ABSTRACT
Dopamine (DA) plays a significant role in regulating hippocampal function, particularly in modulating synaptic plasticity. Despite this, a comprehensive understanding of the molecular mechanisms involved in neuroplasticity-related signaling influenced by DA remains incomplete. This study aimed to elucidate the changes in the expression of key molecules related to hippocampal neuroplasticity following DA depletion in rats. To induce DA depletion, unilateral striatal infusions of 6-hydroxydopamine (6-OHDA) were administered to adult Sprague-Dawley rats. The subsequent loss of nigrostriatal DAergic signaling in these 6-OHDA-lesioned rats was confirmed using an apomorphine-induced rotation test at 4 weeks post-infusion and by assessing the expression levels of tyrosine hydroxylase (TH) through immunohistochemistry and western blotting at 7 weeks post-infusion. A decrease in DAergic signaling, evidenced by reduced TH-positive immunoreactivity, was also noted in the ipsilateral hippocampus of the lesioned rats. Interestingly, 6-OHDA infusion led to increased phosphorylation of pivotal hippocampal plasticity-related proteins, including extracellular signal-regulated kinase (ERK), protein kinase B (Akt), glycogen synthase kinase 3β (GSK3β), and cAMP response element-binding protein (CREB), in the ipsilateral hippocampus 7 weeks following the infusion. To extend these findings, in vitro experiments were conducted on primary hippocampal neurons exposed to DA and/or the active D1/D2 DA receptor antagonist, flupentixol (Flux). DA inhibited the constitutive phosphorylation of ERK, Akt, GSK3, and CREB, while Flux restored these phosphorylation levels. Taken together, these findings indicate that DA depletion triggers an increase in plasticity-related signaling in the hippocampus, suggesting a possible compensatory mechanism that promotes activity-independent neuroplasticity following DA depletion.
Introduction
Dopamine (DA) is a critical neurotransmitter that plays a central role in a range of physiological processes in the brain, including motor control, emotions, reward processing, behavior, and cognition (Juarez Olguin et al. Citation2016). Disturbances in DA transmission are implicated in a diverse array of disorders, encompassing both neuropsychiatric conditions – such as attention deficit hyperactivity disorder, Tourette syndrome, schizophrenia, psychosis, and depression – and neurodegenerative diseases like Parkinson’s disease (PD) (Tanaka et al. Citation2022). Specifically, PD is characterized by the degeneration of DAergic neurons, which results in a reduced availability of DA (Klein et al. Citation2019; Masato et al. Citation2019). This depletion of DA triggers a series of morphological, synaptic, and signaling alterations in dopaminoreceptive neurons located in various brain regions, including the hippocampus (Kim et al. Citation2022; Madadi Asl et al. Citation2022).
DA modulates both short-term neuronal excitability and long-term synaptic plasticity through the regulation of ion channels and gene expression (Rangel-Barajas et al. Citation2015). DA’s effects are mediated via G-protein-coupled receptors, specifically D1-like (stimulatory) and D2-like (inhibitory) receptors, which in turn modulate cAMP production (Neve et al. Citation2004; Rangel-Barajas et al. Citation2015). This bidirectional modulation influences various signaling pathways, such as the protein kinase A (PKA)/DA- and cAMP-regulated phosphoprotein-32 pathway (Greengard et al. Citation1999), the protein kinase B (Akt)/glycogen synthase kinase 3 (GSK3) pathway (Beaulieu et al. Citation2005), and the mitogen-activated protein kinase (MAPK)/cAMP response element-binding protein (CREB) pathway (Roberson et al. Citation1999). Additionally, both D1-like and D2-like DA receptors collaboratively induce immediate early gene expression in the striatum and influence rotational behavior in the context of striatal DA depletion, features that are sensitive to glutamatergic modulation (Paul et al. Citation1992).
The hippocampus, essential for both cognition and emotions, receives DAergic inputs from the ventral tegmental area and the substantia nigra (Weerasinghe-Mudiyanselage et al. Citation2022). DA serves as a modulator for hippocampal long-term potentiation (LTP) (Swanson-Park et al. Citation1999). In the context of PD, the DAergic system influences synaptic plasticity in the hippocampus (Calabresi et al. Citation2013), implicating this brain region in non-motor dysfunctions (Weerasinghe-Mudiyanselage et al. Citation2022). Multiple signaling molecules are involved in hippocampal neuroplasticity. For instance, extracellular signal-regulated kinase (ERK) is critical in memory, synaptic plasticity, and molecular-level information processing (Scott Bitner Citation2012). Upon activation, ERK modulates cellular functions through protein phosphorylation, transcription, and translation (Lavoie et al. Citation2020). Another key molecule is CREB, which is instrumental in learning, memory, synaptic transmission, neuron development, survival, and axon growth (Lu and Chow Citation1999). Both ERK and CREB are essential for neuroplasticity related to memory formation and schizophrenia (Yang et al. Citation2004). DA receptor regulation of the Akt/GSK3β signaling pathway also modulates specific aspects of synaptic plasticity (Jaworski et al. Citation2019). Changes in GSK3β activity influence the development of both LTP and long-term depression (LTD) in rat hippocampal slices, processes regulated by ionotropic glutamate receptors (Golpich et al. Citation2015). Furthermore, CREB is among the transcription factors that GSK3β potentially regulates (Johannessen and Moens Citation2007), participating in crucial cellular processes (Brami-Cherrier et al. Citation2002). However, unanswered questions persist regarding the alterations in neuroplasticity-related signaling and hippocampal neuron function following DA depletion.
In the realm of animal models for PD, DAergic neurons are targeted through the administration of neurotoxic substances to the nigrostriatal system (Hernandez-Baltazar et al. Citation2017). The rat model employing striatal infusion of 6-hydroxydopamine (6-OHDA) provides a suitable platform for examining DA depletion across various brain regions, including the hippocampus (Kim et al. Citation2022). In this study, we utilized a hemiparkinsonian rat model with ipsilateral striatal infusion of 6-OHDA to induce DA depletion in the hippocampus. The study aims to investigate nigrostriatal impairment and alterations in neuroplasticity-related signaling in the hippocampus, along with the correlations between these factors in this animal model.
Materials and methods
Animals, surgical procedures, and behavioral tests
Male Sprague-Dawley (SD) rats were sourced from Charles River Laboratories (Wilmington, MA, USA) for the present study. All animal care and experimental protocols adhered to the guidelines set forth by Chonnam National University (17 June 2021, CNU IACUC-YB-2021-71; 1 June 2023, CNU IACUC-YB-2023-70) and the NIH Guide for the Care and Use of Laboratory Animals. Measures were taken to minimize both the number of animals used and any associated suffering.
For the experiments, rats (n = 6 per group) were placed in a stereotaxic apparatus (SR-6; NARISHIGE, Tokyo, Japan) with their heads level. Surgical coordinates used were as follows: anteroposterior (AP) at +1.3, + 0.4, −0.4, −1.3 mm relative to the bregma; mediolateral (ML) at −2.6, −3.0, −4.2, −4.5 mm from the midline; and dorsoventral (DV) at −5.0 mm from the skull surface. A 6-OHDA solution with a concentration of 3.5 mg/mL (Sigma-Aldrich, St. Louis, MO, USA) was infused into the right striatum of each rat using an infusion pump. The 6-OHDA was dissolved in an 8 µL saline solution containing 0.02% ascorbic acid (Wako, Osaka, Japan) and delivered through a 10.5 μL microinjection cannula at a flow rate of 1 μL/min, with 2 μL delivered at each specified coordinate. For the sham-operated control group, identical surgical procedures were conducted, but 8 μL of vehicle solution (0.9% saline with 0.02% ascorbic acid) was infused into the striatum instead of 6-OHDA.
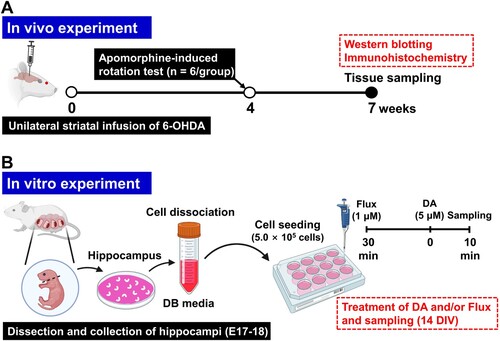
Four weeks post-striatal 6-OHDA infusion, rats were administered intraperitoneal injections of apomorphine hydrochloride (1 mg/kg; Wako). The incidence of contralateral rotations, identified as left-handed rotations, was subsequently observed and documented for each rat over a span of 30 min. Brain samples were collected seven weeks after the initial 6-OHDA infusion for both immunohistochemistry and western blot analyses. A illustrates the experimental procedure utilized in the hemiparkinsonian rat model.
Immunohistochemistry
For the immunohistochemistry procedures, we adhered to established protocols as described in a previous study (Hong et al. Citation2023). Brains (n = 3 per group) were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and sectioned coronally into 4 µm slices. To inhibit endogenous peroxidase activity, the paraffin-embedded sections were treated with 0.3% hydrogen peroxide solution for 20 min. Following this, the sections were blocked using 5% normal goat serum (NGS; Vector ABC Elite Kit, Vector Laboratories, Burlingame, CA, USA) in PBS containing 0.1% Tween 20 (PBS-T; pH 7.4) for 1 h at room temperature (RT; approximately 22°C ± 2°C). The sections were then incubated with rabbit anti-tyrosine hydroxylase (TH) antibody (dilution ratio of 1:500; Millipore, Burlington, MA, USA) overnight at 4°C. Afterward, the sections were exposed to biotinylated goat anti-rabbit IgG (Vector ABC Elite Kit) for 1 h at RT, followed by incubation with an avidin-biotin-peroxidase complex (Vector ABC Elite Kit) for an additional hour. The peroxidase reaction was visualized using a diaminobenzidine substrate (DAB kit; Vector Laboratories).
Western blot analysis
Upon euthanizing the animals, brain tissues, specifically from the striatum, substantia nigra, and hippocampus, were promptly extracted (n = 3 per group) and preserved at −80°C. Proteins were subsequently isolated from these samples and subjected to electrophoretic separation on 7–15% SDS-PAGE gels. These separated proteins were then transferred onto polyvinylidene difluoride membranes. For blocking, the membranes were incubated for 1 h at RT in a solution composed of 1% BSA (Sigma-Aldrich) and 2% NGS (Vector Laboratories) diluted in PBS-T. The membranes were then exposed to overnight incubation at 4°C with primary antibodies, including rabbit anti-TH (1:1,000; Millipore), anti-p-ERK (1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti-ERK (1:1,000; Cell Signaling Technology), anti-p-AKT (1:1,000; Cell Signaling Technology), anti-AKT (1:1,000; Cell Signaling Technology), anti-p-GSK3β (Ser9) (1:1,000; Cell Signaling Technology), anti-GSK3β (1:1,000; Cell Signaling Technology), anti-p-CREB (1:1,000; Cell Signaling Technology), and anti-CREB (1:1,000; Cell Signaling Technology). Following primary antibody incubation, membranes were treated with a horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5,000; Thermo Fisher Scientific, Waltham, MA, USA) for 2 h at RT. Chemiluminescent signals were captured using an EZ-Western Lumi Femto kit (DoGenBio, Seoul, Republic of Korea). After membrane stripping, re-probing was conducted with a mouse anti-β-actin antibody (1:5,000; Sigma-Aldrich) for an additional 2 h at RT. Finally, the optical density (OD) of each band was quantified using an iBrightTM CL750 Imaging System (Thermo Fisher Scientific).
Primary hippocampal cell culture and drug treatment
Hippocampi were surgically excised from SD rat pups on prenatal day 17.5 and prepared for culturing. Following dissection, the tissue samples underwent a multi-step protocol. First, the tissues were minced and enzymatically digested in a dissociation buffer containing 10 units/mL of papain (Worthington Biochemical, Lakewood, NJ, USA) and 100 units/mL of DNase I (Roche, Basel, Switzerland) at 37°C for 30 min. The digested material was then triturated using Neurobasal A medium (Life Technology, Carlsbad, CA, USA). The resulting cell suspension was plated at a density of 5.0 × 105 cells per well in 12-well plates (Nunc; Thermo Fisher Scientific) pre-coated with poly-D-lysine hydrobromide (150 μg/mL; Sigma-Aldrich). The medium was replaced with growth Neurobasal A medium supplemented with 1× B27 (Invitrogen, Carlsbad, CA, USA), 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 0.5 mM glutamine within 1 h of plating. Cultures were maintained at 37°C in a 5% CO2 atmosphere.
To assess the inhibitory influence of the DA signaling pathway, flupentixol (Flux; 1 μM; Abcam, Cambridge, UK) was administered 30 min before exposing the cultures to DA treatment (5 μM; Abcam) on day 14 in vitro (DIV). Cells were analyzed or harvested 10 min post-DA treatment. Both DA and Flux were prepared in sterile 0.9% saline solution. B presents a schematic of the in vitro experimental design utilizing rat primary hippocampal neuron cultures.
Statistical analysis
Statistical analyses were performed using Prism software (GraphPad Software, San Diego, CA, USA; RRID: SCR_002798). Data are presented as means ± standard errors (SEs) along with the sample sizes. To compare the sham-operated control group with the 6-OHDA-lesioned group, unpaired Student’s t-tests were used in all pertinent analyses. For the in vitro study, data were analyzed using one-way analysis of variance (ANOVA), followed by a Student-Newman-Keuls post hoc test for multiple comparisons. A significance level of P < 0.05 was established for all tests to denote statistical significance.
Results
Unilateral striatal 6-OHDA infusion impairs ipsilateral nigrostriatal DAergic signaling in rats
Four weeks post-infusion, the rats exhibited pronounced contralateral circling behavior upon systemic administration of apomorphine (n = 6 rats/group). Rats that demonstrated more than 7 rotations/min in response to apomorphine administration were considered to have effectively experienced ipsilateral DA depletion in this model (A; t(10) = 26.00; P < 0.001). Immunohistochemical results (B; n = 3 per group) showed a remarkable reduction in TH-positive immunoreactive cells and fibers in both the ipsilateral striatum and substantia nigra after unilateral 6-OHDA treatment, consistent with a prior study (Kim et al. Citation2022). Western blot data (C; n = 3 per group) revealed a significant decrease in TH protein levels in both the ipsilateral striatum (t(4) = 9.244, P < 0.001) and substantia nigra (t(4) = 21.45, P < 0.001).
Figure 2. Ipsilateral impairments of nigrostriatal DAergic signaling in unilateral 6-OHDA-lesioned hemiparkinsonian rat models. (A) Apomorphine rotation test (n = 6 rats/group). (B) Representative photomicrographs (n = 3 rats/group) showing immunoreactivity of TH-positive cell bodies and fibers in the STR (scale bar = 1,000 μm) and SN (scale bar = 100 μm). (C) Immunoblots of TH expression in STR and SN (n = 3 rats/group). Data are expressed as the means ± SEs. 6-OHDA, 6-hydroxydopamine-lesioned group; DA, dopamine; Sham, sham-operated controls; STR, striatum; SN, substantia nigra; TH, tyrosine hydroxylase. ***P < 0.001.
Unilateral striatal 6-OHDA infusion affects DAergic signaling in ipsilateral rat hippocampi
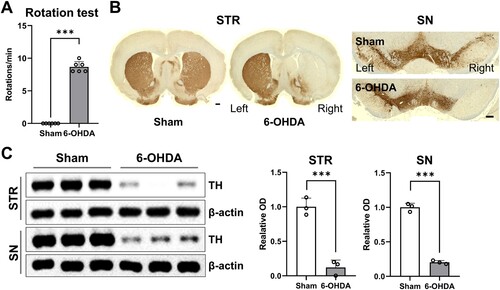
The western blot analysis revealed a statistically significant difference in TH protein levels between the sham-operated controls and the 6-OHDA-treated rats in the hippocampus (A; t(4) = 5.018, P < 0.01). Immunohistochemical observations revealed a notable reduction in TH-positive catecholaminergic terminals within the CA1, CA3, and dentate gyrus subregions of the hippocampus following unilateral striatal 6-OHDA administration (B).
Figure 3. Reduction in DAergic signaling in the rat hippocampus. (A) Immunoblots of TH expression in the hippocampus (n = 3 rats/group). (B) Representative photos (n = 3 rats/group) showing the TH-positive nerve fibers in the CA1, CA3, and dentate gyrus subregions in the hippocampus (scale bar = 100 and 400 μm). Data are expressed as means ± SEs. 6-OHDA, 6-hydroxydopamine-lesioned group; DA, dopamine; Sham, sham-operated controls; HP, hippocampus; TH, tyrosine hydroxylase. **P < 0.01.
DA depletion activates neuroplasticity-related signaling in the rat hippocampus
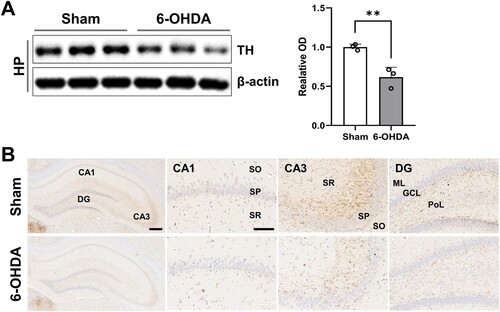
As shown in A, unilateral 6-OHDA treatment notably elevated the phosphorylation levels of ERK in the ipsilateral hippocampus (t(4) = 3.778, P < 0.05). Additionally, Akt phosphorylation levels in the 6-OHDA-treated hippocampi were significantly elevated compared to those in sham-operated controls (B; t(4) = 7.324, P < 0.01). We further identified a significant rise in the inhibitory phosphorylation (Ser 9) of GSK3β in 6-OHDA-treated rat hippocampi in comparison to sham-operated controls (C; t(4) = 3.124, P < 0.05). 6-OHDA treatment also significantly activated CREB in the hippocampus (D; t(4) = 4.532, P < 0.05).
Figure 4. Alterations in basal phosphorylation of neuroplasticity-related proteins in the hippocampus following DA depletion. (A–D, left panels) Immunoblot images of p-ERK/ERK (A), p-Akt/Akt (B), p-GSK3β/GSK3β (C), and p-CREB/CREB (D) expression in the hippocampus. (A–D, right panels) Bar graphs showing semi-quantitative data analysis (relative OD, n = 3 rats/group). Data are expressed as the means ± SEs. 6-OHDA, 6-hydroxydopamine-lesioned group; Sham, sham-operated controls. *P < 0.05. **P < 0.01. 6-OHDA, 6-hydroxydopamine-lesioned group; DA, dopamine; Sham, sham-operated controls.
Phosphorylation changes of neuroplasticity-associated proteins in cultured hippocampal neurons exposed to DA and/or a DA antagonist
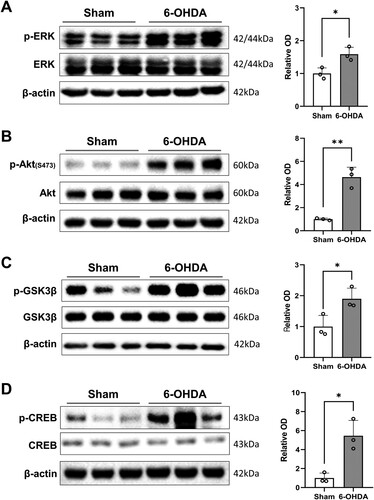
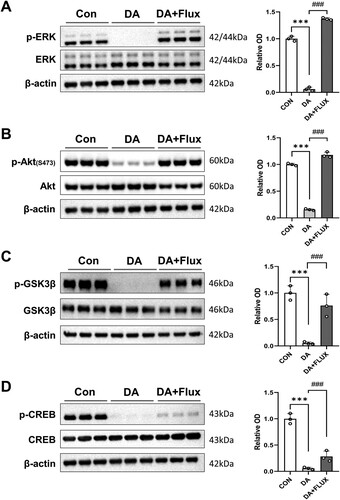
Using western blot analysis (; n = 3 per group), we assessed the phosphorylation status of relevant proteins in cultured rat hippocampal neurons. Initial treatment with DA led to a significant reduction in the basal phosphorylation levels of neuroplasticity-associated proteins, such as ERK (A; Finteraction [2, 6] = 1082, P < 0.001), Akt (B; Finteraction [2, 6] = 775.3, P < 0.001), GSK3β (Ser 9) (C; Finteraction [2, 6] = 34.15, P < 0.001), and CREB (D; Finteraction [2, 6] = 105.1, P < 0.001). However, subsequent treatment with a dopamine antagonist, Flux, substantially restored the phosphorylation levels of these proteins in the cultured neurons (P < 0.001). Importantly, neither DA nor Flux treatment led to damage in primary hippocampal neurons, as confirmed by microscopic evaluation and lactate dehydrogenase assay (data not shown).
Figure 5. Effects of DA and/or DA antagonists on the phosphorylation of neuroplasticity-related proteins in primary cultured rat hippocampal neurons. (A–D, left panels) Immunoblot images of p-ERK/ERK (A), p-Akt/Akt (B), p-GSK3β/GSK3β (C), and p-CREB/CREB (D) expression in the hippocampus. (A–D, right panels) Bar graphs displaying semi-quantitative data analyses (relative OD, n = 3 samples/group). CON, control group; DA, DA-treated groups, Flux, Flux-treated groups. ***P < 0.001 (CON vs. DA), ###P < 0.001 (DA vs. DA + Flux).
Discussion
In this study, we utilized a hemiparkinsonian rat model involving ipsilateral striatal infusion of 6-OHDA to investigate DA depletion in multiple ipsilateral brain regions, including the hippocampus. Our initial assessment used an apomorphine-induced rotation test conducted four weeks following 6-OHDA infusion, which demonstrated significant contralateral rotations in the treated rats. Subsequent analyses examined TH expression levels in the striatum, substantia nigra, and hippocampus. The results corroborated a reduction in TH expression within the ipsilateral brain regions, confirming the presence of ipsilateral DA depletion in both the nigrostriatal system and hippocampus of the rat model.
DA serves as a crucial neurotransmitter implicated in a broad spectrum of physiological processes (Juarez Olguin et al. Citation2016). Impairment of DA function leads to various changes in morphology, synaptic connectivity, and signaling pathways in DAergic target neurons across various brain areas, including the hippocampus (Kim et al. Citation2022; Madadi Asl et al. Citation2022). Disruptions in DA transmission have also been linked to an array of disorders ranging from neuropsychiatric conditions such as depression to neurodegenerative diseases like PD (Tanaka et al. Citation2022). Although much research has concentrated on the nigrostriatal system due to the central role of DAergic impairment in PD (Nam et al. Citation2021), it is imperative to examine the effects of DAergic dysfunction in other brain regions as well. These regions may have critical interactions with the nigrostriatal system and could contribute to the underlying mechanisms of non-motor dysfunctions. The hippocampus is especially relevant for non-motor symptoms, as it is involved in cognitive and emotional regulation (Calabresi et al. Citation2013). Previous anatomical studies have shown that midbrain DA neurons project directly to the hippocampus (Kumaran and Duzel Citation2008; Shohamy and Adcock Citation2010), underscoring its significance. Therefore, our study prioritized the hippocampus to explore its role in DAergic signaling and its interconnectedness with the DAergic system.
Neuroplasticity refers to the ability of neuronal networks in the brain to change through development and reorganization (Weerasinghe-Mudiyanselage et al. Citation2022). Molecules such as ERK, Akt, GSK3β, and CREB have been shown to play roles in DA signaling within the hippocampus. ERK activation can differ within the same cell or among various cell types, influenced by factors like signal duration, subcellular localization of signaling components, interactions with other signaling pathways, and cellular energy status (Colucci-D'Amato et al. Citation2003). Importantly, the activation of ERK, followed by its translocation to the nucleus, is associated with synaptic plasticity and LTP (Wiegert and Bading Citation2011). The Akt signaling pathway, which is activated by phosphatidylinositol 3-kinase in response to various stimuli such as insulin, growth factors, cytokines, and cellular stress (Manning and Cantley Citation2007), initiates the recruitment of Akt to the plasma membrane. Upon phosphorylation, Akt becomes activated (Timmons et al. Citation2009) and influences several substrates, including GSK3β and CREB, which have pivotal roles in cell survival, metabolism, and neuronal function (Rai et al. Citation2019). GSK3 is a serine/threonine kinase originally identified for its role in glycogen synthesis regulation but is now acknowledged for its broader involvement in cellular processes like gene expression, microtubule organization, development, and cell survival (Forde and Dale Citation2007). The activity of GSK3β is mainly regulated by inhibitory phosphorylation at specific serine residues – Ser21-GSK3α and Ser9-GSK3β (Pardo et al. Citation2016; Hong et al. Citation2022). Stimulation of D2 receptors has been found to modulate the regulatory phosphorylation of the Akt/GSK3 pathway (Mines and Jope Citation2012). Additionally, phosphorylation of ERK and CREB can be induced in hippocampal neurons during both D1 and D2 receptor stimulation but through separate pathways (Wu et al. Citation2001). Activation of CREB is dependent on the ERK and Akt/GSK3 pathways (Rai et al. Citation2019). Our study found that DA depletion impacts the constitutive phosphorylation of these proteins in the hippocampus. Given their roles in neuronal function, these proteins may serve as protective factors against DA depletion, thus contributing to the maintenance of hippocampal function.
Our findings reveal that phosphorylation of neuroplasticity-related molecules was significantly upregulated in hippocampal neurons following DA depletion. These results align with a prior study, which demonstrated that severe DA depletion led to elevated levels of Akt and GSK phosphorylation in the hippocampus (Morris et al. Citation2008). Such evidence supports the notion that even minor reductions in DA concentration can enhance Akt/GSK3β signaling in the hippocampus, whether directly or indirectly through network interactions (Li and Gao Citation2011). This suggests that intrinsic, activity-independent variations in plasticity mechanisms might result in a diverse range of neuroplastic outcomes at physiologically relevant levels.
Additionally, molecules related to neuroplasticity have been shown to contribute to structural modifications within the hippocampus (Weerasinghe-Mudiyanselage et al. Citation2022). In the context of a PD animal model, sustained inhibition of the DAergic pathway led to changes in neuronal architecture in the hippocampus (Kim et al. Citation2022). Notably, a prior study demonstrated that reductions in synaptic counts and arbor complexity coincided with synapse enlargement in specific forebrain regions (Sando et al. Citation2017). Such findings point to reciprocal and possibly self-sustaining relationships among synaptic size, size distributions, and synaptic counts. These relationships are modulated by the ongoing dynamics of synaptic molecules that are constantly relocating within and between synapses. Therefore, the activation of neuroplasticity signaling pathways observed in our study could be instrumental in reinforcing both structural and functional plasticity, which are crucial for maintaining hippocampal function.
While our study yields crucial insights into the molecular mechanisms contributing to neuropsychiatric symptoms in PD, it has some limitations. The small sample size necessitates caution in interpreting our significant findings. Furthermore, our use of the non-selective D1/D2 receptor antagonist, flupentixol, in a dopamine-depleted condition provides a broad overview of neuroplasticity-related signaling pathways in hippocampal neurons. However, a more nuanced understanding of the specific contributions of D1 and D2 receptors is required. Future investigations employing selective D1 and D2 receptor antagonists are warranted to dissect their individual roles in these pathways, which would refine our comprehension of dopamine receptor subtype interactions. Therefore, while our study provides a foundational understanding of neuropsychiatric symptoms in PD, limitations in sample size and receptor specificity highlight the necessity for future research with larger cohorts and selective receptor modulation to validate and refine our findings.
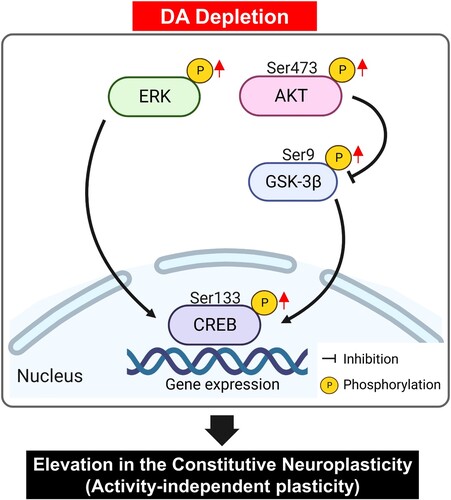
In summary, our study aimed to examine the effects of DA depletion on signaling pathways related to neuroplasticity in the hippocampus, a region integral to cognitive and emotional regulation. We found significant changes in the levels of molecules associated with neuroplasticity, such as ERK, Akt, GSK3β, and CREB, in hippocampal neurons following DA depletion (). Notably, phosphorylation levels of these molecules were elevated in response to decreased DA levels. These alterations may be indicative of varied responses from DA receptors and transporters to neurotransmitter fluctuations, activation of compensatory pathways, and the phase of disease development. Therefore, DA depletion appears to elevate the basal levels of neuroplasticity-related signaling pathways, leading to activity-independent plasticity in the rat hippocampus. These findings may enhance our understanding of the molecular mechanisms implicated in DA-associated neuropsychiatric and neurological conditions. However, additional studies are necessary to elucidate the specific mechanisms responsible for changes in neuroplasticity-related signaling in the hippocampus due to DA depletion and to investigate alterations in other related signaling pathways.
Figure 6. Schematic illustration of the proposed molecular mechanism underlying neuroplasticity in the hippocampus due to DA depletion. In hippocampal neurons, activity-independent plasticity involves the phosphorylation of ERK and the subsequent activation of CREB specific transcription factors, which in turn regulate gene transcription. Concurrently, DA depletion influences the phosphorylation of Akt at the Ser473 residue, leading to inhibitory phosphorylation of GSK-3β at the Ser9 residue. A pivotal result of this signaling cascade is the activation of CREB, which influences synaptic plasticity by modulating gene expression in hippocampal neurons. It should be noted that this representation omits some critical pathways in hippocampal neurons for the sake of clarity. Akt, protein kinase B; CREB, cAMP response element-binding protein; DA, dopamine; ERK, extracellular signal-regulated kinase; GSK3, glycogen synthase kinase 3.
CRediT authorship contribution statement
BK and CM participated in research design. BK, JSK, BY, and CM performed the study. BK and CM contributed in analysis and writing first draft of manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (2.8 MB)Data availability
Data will be made available on request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. 2005. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 122(2):261–273.
- Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. 2002. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 22(20):8911–8921.
- Calabresi P, Castrioto A, Di Filippo M, Picconi B. 2013. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson's disease. Lancet Neurol. 12(8):811–821.
- Colucci-D'Amato L, Perrone-Capano C, di Porzio U. 2003. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 25(11):1085–1095.
- Forde JE, Dale TC. 2007. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 64(15):1930–1944.
- Golpich M, Amini E, Hemmati F, Ibrahim NM, Rahmani B, Mohamed Z, Raymond AA, Dargahi L, Ghasemi R, Ahmadiani A. 2015. Glycogen synthase kinase-3 beta (GSK-3β) signaling: Implications for Parkinson's disease. Pharmacol Res. 97:16–26.
- Greengard P, Allen PB, Nairn AC. 1999. Beyond the dopamine receptor. Neuron. 23(3):435–447.
- Hernandez-Baltazar D, Zavala-Flores LM, Villanueva-Olivo A. 2017. The 6-hydroxydopamine model and parkinsonian pathophysiology: novel findings in an older model. Neurología. 32(8):533–539.
- Hong N, Park JS, Kim HJ. 2022. Synapto-protective effect of lithium on HIV-1 Tat-induced synapse loss in rat hippocampal cultures. Anim Cells Syst (Seoul). 26(1):1–9.
- Hong S, Weerasinghe-Mudiyanselage PDE, Kang S, Moon C, Shin T. 2023. Retinal transcriptome profiling identifies novel candidate genes associated with visual impairment in a mouse model of multiple sclerosis. Anim Cells Syst (Seoul). 27(1):219–233.
- Jaworski T, Banach-Kasper E, Gralec K. 2019. GSK-3beta at the intersection of neuronal plasticity and neurodegeneration. Neural Plast. 2019:4209475.
- Johannessen M, Moens U. 2007. Multisite phosphorylation of the cAMP response element-binding protein CREB by a diversity of protein kinases. Front Biosci. 12:1814–1832.
- Juarez Olguin H, Calderon Guzman D, Hernandez Garcia E, Barragan Mejia G. 2016. The role of dopamine and Its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. 2016:9730467.
- Kim B, Weerasinghe-Mudiyanselage PDE, Ang MJ, Lee J, Kang S, Kim JC, Kim SH, Kim JS, Jung C, Shin T, et al. 2022. Changes in the neuronal architecture of the hippocampus in a 6-hydroxydopamine-lesioned Rat model of Parkinson disease. Int Neurourol J. 26(Suppl 2):S94–105.
- Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. 2019. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol. 39(1):31–59.
- Kumaran D, Duzel E. 2008. The hippocampus and dopaminergic midbrain: old couple, new insights. Neuron. 60(2):197–200.
- Lavoie H, Gagnon J, Therrien M. 2020. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 21(10):607–632.
- Li YC, Gao WJ. 2011. GSK-3β activity and hyperdopamine-dependent behaviors. Neurosci Biobehav Rev. 35(3):645–654.
- Lu B, Chow A. 1999. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 58(1):76–87.
- Madadi Asl M, Vahabie AH, Valizadeh A, Tass PA. 2022. Spike-Timing-Dependent plasticity mediated by dopamine and its role in Parkinson's disease pathophysiology. Front Netw Physiol. 2:817524.
- Manning BD, Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell. 129(7):1261–1274.
- Masato A, Plotegher N, Boassa D, Bubacco L. 2019. Impaired dopamine metabolism in Parkinson's disease pathogenesis. Mol Neurodegener. 14(1):35.
- Mines MA, Jope RS. 2012. Brain region differences in regulation of Akt and GSK3 by chronic stimulant administration in mice. Cell Signal. 24(7):1398–1405.
- Morris JK, Zhang H, Gupte AA, Bomhoff GL, Stanford JA, Geiger PC. 2008. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson's disease. Brain Res. 1240:185–195.
- Nam D, Kim A, Han SJ, Lee SI, Park SH, Seol W, Son I, Ho DH. 2021. Analysis of α-synuclein levels related to LRRK2 kinase activity: from substantia nigra to urine of patients with Parkinson’s disease. Anim Cells Syst (Seoul). 25(1):28–36.
- Neve KA, Seamans JK, Trantham-Davidson H. 2004. Dopamine receptor signaling. J Recept Signal Transduct Res. 24(3):165–205.
- Pardo M, Abrial E, Jope RS, Beurel E. 2016. GSK3β isoform-selective regulation of depression, memory and hippocampal cell proliferation. Genes Brain Behav. 15(3):348–355.
- Paul ML, Graybiel AM, David JC, Robertson HA. 1992. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci. 12(10):3729–3742.
- Rai SN, Dilnashin H, Birla H, Singh SS, Zahra W, Rathore AS, Singh BK, Singh SP. 2019. The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res. 35(3):775–795.
- Rangel-Barajas C, Coronel I, Floran B. 2015. Dopamine receptors and neurodegeneration. Aging Dis. 6(5):349–368.
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. 1999. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 19(11):4337–4348.
- Sando R, Bushong E, Zhu Y, Huang M, Considine C, Phan S, Ju S, Uytiepo M, Ellisman M, Maximov A. 2017. Assembly of excitatory synapses in the absence of glutamatergic neurotransmission. Neuron. 94(2):312–321e313.
- Scott Bitner R. 2012. Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer's disease therapeutics. Biochem Pharmacol. 83(6):705–714.
- Shohamy D, Adcock RA. 2010. Dopamine and adaptive memory. Trends Cogn Sci. 14(10):464–472.
- Swanson-Park JL, Coussens CM, Mason-Parker SE, Raymond CR, Hargreaves EL, Dragunow M, Cohen AS, Abraham WC. 1999. A double dissociation within the hippocampus of dopamine D1/D5 receptor and β-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 92(2):485–497.
- Tanaka M, Spekker E, Szabo A, Polyak H, Vecsei L. 2022. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J Neural Transm. 129(5-6):627–642.
- Timmons S, Coakley MF, Moloney AM, ON C. 2009. Akt signal transduction dysfunction in Parkinson's disease. Neurosci Lett. 467(1):30–35.
- Weerasinghe-Mudiyanselage PDE, Ang MJ, Kang S, Kim JS, Moon C. 2022. Structural plasticity of the hippocampus in neurodegenerative diseases. Int J Mol Sci. 23(6).
- Wiegert JS, Bading H. 2011. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 49(5):296–305.
- Wu GY, Deisseroth K, Tsien RW. 2001. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 98(5):2808–2813.
- Yang BH, Son H, Kim SH, Nam JH, Choi JH, Lee JS. 2004. Phosphorylation of ERK and CREB in cultured hippocampal neurons after haloperidol and risperidone administration. Psychiatry Clin Neurosci. 58(3):262–267.