by mating C57BL/6J (N30) PLCβ1+/– and 129S4/SvJae (N40) PLCβ1+/– mice (Kim and Koh Citation2016). All sires used in mating pairs were WT. Subjects were maintained on a 12:12 – light/dark schedule (light on at 8:30 AM). All experimental protocols using animals followed the institutional guidelines and regulations of Korea Institute of Science and Technology (KIST) and were approved by the Institutional Animal Care and Use Committee (IACUC) of KIST (AP201149).
Pup survival rate
Pup survival rate was calculated by dividing the number of pups weaned 3 weeks after parturition by the number of pups born in each mating cage. We examined pups reared under these four different parental conditions: a single KO (N = 10) or WT (N = 10) dam whose WT male mate was removed from the mating cage at about day 14 of pregnancy; a KO (N = 8) or WT (N = 5) dam with its WT male mate (sire) remaining in the mating cage.
Recording and analysis of maternal and paternal care behavior
The basic protocol was previously described (Kim et al. Citation2022). Virgin females 15–20 weeks old (WT, N = 10; KO, N = 11) were mated with WT virgin males 20–30 weeks old to video-record the parental care behaviors of the two members from each pair. In the present study, grooming (self-grooming) was not included in the total maternal (or paternal) care behavior equation: therefore, ‘relative time spent in total maternal (or paternal) behavior’ is defined here as [time spent in nesting + licking + hovering] / [total recording time]. The data were obtained every 6 h during the 48-h recording period (24 h before and after parturition) ((C,D)). Measurable events were only those behavioral events where one parameter was performed longer than 3 s. The total recording time at every time point was 10 min, but the 10-min window was shifted in either direction in cases when sleep occurred around the designated time point. Numbers of mice (N) used at each time point are: (WT dam: −24, N = 8; −18, N = 8; −12, N = 8; −6, N = 8; + 0, N = 10; + 6, N = 9; + 12, N = 7; + 18, N = 7; + 24, N = 7), (KO dam: −24, N = 9; −18, N = 9; −12, N = 9; −6, N = 9; + 0, N = 11; + 6, N = 11; + 12, N = 8; + 18, N = 9; + 24, N = 9).
To analyze paternal plasticity and decide the compensation type, ‘sire care’ and ‘total care’ (sum of dam care and sire care in a pair) were obtained as the relative time spent in nesting, licking, or hovering, during the 10-min periods at PPH 12, 18, and 24. This sampling method was based on the observation that typically the paternal plastic response peaks at around PPH 24; thereafter, the activities get gradually rarer and shorter as pups grow and mature. Data at PPH 12, 18 and 24 with significant differences between the two groups ((C,D)) represent the relative time spent in each parameter during the most active period. Numbers of mice (N) used are: (With WT dam: N = 7), (With KO dam: N = 8).
Pup retrieval test (PRT)
The basic procedure of the pup retrieval test (PRT) was described previously (Kim et al. Citation2022). Two groups of nine sires mated with either WT or KO dams were tested during the period within 12 h postpartum (PPH12) or after PPH 18 (PPH18). A sire was placed alone first with nesting material scattered around in the home cage for 1 h to build a nest. Three of its pups were then placed at the three corners for the sire to finish retrieving all pups to the nest within 10 min. The latency for the sire to pick and carry each pup to the nest was measured. Numbers of mice (N) used are: With WT PPH12, N = 7; With WT PPH18, N = 5; With KO PPH12, N = 3; With KO PPH18, N = 8.
Immunohistochemistry for FosB expression in the medial prefrontal cortex, nucleus accumbens and medial preoptic area
Brains from sires whose behavior was monitored and not asleep during the 60–120 min period prior to euthanasia were sampled at around PPH 24. The basic histology procedure was previously described (Kim et al. Citation2022). After transcardial perfusion, sampled brains were fixed in 10% formalin for 24 h. Fixed brains were infiltrated with 30% sucrose overnight, frozen, and sectioned into 40 µm thick sections. Consecutive sections were placed in six-well plates containing PBS, floated in 3% H2O2 in methanol for 10 min, and later in PBS with 0.3% Triton X-100. After treatment with blocking serum in PBS for 1 h, the sections were incubated with a polyclonal antibody to the FosB protein (Santa Cruz Biotechnology; diluted 1:1000) in blocking serum at 4°C for 3 days. After washing in PBS, sections were incubated in the biotinylated secondary antibody, anti-rabbit IgG (VECTASTAIN® Elite ABC kit) for 1 h, followed by an avidin and biotinylated horseradish peroxidase macromolecular complex (VECTASTAIN® Elite ABC kit) for 1 h. After rinsing in PBS, sections were mounted on subbed slides and cover-slipped with mounting medium (VECTOR, VectaMount). The medial prefrontal cortex (mPFC), nucleus accumbens (NAcc) and medial preoptic area (mPOA) were collected by a systematic and random sample of sections according to a brain atlas. Every 6–8th section from bregma 1.98∼1.42 mm for the mPFC, 1.54∼0.74 mm for the NAcc, and 0.14∼ −0.1 mm for the mPOA, provided on average three random sections for each area per animal. Each brain area was photographed on a microscope equipped with a digital camera (Olympus, Tokyo, Japan). The number of FosB-positive cells within each brain area was counted using Image-J free software (NIH, MD, United States). Bilateral counts per square mm from each section constituted a data point. Damaged sections or those where it was difficult to count stained cells were excluded. Numbers of mice (N) and sections (n) used are: (With WT: mPFC, N = 14, n = 38; NAcc, N = 19, n = 51; mPOA, N = 8, n = 22), (With KO: mPFC, N = 9, n = 24; NAcc, N = 8, n = 21; mPOA, N = 3, n = 9).
Statistical analysis
The same software and methods were used as in the previous study (Kim et al. Citation2022). Data were analyzed using GraphPad Prism 7.03. Data for (C,D) were analyzed using a repeated analysis of variance (ANOVA), and post hoc comparisons were performed using a Tukey’s or Sidak’s multiple comparisons test. Comparisons between the two groups were performed using a t-test. All data are expressed as mean ± SEM. p values less than 0.05 were considered statistically significant.
Results
Sires increased care to fully compensate for reduced dam care
With a PLCβ1-KO dam alone as a single parent, all pups die within a couple of days after birth (Kim et al. Citation2022). First, we examined whether sire presence can affect pup survival by measuring the weaning rate for pups reared by: a KO or WT dam alone, and either dam with its WT male partner (sire) remaining in the mating cage. Snapshots at PPH 24 showed that pups reared by KO dams with WT sire were grouped in the nest, while those reared by single KO dams were scattered all over ((A)). When the WT sire was present, the pup weaning rate for KO dams (85 ± 15%) was not significantly different from those for WT dams (100%) (Alone, t(16) = 1.364, p = 0.1915; With sire, t(11) = 0.9410, p = 0.3669; t-test) ((B)). This result suggests that sires improved pup survival, presumably by providing missing care from pup-neglecting KO dams. To decide whether and to what extent sires increase their care activities in response to reduced dam care, we examined the overall parental care behaviors of both dams and sires in mating pairs by video-recording them from late pregnancy to a couple days after parturition. Plotting the relative time spent on total maternal care behavior (nesting, licking, and hovering) every 6 h from 24 h prepartum to PPH 24 showed a normal onset of maternal behavior followed by a sharp decrease until the beginning of neglect at PPH 18 in KO dams ((C)). The overall shape of these time courses is similar to that of total maternal behavior observed in single dams, even though grooming (self-grooming) was not included in the total maternal care behavior equation (Kim et al. Citation2022). An ANOVA [(genotype – WT, KO) X (time – nine-time points from parturition)] on total maternal behavior found main effects of genotype [F(1,138) = 90.16, p < 0.0001] and time [F(8,138) = 24.71, p < 0.0001], and a significant genotype X time interaction [F(8,138) = 4.925, p < 0.0001]. Post hoc comparisons identified significant differences between WT and KO at PPH 12 (p < 0.01), 18, and 24 (ps < 0.0001). Plotting the total paternal behavior indicated that sires paired with a KO dam (With KO) were becoming more active than sires paired with a WT dam (With WT) during PPH 12 ∼ 24 ((D)). An ANOVA [(dam – WT, KO) X time] for total paternal behavior found main effects of dam [F(1,138) = 35.93, p < 0.0001] and time [F(8,138) = 3.207, p < 0.0023], and a significant dam X time interaction [F(8,138) = 6.749, p < 0.0001]. Post hoc comparisons identified significant differences between With WT and With KO groups at PPH 12 (p < 0.001), 18, and 24 (ps < 0.0001). The overall shape of the time courses of sires’ paternal behavior appears complementary to that of their mates’ maternal behavior plots: sires increased care behavior while their KO mates decreased theirs, and stayed inactive when their WT mates maintained a higher activity, which suggests the existence of sires’ compensatory-like responses to their partner’s care behavior. One-way ANOVA for the total maternal and total paternal behavior in the With WT group at PPH 6 ∼ 24 found no effect of time [maternal, F(3, 26) = 2.813, p = 0.059; paternal, F(3, 26) = 1.289, p = 0.2992] ((C,D)), nor did any of the three parameters change significantly, but With KO group sires increased nesting activity while their KO mates decreased theirs ().
Figure 2. Parametric details of the total maternal and total paternal behavior of With WT and With KO groups at PPH 6 ∼ 24 ((C,D)). One-way ANOVAs for nesting, licking and hovering of With WT group found no effect of time [♀ maternal: nesting, F(3, 26) = 0.5175, p = 0.6739; licking, F = 1.435, p = 0.2553; hovering, F = 0.2074, p = 0.8903], [♂ paternal: nesting, F = 0.6373, p = 0.5978; licking, F = 0.8220, p = 0.4936; hovering, F = 0.7993, p = 0.5055]. One-way ANOVAs of KO dams (♀) found a main effect of time for nesting [F(3, 33) = 16.81, p < 0.0001] and hovering [F = 4.917, p < 0.05], but no effect for licking [F = 1.869, p = 0.1540]. In With KO group sires (♂), there was a main effect of time only for nesting [F = 3.069, p < 0.01], [licking, F = 0.7528, p = 0.5286; hovering, F = 2.476, p = 0.0786].
![Figure 2. Parametric details of the total maternal and total paternal behavior of With WT and With KO groups at PPH 6 ∼ 24 (Figure 1(C,D)). One-way ANOVAs for nesting, licking and hovering of With WT group found no effect of time [♀ maternal: nesting, F(3, 26) = 0.5175, p = 0.6739; licking, F = 1.435, p = 0.2553; hovering, F = 0.2074, p = 0.8903], [♂ paternal: nesting, F = 0.6373, p = 0.5978; licking, F = 0.8220, p = 0.4936; hovering, F = 0.7993, p = 0.5055]. One-way ANOVAs of KO dams (♀) found a main effect of time for nesting [F(3, 33) = 16.81, p < 0.0001] and hovering [F = 4.917, p < 0.05], but no effect for licking [F = 1.869, p = 0.1540]. In With KO group sires (♂), there was a main effect of time only for nesting [F = 3.069, p < 0.01], [licking, F = 0.7528, p = 0.5286; hovering, F = 2.476, p = 0.0786].](/cms/asset/9aabf987-4f88-4fe1-8891-f8496a8770f1/tacs_a_2266006_f0002_ob.jpg)
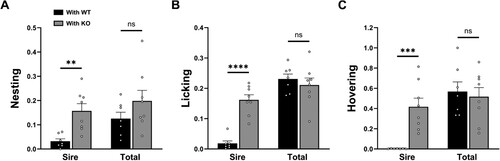
To determine how much sires increase their care behavior in response to reduced care by their partner, which indicates the plasticity pattern (e.g. partial or full compensation), we compared both sire care and total care between With WT and With KO groups. Sire care and total care were obtained as relative time spent in nesting, licking or hovering, during the 10-min periods at PPH 12, 18 and 24, measured from the video-recording data of the experiments done for (C,D). Sire’s nesting (t = 3.800, p < 0.01), licking (t = 7.356, p < 0.0001), and hovering (t = 4.571, p < 0.001) in the With KO group were 5–174 fold higher than in the With WT group (df = 13, t-test, ). There was no significant difference in total care for nesting (t = 1.376, p = 0.1922), licking (t = 0.6751, p = 0.5114), and hovering (t = 0.3946, p = 0.6995) between groups (df = 13, t-test, ). These results show that, in response to reduced partner care, sires increased their care sufficiently for the total care to match that provided by WT control pairs, demonstrating a full compensation.
Figure 3. Sires increased care to fully compensate for reduced dam care. Sire care and total care (sum of dam care and sire care in a mating pair) obtained as the relative time spent in nesting (A), licking (B) or hovering (C), during the 10-min periods at PPH 12, 18 and 24, measured from the video-recording data of the experiments done for (C,D) compared between ‘sire with WT dam’ pairs (With WT, black) and ‘sire with KO dam’ pairs (With KO, gray). All values are Mean ± SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001; ns indicates ‘not significant’.
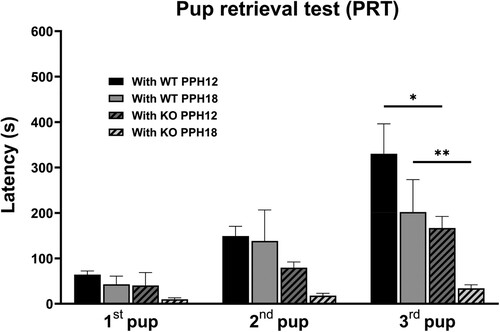
Sires paired with a KO dam retrieved pups faster on PRT
As shown in , sires paired with a KO dam were in close contact with pups most of the time. Casual inspection of the overall behavior of sires paired with a KO dam gave the impression that they were highly aroused and generally motivated, in contrast to sires paired with a WT dam that were mostly inactive. Further, they showed some highly motivated behaviors never observed in sires paired with a WT dam. For example, when a KO dam was sleeping away from the pups in the nest, they (likely responding to specific pup calls) carried and transported the pups to place them right under the KO dam’s belly to suckle, and worked to build a new nest surrounding the newly-located pups with their KO dam (). To address whether enhanced motivation is involved in paternal plasticity, we performed a pup retrieval test (PRT) to compare between sire groups. Shorter latency to retrieve pups is indicative of higher parental motivation.
Figure 4. Sequential cut images of a sire in successive motions while he relocates nest site at the spot where the KO dam is sleeping (PPH 20). KO dam was sleeping alone away from the pups grouped in the old nest (under the water bottle). The sire (♂, likely responding to pups’ specific calls) carries and transports pups to place them right under the KO dam’s belly in order for them to be able to suckle, while simultaneously working diligently to build a new nest surrounding the pups newly-located now with their KO dam. Between 21:28 and 33:15, a more detailed 4-s sequence (right, 26:24 ∼ 26:28) is inserted. At 33:15, a pup dropped at the center in the middle of the pup-transport process that was soon to be retrieved to the sleeping KO dam. At 56:47, KO dam (♀) hovering over and nursing pups in the new nest.

Both during the period within PPH 12 (samples at PPH 6∼12) and after PPH 18 (samples at around PPH 24), With KO group sires completed retrieving all three pups significantly faster than With WT group (). An ANOVA [(dam – WT, KO) X (time – PPH12, PPH18)] for latency found main effects of dam [F(1,19) = 8.967, p < 0.01] and time [F(1,19) = 5.554, p < 0.05], but no dam X time interaction [F(1,19) = 0.0017, p = 0.9680]. Post hoc comparisons identified significant differences in these pairs: 3rd pup, With WT PPH18 vs With KO PPH18 (p < 0.01), With WT PPH12 vs With KO PPH12 (p < 0.05). The 3rd pup latency of With KO group sires after PPH 18 (33.88 ± 7.85, Mean ± SEM) was even shorter (t = 2.383, df = 17, p < 0.05, t-test) than that of the competent WT single dams in our previous study (172.5 ± 48.92) (Kim et al. Citation2022), which suggests an unusually heightened paternal motivation.
Increased neural activity in relevant brain regions of sires paired with KO dam
The strong paternal plasticity indicated by the full compensation observed in our experiments suggests increased activity in a paternal neurocircuitry flexibly modulated by environmental factors. In mothers, activity in the medial preoptic area of hypothalamus (mPOA) and its projections sensitizes the subcortical mammalian parenting network (Numan and Stolzenberg Citation2009; Dobolyi et al. Citation2014; Kohl et al. Citation2018). mPOA projections to the nucleus accumbens (NAcc) and the mesolimbic dopamine reward system are involved in maternal motivation (Lee et al. Citation2000; Numan Citation2006; Brunton and Russell Citation2008; Numan and Stolzenberg Citation2009). The medial prefrontal cortex (mPFC) is proposed to function as an executive control system for parental behavior (Li Citation2022), and fMRI studies on human fathers show it as a main cortical network for mentalization (Provenzi et al. Citation2021).
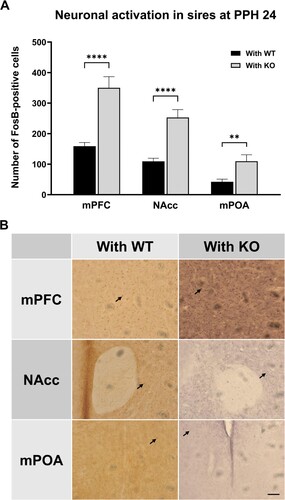
To find the neural basis for paternal plasticity responding to reduced partner care, we first compared neural activation levels in the mPOA, NAcc, and mPFC of With KO and With WT groups. FosB expression was measured at around PPH 24, at which time point sires’ plastic response to reduced dam care appears to be stabilized at its peak. The immediate early gene product FosB has been used as a neural activity marker in reward and addiction systems (Hope et al. Citation1992; Perrotti et al. Citation2004; Harris et al. Citation2007; Gajewski et al. Citation2016) and is also known to act as a major signal in parental behavior (Kuroda et al. Citation2007; Kuroda et al. Citation2008). At PPH 24, FosB expression was about twofold higher in all three regions of the With KO group than in those of the With WT group (mPFC, t(22) = 6.304, p < 0.0001; NAcc, t(23) = 6.298, p < 0.0001; mPOA, t(8) = 3.670, p < 0.01) (t-test, ). Interestingly, activity levels in the NAcc and mPOA of With KO group sires at PPH 24 were similar (NAcc, t(48) = 0.5192, p = 0.6060; mPOA, t(18) = 0.4459, p = 0.6610; t-test) to the counterparts of the competent WT single dams reported in our previous study (Kim et al. Citation2022). In addition, activity level in the mPFC of With KO group sires (350.3 ± 36.26) was significantly higher (t = 3.910, df = 39, p < 0.001, t-test) than that of WT single dams (219.0 ± 14.08) (Kim et al. Citation2022). This pronounced mPFC activity in With KO group sires seems associated with the paternal behavior shown in that proactively places pups under the KO dam sleeping away, which might require executive and empathy-like functions as well as heightened motivation.
Figure 6. Increased neural activity in relevant brain regions of sires paired with KO dam. (A) Number of FosB-positive cells (counts per square mm from each section) in the medial preoptic area (mPOA), nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC) of sires paired with WT dam (With WT, black) and those paired with KO dam (With KO, gray) at PPH 24. (B) Representative images of FosB immunohistochemistry for the six groups from (A). Arrows indicate the stained cells representing those included in the counts. Calibration bar is 100 µm. All values are Mean ± SEM. **p < 0.01, ****p < 0.0001.
Discussion
Toward unveiling neural correlates of paternal plasticity
Over the course of mating and cohabitation with a female during pregnancy and parturition, male mice undergo physiological changes in their brain to become fathers. Males have mating-induced transition from infanticide to parental care (Labov Citation1980; Elwood Citation1985; vom Saal Citation1985) possibly through the reduction of vomeronasal sensory activation by pup cues (Tachikawa et al. Citation2013; Wu et al. Citation2014). Once parental, they display care behaviors similar to those of mothers except for nursing, until pup weaning (Priestnall and Young Citation1978; vom Saal Citation1985; Tachikawa et al. Citation2013). Due to the relatively low level of mouse paternal care under ordinary conditions with normal female mates, the neuroanatomical and hormonal mechanisms of the paternal brain have been studied mainly in obligate biparental rodents (Bales and Saltzman Citation2016; Feldman et al. Citation2019). The present study reports paternal plasticity in mice whereby fathers respond by working harder when mother’s caregiving is reduced, and demonstrates that paternal care behavior can be dynamically modulated to a relatively high degree, which is adaptive enough to compensate completely for fatal family/social environment such as a pup-neglecting mother. Given this strong paternal plasticity in mice, better approaches to mice paternal care behavior itself will now be available by experimentally reducing the level of female partner’s care to amplify paternal care behavior so as to be studied. Experimental manipulation of dam care level could be done by using known genetic mouse models of poor maternal behavior or other non-genetic methods.
Further to ask in our study is: whether sires respond directly to dam’s poor care behavior or indirectly due to the resultant offspring condition and/or behavior, or both; how partner’s care behavior is perceived by sire for the adaptive level of plasticity to occur. As to the neural correlates of paternal plasticity in mice, among candidate neural circuits that our future studies should look at could be those around galanin-positive mPOA neurons (mPOAGal) (Wu et al. Citation2014; Kohl et al. Citation2018), since they seem to integrate internal and external signals to coordinate multiple components of parenting. We pay attention especially to mPOAGal neurons presynaptic to the paraventricular nucleus neurons expressing arginine vasopressin (PVNAVP) or corticotropin-releasing factor (PVNCRF), as they are significantly more abundant in males than in females (Kohl et al. Citation2018). Besides, our preliminary experiments in With KO and With WT group sires showed differential time courses of CRF expression in the PVN during the first day postpartum. Moreover, postpartum upregulation of the calcitonin receptor (Calcr) in the central mPOA neurons is known to be involved in the heightened maternal motivation to retrieve pups under risky conditions (Yoshihara et al. Citation2021). The unusually short pup retrieval latency in With KO group sires suggests heightened paternal motivation; we are currently testing if they can retrieve pups on elevated plus-maze (Ret-EPM test), and whether Calcr expression is upregulated in the central mPOA neurons compared to With WT group.
Plasticity patterns and parental care strategies
The present study provides an example of parental plasticity where the ‘less-caring’ father increases care to fully compensate for reduced care by the ‘main-caring’ mother in a female-biased facultative biparental species. A similar yet stronger plasticity is observed in the poison frog Allobates femoralis, where the obligatory tadpole transport is performed normally by males alone while the female partner leaves immediately after oviposition to stay away within 20 m outside the male territory: the female partner would take over the parental duty when males disappear during clutch development (Ringler et al. Citation2015). The strategy of A. femoralis, currently classified as male uniparental, could be regarded as an extreme end case of male-biased facultative biparental care with full compensation by maternal plasticity, only that the non-caring mother stays remote while monitoring the father’s presence, most likely via acoustic cues. On the other hand, mouse fathers in our study appeared almost to take over the main-caring mother’s role (except for nursing) (), only that the less-caring father stays in close proximity to his family. Instead of the simple uniparental-biparental distinction, parental care strategies may now be seen rather along a multidimensional continuum of relative contribution (zero to a half), plasticity (zero, partial to full compensation), timing (early to late parental role assumption) (Ringler et al. Citation2015), and even spacing (remote to close to family, spatially and/or socially).
There are experimental studies on obligate biparental rodents that did not look at the degree of plasticity per se but allow ones to make inferences about compensation. In a mate removal study, female California mice (Peromyscus californicus) did not compensate for the care loss in the absence of male mates, eventually to raise fewer pups (Cantoni and Brown Citation1997). Prairie voles (Microtus ochrogaster) show opposing trends between mothers and fathers in their patterns of change in parental investment across the first four liters, with a compensatory increase in paternal care while maternal care decreases, but the total care value at each liter turns out to be overall partial compensation (Rogers et al. Citation2018). In a study on paternal deprivation, female prairie voles did compensate partially for the removal of male partner, and did not compensate for reduced care by the male partner under a condition of difficult food access (Kelly et al. Citation2020). Together with the research on obligate biparental birds, these rodent studies suggest partial or zero compensation in obligate biparental care.
In terms of energy reserves for future reproduction, selection may favor obligate biparental individuals to increase care effort not much or not at all in response to reduced partner effort, given the already high innate care level and the prospective care duty in future breeding; while the less/non-caring parent of female/male-biased facultative biparental species appears more willing to incur costs to their own future fitness for the benefits of increasing the well-being of current offspring, given the lesser burden in next breeding. Although the actual shape of cost–benefit functions or negative correlation between innate care level and degree of plasticity should be drawn, observing how the less-cooperating sex of facultative biparental species responds to reduced partner effort differently from obligate biparental species allows us to test this theoretical prediction. This observation encourages us to seek more examples across different taxa, which may serve as basis to understand the evolution of behavioral plasticity and parental care behaviors.
Supplemental Material
Download Zip (38.6 KB)Acknowledgements
H.-Y.K conceptualized and designed the study, interpreted results and wrote the paper. H.K set up and performed experiments for all figures except for (for (C,D), and : With WT, N = 5; With KO, N = 4). J.J performed experiments for (A,C,D), (With WT, N = 5; With KO, N = 7) and did data analysis for all figures. All three authors discussed on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Bales KL, Saltzman W. 2016. Fathering in rodents: neurobiological substrates and consequences for offspring. Horm Behav. 77:249–259. doi:10.1016/j.yhbeh.2015.05.021.
- Barnett SA, Dickson RG. 1985. A paternal influence on survival of wild mice in the nest. Nature. 317(6038):617–618. doi:10.1038/317617a0.
- Brunton PJ, Russell JA. 2008. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 9(1):11–25. doi:10.1038/nrn2280.
- Cantoni D, Brown RE. 1997. Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Anim Behav. 54(2):377–386. doi:10.1006/anbe.1996.0583. Eng.
- Dobolyi A, Grattan DR, Stolzenberg DS. 2014. Preoptic inputs and mechanisms that regulate maternal responsiveness. J Neuroendocrinol. 26(10):627–640. doi:10.1111/jne.12185.
- Elwood RW. 1985. Inhibition of infanticide and onset of paternal care in male mice (Mus musculus). J Comp Psychol. 99(4):457. doi:10.1037/0735-7036.99.4.457.
- Feldman R, Braun K, Champagne FA. 2019. The neural mechanisms and consequences of paternal caregiving. Nat Rev Neurosci. 20(4):205–224. doi:10.1038/s41583-019-0124-6.
- Gajewski PA, Turecki G, Robison AJ. 2016. Differential expression of FosB proteins and potential target genes in select brain regions of addiction and depression patients. PLoS One. 11(8):e0160355. doi:10.1371/journal.pone.0160355. English.
- Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G. 2007. Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience. 147(3):583–591. doi:10.1016/j.neuroscience.2007.04.050.
- Harrison F, Barta Z, Cuthill I, SzÉKely T. 2009. How is sexual conflict over parental care resolved? A meta-analysis. J Evol Biol. 22(9):1800–1812. doi:10.1111/j.1420-9101.2009.01792.x.
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. 1992. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA. 89(13):5764–5768. doi:10.1073/pnas.89.13.5764.
- Houston AI, Székely T, McNamara JM. 2005. Conflict between parents over care. Trends Ecol Evol. 20(1):33–38. doi:10.1016/j.tree.2004.10.008. Eng.
- Kelly AM, Ong JY, Witmer RA, Ophir AG. 2020. Paternal deprivation impairs social behavior putatively via epigenetic modification to lateral septum vasopressin receptor. Sci Adv. 6(36):eabb9116. doi:10.1126/sciadv.abb9116.
- Kim HJ, Jang J, Koh HY. 2022. Abnormal maternal behavior in mice lacking phospholipase Cbeta1. Anim Cells Syst (Seoul). 26(6):291–299. doi:10.1080/19768354.2022.2141319.
- Kim HJ, Koh HY. 2016. Impaired reality testing in mice lacking phospholipase Cbeta1: observed by persistent representation-mediated taste aversion. PLoS One. 11(1):e0146376.
- Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, et al. 2018. Functional circuit architecture underlying parental behaviour. Nature. 556(7701):326–331. doi:10.1038/s41586-018-0027-0.
- Korgan AC, O'Leary E, Bauer J, Fortier A, Weaver IC, Perrot TS. 2016. Effects of paternal predation risk and rearing environment on maternal investment and development of defensive responses in the offspring. eNeuro. 3(6). doi:10.1523/ENEURO.0231-16.2016. Eng
- Korgan AC, O'Leary E, King JL, Weaver ICG, Perrot TS. 2018. Effects of paternal high-fat diet and rearing environment on maternal investment and development of defensive responses in the offspring. Psychoneuroendocrinology. 91:20–30. doi:10.1016/j.psyneuen.2018.02.010. Eng.
- Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. 2007. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol Cell Neurosci. 36(2):121–131. doi:10.1016/j.mcn.2007.05.010.
- Kuroda KO, Meaney MJ, Uetani N, Kato T. 2008. Neurobehavioral basis of the impaired nurturing in mice lacking the immediate early gene FosB. Brain Res. 1211:57–71. doi:10.1016/j.brainres.2008.02.100.
- Labov JB. 1980. Factors influencing infanticidal behavior in wild male house mice (Mus musculus). Behav Ecol Sociobiol. 6:297–303. doi:10.1007/BF00292772.
- Lee A, Clancy S, Fleming AS. 2000. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 108(2):215–231. doi:10.1016/S0166-4328(99)00170-9.
- Li M. 2022. The medial prefrontal regulation of maternal behavior across postpartum: a triadic model. Psychol Rev. 130(4):873. doi:10.1037/rev0000374.
- Mashoodh R, Franks B, Curley JP, Champagne FA. 2012. Paternal social enrichment effects on maternal behavior and offspring growth. Proc Natl Acad Sci USA. 109(Suppl 2):17232–17238. doi:10.1073/pnas.1121083109. Eng.
- McNamara JM, Gasson CE, Houston AI. 1999. Incorporating rules for responding into evolutionary games. Nature. 401(6751):368–371. Eng.
- McNamara JM, Houston AI, Barta Z, Osorno JL. 2003. Should young ever be better off with one parent than with two? Behav Ecol. 14(3):301–310. doi:10.1093/beheco/14.3.301.
- McNamara JM, Wolf M. 2015. Sexual conflict over parental care promotes the evolution of sex differences in care and the ability to care. Proc Biol Sci. 282(1803):20142752.
- Numan M. 2006. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 5(4):163–190. doi:10.1177/1534582306288790.
- Numan M, Stolzenberg DS. 2009. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 30(1):46–64. doi:10.1016/j.yfrne.2008.10.002.
- Olson VA, Liker A, Freckleton RP, Székely T. 2008. Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proc Biol Sci. 275(1632):301–307. Eng.
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. 2004. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 24(47):10594–10602. doi:10.1523/JNEUROSCI.2542-04.2004.
- Priestnall R, Young S. 1978. An observational study of caretaking behavior of male and female mice housed together. Dev Psychobiol. 11(1):23–30. doi:10.1002/dev.420110107. Eng.
- Provenzi L, Lindstedt J, De Coen K, Gasparini L, Peruzzo D, Grumi S, Arrigoni F, Ahlqvist-Björkroth S. 2021. The paternal brain in action: a review of human fathers’ fMRI brain responses to child-related stimuli. Brain Sci. 11(6):816. doi:10.3390/brainsci11060816. Eng.
- Remes V, Freckleton RP, Tokolyi J, Liker A, Szekely T. 2015. The evolution of parental cooperation in birds. Proc Natl Acad Sci USA. 112(44):13603–13608. doi:10.1073/pnas.1512599112.
- Ringler E, Pašukonis A, Fitch WT, Huber L, Hödl W, Ringler M. 2015. Flexible compensation of uniparental care: female poison frogs take over when males disappear. Behav Ecol. 26(4):1219–1225. doi:10.1093/beheco/arv069.
- Rogers FD, Rhemtulla M, Ferrer E, Bales KL. 2018. Longitudinal trajectories and inter-parental dynamics of prairie vole biparental care. Front Ecol Evol. 6:73. doi:10.3389/fevo.2018.00073.
- Royle NJ, Russell AF, Wilson AJ. 2014. The evolution of flexible parenting. Science. 345(6198):776–781. doi:10.1126/science.1253294.
- Tachikawa KS, Yoshihara Y, Kuroda KO. 2013. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci. 33(12):5120–5126. doi:10.1523/JNEUROSCI.2364-12.2013. Eng.
- vom Saal FS. 1985. Time-contingent change in infanticide and parental behavior induced by ejaculation in male mice. Physiol Behav. 34(1):7–15. doi:10.1016/0031-9384(85)90069-1. Eng.
- Wright SL, Brown RE. 2000. Maternal behavior, paternal behavior, and pup survival in CD-1 albino mice (Mus musculus) in three different housing conditions. J Comp Psychol. 114(2):183–192. doi:10.1037/0735-7036.114.2.183.
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 509(7500):325–330. doi:10.1038/nature13307. Eng.
- Yoshihara C, Tokita K, Maruyama T, Kaneko M, Tsuneoka Y, Fukumitsu K, Miyazawa E, Shinozuka K, Huang AJ, Nishimori K, et al. 2021. Calcitonin receptor signaling in the medial preoptic area enables risk-taking maternal care. Cell Rep. 35(9):109204. doi:10.1016/j.celrep.2021.109204. Eng.