ABSTRACT
Myeloid ecotropic virus insertion site 1 (MEIS1) is a HOX co-factor necessary for organ development and normal hematopoiesis. Recently, MEIS1 has been linked to the development and progression of various cancers. However, its role in gliomagenesis particularly on glioma stem cells (GSCs) remains unclear. Here, we demonstrate that MEIS1 is highly upregulated in GSCs compared to normal, and glioma cells and to its differentiated counterparts. Inhibition of MEIS1 expression by shRNA significantly reduced GSC growth in both in vitro and in vivo experiments. On the other hand, integrated transcriptomics analyses of glioma datasets revealed that MEIS1 expression is correlated to cell cycle-related genes. Clinical data analysis revealed that MEIS1 expression is elevated in high-grade gliomas, and patients with high MEIS1 levels have poorer overall survival outcomes. The findings suggest that MEIS1 is a prognostic biomarker for glioma patients and a possible target for developing novel therapeutic strategies against GBM.
KEYWORDS:
Introduction
Glioblastoma multiforme (GBM) is classified as a WHO grade IV glioma and is the most malignant type of primary brain tumor. Individuals with GBM have an average overall survival of 15–18 months after diagnosis (Alves et al. Citation2021; Wu et al. Citation2021). Despite multimodal treatment options like surgery, radiotherapy, and chemotherapy, current approaches have had extremely limited success (Davis Citation2016; Taylor et al. Citation2019; Roh et al. Citation2023). In recent years, glioma stem cells (GSCs) have been linked to the aggressive characteristics of GBM (Alves et al. Citation2021). GSCs are identified by their capacity to self-renew and produce more differentiated progenies, which constitute most of the tumor mass (Suvà and Tirosh Citation2020). GSCs can recapitulate the heterogeneity of the parental tumor, drive resistance to treatments, and survive in harsh and complex microenvironmental niches (Park and Rich Citation2009; Prager et al. Citation2020). Maintaining the pluripotency and self-renewal abilities of cancer stem cells (CSC) mostly depends on chromatin regulators, master transcription factors (TFs), and related cellular networks. These regulators frequently act as oncogenes by encouraging the reacquisition of programs necessary for CSC dedifferentiation (Suvà and Tirosh Citation2020).
The HOX genes encode a family of homeodomain (HD)–containing TFs that play significant functions in embryogenesis and morphogenesis during development and adulthood. These genes are also involved in the formation of segmental identity along the anterior-posterior (AP) body axis, as well as the regulation of cell proliferation, differentiation, and survival (Mallo et al. Citation2010; Gonçalves et al. Citation2020; Feng et al. Citation2021; Arunachalam et al. Citation2022). Transcriptional regulation, nuclear dynamics, RNA processing, long noncoding RNAs (lncRNAs), miRNAs, and translational processes all influence HOX gene expression (Girgin et al. Citation2020). Several studies showed that the expression of HOX genes is frequently dysregulated in leukemia, breast, brain, lung, liver, colon, cholangiocarcinoma, pancreatic, skin, head and neck, kidney, bladder, prostate, cervix, endometrial, and ovarian cancer (Brotto et al. Citation2020; Gonçalves et al. Citation2020; Feng et al. Citation2021).
One of the key HD proteins is the triple amino acid loop extension (TALE) homeobox family, which has 27 members (MEIS, PBX, IRX, MKX, PKNOX, TGIF, and pseudogenes) (Girgin et al. Citation2020). MEIS and related Pre-B-cell Leukemia Homeobox (PBX)-HOX proteins can act as both oncogenes and tumor suppressors in certain biological contexts (Girgin et al. Citation2020). Co-factors like the PBX form heterodimers with HOX1–9 proteins, while the MEIS family dimerizes with HOX9–13 proteins. The binding of these co-factors improves the attachment and interaction of the highly conserved HD of HOX proteins with the DNA. These co-factors facilitate the recruitment of transcriptional inhibitors such as histone deacetylase (HDAC) and RNA polymerase II or III, leading to differential gene regulation based on the target site's location and sequence in the enhancer or promoter region (Arunachalam et al. Citation2022).
MEIS1 plays a crucial role in cellular development and proliferation (Li et al. Citation2022). The MEIS1 locus covers around 175 kb of the genome containing several regulatory domains and enhancers (Xiang et al. Citation2022). MEIS1 expression has been linked as an oncogene by regulating the proliferation of acute myeloid leukemia (Li et al. Citation2016; Xiang et al. Citation2022) and prostate cancer (Johng et al. Citation2019), migration in pancreatic cancer (von Burstin et al. Citation2017), and stemness and epithelial-mesenchymal transition (EMT) capability in esophageal squamous cell carcinoma (Mahmoudian et al. Citation2019; Zargari et al. Citation2020). Conversely, its expression suppresses colorectal (Li et al. Citation2022), prostate (Bhanvadia et al. Citation2018; VanOpstall et al. Citation2020; Whitlock et al. Citation2020), lung (Li et al. Citation2014), and esophageal (Rad et al. Citation2016) cancers. Previous studies have shown that MEIS1 is upregulated in gliomas (Berdasco et al. Citation2009; Vastrad et al. Citation2017) and its expression influences glioma survival and proliferation (Zha et al. Citation2014). Recently, it was found that the erythroblast transformation specific (ETS) domain ELF1 TF promotes glioma proliferation, migration, and invasion by increasing Zinc finger protein GFI1 and MEIS1 interaction (Cheng et al. Citation2021).
In our study, we focused on the role of MEIS1 in the development of GSCs. In both in vitro and in vivo experiments, silencing MEIS1 expression greatly reduced the proliferation of GSCs. Moreover, MEIS1 was found to be linked with cell cycle-related genes in integrated transcriptomics analysis. In clinical data, high MEIS1 expression is related to malignancy and poor outcomes in glioma patients. The findings suggest MEIS1 as a novel biomarker for glioma patients and a potential therapeutic target for GBM.
Materials and methods
Cell culture
Normal (healthy) human astrocyte (NHA) cells were grown in an astrocyte medium (AM; ScienCell Research Laboratories, USA) with 1% astrocyte growth supplement (AGS; ScienCell Research Laboratories, USA), 10% fetal bovine serum (FBS; Gibco, USA), and 1% penicillin/streptomycin (P/S; Welgene, South Korea). Human glioma cell lines (A172, A1207, U87MG, and LN229) were cultured in Dulbecco's modified media (DMEM/F12; Welgene, South Korea), with 1% P/S and 10% FBS. Glioma stem cell lines (GSC11 and GSC23) were received from The University of Texas MD Anderson Cancer Center (Bhat et al. Citation2013). GSC11 and GSC23 were originally derived from surgical GBM specimens. These lines demonstrate the typical in vitro stem cell traits, such as extensive self-renewal, the capacity to differentiate into neurons and astrocytes, and the capacity to initiate tumors in vivo (Yuan et al. Citation2015). GSC11 and GSC23 were grown in a neurobasal (NBE) media which comprised DMEM/F12 media, 20 ng/ml of epidermal growth factor (EGF; R&D Systems, USA), and basic fibroblast growth factor (bFGF; R&D Systems, USA), 2% B27 (Gibco, USA), and 1% P/S. All cells were maintained at 37°C with 5% CO2.
Lentivirus preparation
Lentiviral vectors generating short-hairpin RNA (shRNA) constructs (shMEIS1_01-TRCN0000434701, shMEIS1_72-TRCN00000159272; Sigma-Aldrich, USA) were used to silence the expression of the MEIS1 gene. A non-targeting shRNA (shNT; Sigma-Aldrich, USA) with a shRNA-coding sequence that does not target any gene served as a negative control. Plasmid DNA for lentivirus production was packaged in 293FT cells using the CalPhosTM Mammalian Transfection Kit (Clontech Laboratories, Inc., USA). pMD2.G and psPAX2 were used as packaging plasmids. After 72 h of transfection, the lentivirus-containing medium was filtered in a 0.45 µm PES membrane Millex®-HP (Merck Millipore, MA, USA). Finally, a 100-fold concentration was achieved with a Lenti-X™ Concentrator (Clontech Laboratories, Inc., USA). The lentivirus was generated following the manufacturer's directions.
Cell viability assay
Glioma stem cells (GSC11 and GSC23) were seeded (3×105 cells/well) in a 6-well plate (laminin-coated). After 24 h, cells were infected with lentivirus (shNT, shMEIS1_01, and shMEIS1_72). Cells were incubated for 48 h and then reseeded in a 96-well plate (3000 cells/well; n = 6). After 2-, 4- and 6-days post-transfection, the cell viability was evaluated using the alamarBlueTM cell viability reagent (Invitrogen, USA), as directed by the manufacturer. The absorbance at 590 nm was measured in a Synergy HTX Multi-Mode Reader (BioTek Instruments Inc., USA).
In vitro limiting dilution assay
The shRNA-transfected GSCs were seeded (96-well plate) at decreasing cell densities (50, 25, 12, 6, 3, and 1 cell(s) per well; n = 30). Growth media (10 µl) was added to the wells every 3 days. After 14 days, wells were observed under a light microscope for tumorsphere formation. Positive wells were identified as having tumorspheres diameter bigger than 20μm. The Extreme Limiting Dilution Analysis (ELDA) tool was used to examine the frequency of GSCs’ ability to produce tumorspheres (Hu and Smyth Citation2009).
Dataset preparation
The 208 homeobox TF-related genes were identified in the Human Protein Atlas database (https://www.proteinatlas.org/). The GSE4536 dataset was used to assess the mRNA expression profile of homeobox TF-related genes in GSCs under stem cell and differentiated conditions (Lee et al. Citation2006). Data normalization and transformation (Log2 CPM+1) was performed in the Integrated Differential Expression and Pathway Analysis (iDEP ver.1.1) website (http://bioinformatics.sdstate.edu/idep11/). The Gliovis website (http://gliovis.bioinfo.cnio.es/) was used to retrieve the TCGA_GBMLGG, Gravendeel (GSE16011), REMBRANDT (GSE108474), and Ivy Glioblastoma Atlas Project (Ivy GAP) datasets.
Bioinformatics analysis
Pearson's R correlation was performed to identify the correlated genes with MEIS1 expression. Positively moderate to strong correlated genes (R ≥ 0.4; p ≤ 0.05) (Akoglu Citation2018; Schober et al. Citation2018) to MEIS1 expression were annotated in the Database for Annotation, Visualization, and Integrated Discovery (DAVID) website (https://david.ncifcrf.gov/), a bioinformatics tool employing gene ontology (GO) and Kyoto Encyclopedia for Genes and Genomes (KEGG) pathways. Gene Set Enrichment Analysis (GSEA) ver. 4.2.2 software was used to identify significantly correlated human MSigDB GO biological processes and KEGG pathway gene sets in patients with high MEIS1 expression in the TCGA_GBMLGG dataset. The ‘Weighted Gene Co-expression Network Analysis (WGCNA)’ package in R was used to identify gene clusters-modules having strong correlations to MEIS1 expression and phenotypes. The ‘survival’ and ‘survminer’ package in R were used for univariate and multivariate COX regression analyses. Other data analyses and visualization were performed in the SRplot (https://www.bioinformatics.com.cn/srplot). The TCGA_GBMLGG dataset was used in all analyses.
Single-cell RNA sequencing analysis
The ‘Seurat’ package in R 4.0.5 was used to analyze the scRNA-seq data of three newly diagnosed GBM samples (GSM5518633, GSM5518634, and GSM5518636) from the GSE182109 dataset (Abdelfattah et al. Citation2022). Low-quality cells with (<200 total feature RNA; <15% mitochondrial RNA) were removed. The SCTransform integration function was used to merge the data from each dataset. The NormalizedData and ScaleData functions were used to normalize and scale the expression of all genes. The influence of the cell cycle, the total number of counts, and the percentages of genes expressed in mitochondria in each cell were all regressed out of the data after identifying 2000 integration anchors that represent the closest neighbors across datasets. After dimension reduction (npcs = 30) using principal component analysis (PCA), it was further reduced (dims = 1:15) using the uniform manifold approximation and projection (UMAP) method. The FindNeighbors and FindClusters (resolution = 0.1) functions were used to identify the nearest neighbors of single-cell and cluster cells based on their similarity using the Louvain algorithm. Differentially expressed genes in each cluster were identified using the FindAllMarkers function and Wilcoxon rank-sum test (cutoff: min.pct = 0.25 and logfc.threshold = 0.25). Marker genes in each cluster were manually entered into CellMarker2.0 (http://bio-bigdata.hrbmu.edu.cn/CellMarker/index.html) for cell type annotation. Gene scores for Mesenchymal (MES)-like Astrocyte (AC)-like, Oligodendrocyte Progenitor cell (OPC)-like, Neural Progenitor cell (NPC)-like, G1/S, and G2/M (Neftel et al. Citation2019) in glioma clusters were identified using the AddModuleScore function. Enrichment analysis was performed using the Fast Gene Set Enrichment Analysis (FGSEA) function. The data were visualized using the DimPlot, FeaturePlot, DotPlot, DOHeatmap, and plotEnrichment function.
RNA-sequencing analysis
GSC11 (3.5×105 cells/well) was seeded in a 6-well plate (laminin-coated). Cells were transfected with either shNT control or shMEIS1_72 lentivirus and then incubated for 48 h. At the end of the incubation period, cells were resuspended in TRIzol® Reagent (Invitrogen; CA, USA) and kept at −80°C. Libraries were constructed using the MGIEasy RNA Directional Library Prep Kit (MGI Tech., Shenzhen, China) following the manufacturer’s procedure. RNA quality control and high-throughput RNA sequencing were performed on the MGISEQ platform (MGI Tech) with 149 bp paired-end reads. Reads were mapped to the hg19 reference genome and expression values were calculated in fragments per kb transcript per million fragments mapped (FPKM). LAS Co., Ltd. (Gimpo, South Korea) performed the RNA purification, library preparation, and sequencing procedures. The new generation of high-throughput sequencing was used to study the differences between the two samples and gene expression in both groups was compared using EdgeR. Significant genes (Log2(FC) cut-off >0.5 and <0.5; p ≤ 0.05) were used for functional annotation on the DAVID website. GSEA analysis was also performed to identify the downregulated gene sets after MEIS1 knockdown.
Quantitative reverse transcription-PCR (RT-qPCR) analysis
Total RNA was isolated using the GeneAll® RiboExTM reagent and purified using the GeneAll® Hybrid-RTM kit as per the manufacturer's instructions (GeneALL Biotechnology, South Korea). Total RNA (500 ng) was converted to its complementary DNA (cDNA) using the RevertAidTM First Strand cDNA Synthesis kit (Thermo-Fisher Scientific, USA). RT-qPCR was completed on a qTOWER3 Real-Time PCR thermocycler (Analytik-Jena, Germany) using Tli RNaseH Plus TB Green® Premix Ex TaqTM (Takara, South Korea). The relative changes in the gene expression were analyzed using the 2−ΔΔCt method and the results were normalized against the expression level of housekeeping gene 18S. The experiment was independently replicated three times. The primer sequences used in this study are listed in Supplementary Table 1.
In vivo orthotopic implantation
GSC11 (5 × 105 cells) transfected with shNT or shMEIS1_72 lentivirus were intracranially implanted into the brains of 6- to 8-week-old immunodeficient nude mice at the coordinates of 2.0 mm right and 1.0 mm anterior of the bregma using a Stereotaxic instrument (Harvard Apparatus; MA, USA) (n = 6 mice/group). Mice with a body weight loss of more than 30% were sacrificed. The overall survival was presented using the Kaplan-Meier curve. After one month of GSC injection, one mouse from each shMEIS1_72 and shNT group was euthanized concurrently for brain histological investigation. Chonnam National University's Institutional Animal Care and Use Committee approved all mouse experiments following the Republic of Korea government and institutional standards and laws (CNU IACUC-YB-2021-99).
In vivo bioluminescence imaging
To maintain nude mice unconscious during imaging procedures, 2% isoflurane was used. Following intraperitoneal injection of D-luciferin (150 mg/kg), mice were photographed in an IVIS® Spectrum in vivo imaging system (PerkinElmer; CT, USA) for 10 min. Total flux values were estimated for each mouse using luminous regions of interest (ROI) and are shown in radiance (p/sec/cm2/sr).
Hematoxylin and eosin (H&E) staining
Whole brain samples were obtained from the shNT and shMEIS1 treatment groups. Samples were fixed in 4% paraformaldehyde, followed by paraffin embedding, then sectioned into 4μm thick slices. Brain sections were stained with H&E solutions. Finally, stained sections were examined under a light microscope (DM 750, Leica Microsystems, Wetzlar, Germany) equipped with a digital camera (ICC50E, Leica Microsystems, Wentzler, Germany).
Statistical analysis
All data analyses were performed in R ver. 4.3.2 and GraphPad Prism version 8.3 (GraphPad Software, San Diego, CA, United States). The statistical significance among groups was determined using a two-tailed t-test and a one-way ANOVA. The data were then compared using Tukey's multiple comparison tests. The mean ± standard error (SE) was used to report all data. The p-value ≤ 0.05 was considered statistically significant (p<0.05 = *; <0.01 = **; <0.001 = ***; <0.0001 = ****; ns = not significant).
Results
MEIS1 is highly expressed in glioma stem cells
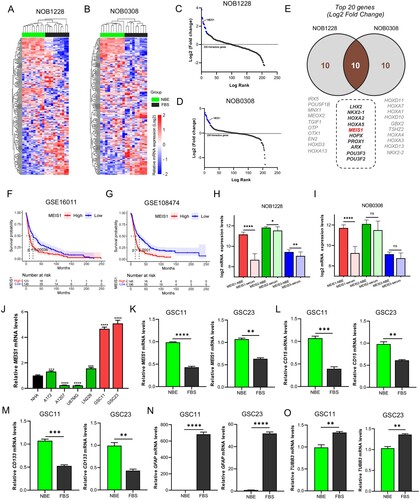
The GSE4536 dataset was used to compare the expression profiles of 208 homeobox TF-related genes in GSCs in stem cell- (NBE) and differentiated- (serum) culture conditions. MEIS1 is among the highly expressed homeobox genes in both NOB1228 and NOB0308 cell lines under NBE-culture conditions (A-D). Filtering out the top 20 upregulated genes, 10 genes (LHX2, NKX2-1, HOXA2, HOXA5, MEIS1, HOPX, PROX1, ARX, POU3F3, POU3F2) were found to overlap in the NOB1228 and NOB0308 cell lines (E). To validate the clinical significance of these genes, we performed a Kaplan-Meier survival analysis using the GSE16011 and GSE108474 glioma datasets (F-G). Results show that high MEIS1 expression correlates to poor outcomes in glioma patients. We also show that high expression of HOXA2, HOXA5, and ARX genes influence poor survival, while NKX2-1 is a good prognostic marker for glioma patients in both datasets (Figure S1). Other overlapped genes showed variable results. The GSE4536 dataset is also used to compare the expression profile of the MEIS family. MEIS1 is significantly higher in gliomas under NBE-culture conditions, while MEIS2 and MEIS3 showed varying expression levels (H-I). To validate these findings, we measured the basal mRNA expression levels of MEIS1 in GSCs (GSC11, GSC23), non-stem cell gliomas (A172, A1207, LN229, U87MG), and NHA cells by RT-qPCR analysis (J). We found that MEIS1 is more highly upregulated in GSCs than in NHA cells and non-stem gliomas. Lastly, we then compared the basal mRNA expression of MEIS1, stemness (CD15, CD133), and differentiation (TUBB3, GFAP) markers in GSC11 and GSC23 under NBE and serum culture conditions. We show that MEIS1 and stemness markers are significantly upregulated (K-M), while differentiation markers are downregulated in GSCs under the NBE-culture condition (N-O). These results show that MEIS1 is highly expressed in GSCs.
Figure 1. MEIS1 is highly expressed in GSCs. (A, B) Cluster heatmap for 208 homeobox-related genes in the GSE4536 dataset. (C, D) Log-rank fold-change for homeobox-related genes in the GSE4536 dataset. (E) The top 20 homeobox-related genes from the NOB1228 and NOB0308 are cell lines displayed in a Ven diagram. (F, G) Kaplan-Meier survival analysis of glioma patients with MEIS1High/Low expression in the GSE16011 and GSE10847 datasets. (H, I) mRNA expression levels of MEIS family genes in the GSE4536 dataset; (J) RT-qPCR analysis of the MEIS1 basal mRNA levels in GBM cell lines (A172, A1207, U87MG, LN229), GSC lines (GSC11, GSC23), and NHA cells. (K-O) RT-qPCR analysis of MEIS1, CD15, CD133, GFAP, and TUBB3 in NBE- and serum-cultured GSC11 and GSC23 cells.
Elevated MEIS1 expression is linked to malignancy and poor survival of glioma patients
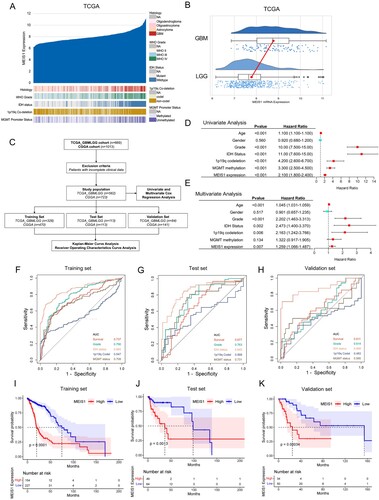
To further understand the clinical relevance of MEIS1 in glioma, we investigated its mRNA expression levels in the TCGA_GBMLGG and CGGA datasets (A, S2A). MEIS1 is highly expressed in GBM (A, 1B, S2A, B), and correlated to IDH wildtype, unmethylated MGMT promoter region, and 1p19q non-co-deletion status phenotypes (A, S2A). To ascertain the prognostic significance of MEIS1 expression, we carried out univariate and multivariate, ROC curve, and Kaplan-Meir survival analyses (C). Both univariate and multivariate analyses show that high MEIS1 expression is a predictor for overall survival for glioma patients (D-E, S2C-D). Age, gender, grade, IDH, 1p19q codeletion, and MGMT status were also independent predictors for overall patient survival. Meanwhile, we used a ROC curve to analyze the predictive efficacy of MEIS1 expression (F-H, S2E-G). We show that MEIS1 expression predicts survival well independently of other clinical variables in the training, test, and validation datasets (AUC >0.6). Kaplan-Meier survival analysis shows that high MEIS1 expression is related to lower patient survival (I-K, S2H-J). These findings imply that high MEIS1 expression may be a predictor of prognosis and overall survival of glioma patients.
Figure 2. Elevated expression of MEIS1 is linked to malignancy and poor survival for patients with gliomas. (A) MEIS1 mRNA expression and histological features in the TCGA_GBMLGG dataset (n = 669). (B) Raincloud plot of MEIS1 expression in GBM and LGG patients. (C) Flow chart showing the patient population in the TCGA and CGGA cohorts. (D, E) Univariate and multivariate Cox proportional hazards regression analysis of variables influencing overall patient survival in the TCGA dataset. (F-H) ROC curve and the risk score distribution stratified by MEIS1 in the TCGA dataset. (I-K) Kaplan-Meier analysis of glioma patients with MEIS1High/Low expression in TCGA dataset.
MEIS1 is increased in the GBM classical subtype and enriched in the cellular tumor region
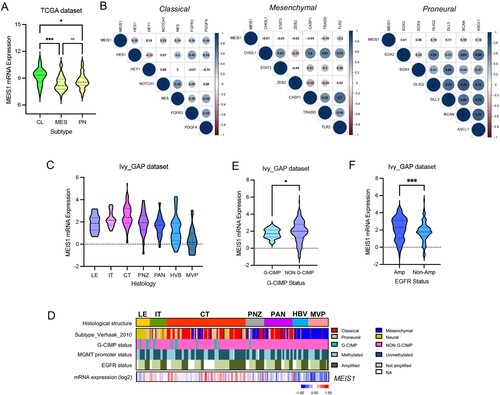
The TCGA dataset was then used to compare the expression of MEIS1 in the different GBM subtypes. We show that MEIS1 is highly expressed in the GBM classical subtype (A). To verify this result, we perform correlation analysis in MEIS1 expression and marker genes for each subtype. We show that MEIS1 expression is positively correlated with classical subtype markers (B). The Ivy GAP dataset was used to identify the GBM anatomical region that significantly expresses MEIS1. We show that MEIS1 is preferentially elevated in the cellular tumor (CT) region (C, D). On the other hand, MEIS1 is significantly expressed in non-G-CIMP and EGFR-expressing gliomas (E, F).
Figure 3. MEIS1 is increased in the classical GBM subtype and enriched in the cellular tumor region. (A) MEIS1 mRNA levels in GBM subtypes from the TCGA dataset (CL - Classical, ME – Mesenchymal, PN – Proneural). (B) Correlation of MEIS1 expression with CL, MES, and PN markers in the TCGA dataset. (C) MEIS1 mRNA levels in various GBM regions as described in the IvyGAP dataset. (LE – leading edge; IT – infiltration tumor; CT – cellular tumor; PNZ – perinecrotic zone; PAN – pseudopalisading cells around necrosis; HBV – hyperplastic blood vessels; MVP – microvascular proliferation). (D) Heatmap of MEIS1 expression and phenotypes in the Ivy GAP dataset. (E, F) MEIS1 expression based on CIMP and EGFR amplification status in the Ivy GAP dataset.
Bioinformatics analysis reveals the role of MEIS1 expression in gliomas
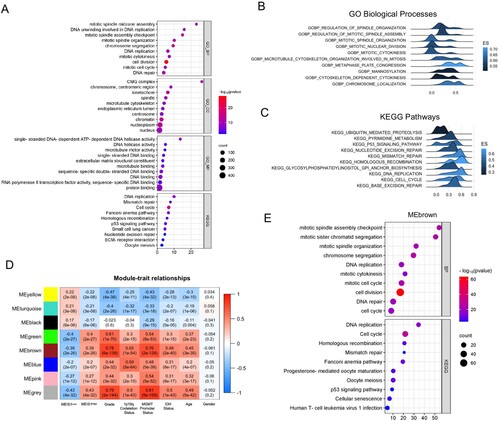
Next, we performed Pearson's R correlation analysis to identify the positively correlated genes with MEIS1 expression in the TCGA dataset. Functional annotation of positively correlated genes (Pearson’s R ≥ 0.40; p-value <0.05) revealed that MEIS1 is associated with DNA replication, DNA repair, cell cycle, and mitosis genes (A). The GSEA analysis corroborates these findings (B, C). We also performed WGCNA analysis to further elucidate the biological roles of MEIS1 in gliomas. Interestingly, the gene hub in the MEbrown module ((R = 0.39; p<0.0001) validates our findings in the correlation and GSEA analyses (D, E, S3). These results show that MEIS1 is crucial in the development and progression of gliomas.
Figure 4. Transcriptomic analysis reveals the role of MEIS1 in gliomas. (A) DAVID functional annotation of correlated genes with MEIS1 in TCGA_GBMLGG (Pearson's R>0.4, p≤ 0.05). (B, C) GSEA analysis of MEIS1High/Low expression in the TCGA_GBMLGG dataset; (D) Relationship between MEIS1 expression and phenotypes analyzed by WGCNA. (E) DAVID functional annotation of gene hub in the MEbrown module.
MEIS1 is enriched in NPC- and OPC-like gliomas according to scRNA-seq analysis
We also performed a single-cell RNA sequencing analysis of 3 newly diagnosed GBM samples (GSE182109). Different cell clusters were identified including gliomas, stromal, and immune cells (Figures S4A-D). Results show that MEIS1 is significantly expressed in glioma (Cluster 1, 3) and oligodendrocyte (Cluster 8) cell clusters among the other cell populations (A, B). Filtering out the glioma and oligodendrocyte cell population (C), we show that MEIS1 is highly expressed in cell clusters 0 and 1 (E). Marker genes for cluster 0 include OLIG1/2, SOX4, and EGFR, while marker genes for cluster 1 include CENPF and TOP2A (D). Filtering out the MEIS1-positive cells, we show that MEIS1 is co-expressed with EGFR, POSTN, CENPF, and TOP2A (F). These genes are known to be highly expressed in the classical and proneural GBM subtypes. Next, we performed the ‘AddModuleScore’ function in Seurat to calculate module scores for feature expression programs of GBM subtypes (Neftel et al. Citation2019) in single cells. We show that MEIS1 is upregulated in the NPC- and OPC-like glioma cells (G, S4E-F). Meanwhile, MEIS1-expressing expressing cells are located in the highly proliferative region, based on G1/S and G2/M scores (G). Lastly, we performed FGSEA analysis to identify the biological processes and functions correlated with MEIS1 expression. We show that MEIS1 is positively correlated with as cell cycle, DNA repair, DNA metabolic process, and mitosis, while negatively correlated to apoptosis, focal adhesion, and oxidative phosphorylation (H, S4G-J). These findings further validate our results in the bulk-transcriptomics analyses.
Figure 5. MEIS1 is enriched in the NPC- and OPC-like gliomas. (A) FeaturePlot of cell clusters expressing MEIS1. (B) DotPlot of MEIS1 expression in different cell clusters. (C) DimPlot of glioma cell clusters. (D) DOHeatmap of top 10 genes in different glioma cell clusters. (E) FeaturePlot of MEIS1 expression in different glioma cell clusters. (F) DOHeatmap of top 10 genes enriched in MEIS1-positive and -negative cells. (G) FeaturePlot of NPC-like, OPC-like, G1/S, and G2/M enrichment in the glioma cell cluster. (H) FGSEA analysis in MEIS1-positive/-negative cells.
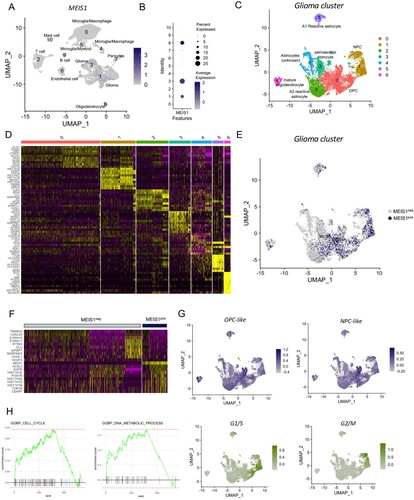
Suppressing MEIS1 inhibits GSC proliferation in vitro and in vivo
To elucidate the function of MEIS1, we silenced the MEIS1 expression in GSC using shRNA. RT-qPCR analysis revealed MEIS1 expression was downregulated by 50% upon shRNA knockdown in both GSC11 and GSC23 cell lines (A). The effects of MEIS1 knockdown on cell viability and tumorsphere formation ability on GSCs were examined in vitro. MEIS1 knockdown significantly reduced cell viability (B) and tumorsphere formation in both GSC11 and GSC23 cells (C-E). Meanwhile, mRNA levels of stemness and differentiation markers were not influenced by MEIS1 knockdown (data not shown). The effect of MEIS1 knockdown on GSC11 tumor formation in vivo was further investigated using intracranial injection in an orthotopic animal model (F). To enable continuous monitoring of tumor volume, GSC11 was transduced with a constitutive luciferase reporter gene. In vivo bioluminescent imaging (G, H) and histological analysis (J) show that mice implanted with MEIS1 knockdown GSC11 had much lower tumor growth compared to the shNT control group. Moreover, Kaplan-Meier survival analysis shows that mice implanted with MEIS1 knockdown GSC11 had significantly greater median survival rates than the shNT group (I). These data indicate that MEIS1 is required for GSC growth.
Figure 6. Suppressing MEIS1 inhibits the GSC proliferation in vitro and in vivo. RT-qPCR analysis of MEIS1 in GSC11 and GSC23 cells after shRNA knockdown. (B) Cell viability of GSC11 and GSC23 after MEIS1 shRNA knockdown. (C) Tumorsphere formation assay in GSC11 and GSC23 after MEIS1 shRNA knockdown. (D) Representative figure of shNT and shMEIS1_72 transduced GSC11 cells (Scale = 100μm). (E) In vivo orthotopic mouse model experimental schedule. (F-G) In vivo bioluminescent imaging of nude mice bearing intracranial tumors derived from shNT and shMEIS1_72 knockdown GSC11 cells. (H) Kaplan–Meier curves of nude mice bearing intracranial tumors derived from shNT and shMEIS1_72 knockdown GSC11 cells. (I) Representative H&E-stained brain sections from shNT and shMEIS1 mice groups (Scale = 100 μm).
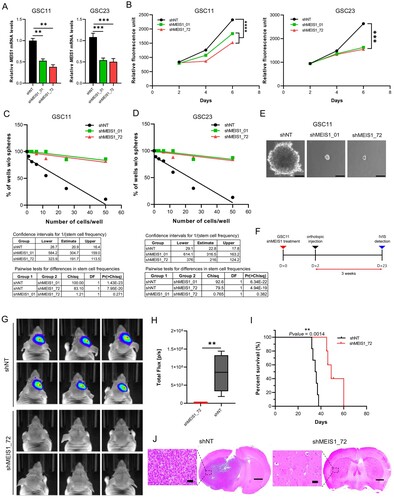
Silencing MEIS1 influences the expression of cell cycle-related genes in GSC
Finally, we performed RNA-sequencing analysis to identify the differentially expressed genes upon MEIS1 knockdown in GSC11 cells. We show that there are 109 downregulated and 176 upregulated genes upon MEIS1 knockdown (A). Functional annotation and GSEA analysis revealed that genes linked to the cell cycle, cell division, mitosis, and DNA replication were downregulated in MEIS1 knockdown cells (B-E). Interestingly, we found that apoptosis-related genes were upregulated upon MEIS1 knockdown. These results show that MEIS1 is important in the maintenance and proliferation of GSCs.
Figure 7. Silencing MEIS1 downregulates the expression of cell-cycle-related genes. (A) Volcano plot of the DEGs in shNT and shMEIS1 treated GSC11 cells. (B) Functional annotation of genes that are significantly downregulated in shMEIS1-treated GSC11 cells (Log2FC <−0.5; p ≤0.05). (C-E) Enrichment plot of downregulated biological processes and pathways in GSC11 cells after MEIS1 shRNA knockdown.
Discussion
MEIS1, a TF belonging to the TALE homeobox family, is essential for cell growth and development (Li et al. Citation2022). In several solid tumors, such as breast, colorectal, human esophageal squamous cell, ovarian, and prostate cancers, MEIS1 plays an oncogenic role (Blasi and Bruckmann Citation2021). Moreover, it has been observed that MEIS1 is upregulated in neuroblastoma and glioma cells, supporting cell survival and proliferation (Berdasco et al. Citation2009; Zha et al. Citation2014; Vastrad et al. Citation2017; Girgin et al. Citation2020). While MEIS1 has been shown to play an oncogenic role in glioma and other cancers, its function in GSC is still unclear. MEIS1 is significantly expressed in GSCs, according to our in-silico and validation experiments, and its expression may have an impact on the aggressive nature of GSCs.
We also found that HOXA2, HOXA5, and ARX are differentially expressed in GSC. HOXA2 and HOXA5 are aberrantly expressed in GBM and contribute to poor outcomes in glioma patients (Liu et al. Citation2020; He et al. Citation2022). Recently, it was found that HOXA5 can transcriptionally activate PTPRZ1 to influence the self-renewal and invasive properties of GSC (He et al. Citation2022). Meanwhile, the oncogenic role of the ARX gene has not been reported to our knowledge, thus further studies are needed. Several studies have shown that the interaction of MEIS and PBX-HOX proteins may act as an oncogene in various cellular settings (Girgin et al. Citation2020; Jiang et al. Citation2021). For example, MEIS1 overexpression and its interaction with HOXA9 or PBX promote proliferation in leukemia cells. Also, MEIS1-HOXB13 and MEIS1-SOX2 promote proliferation in prostate cancer and esophageal squamous cell carcinoma, respectively (Yao et al. Citation2021). The co-expression and possible interaction of MEIS1 and its co-factors might influence the aggressive phenotype of GSC, thus more research is needed.
Accordingly, MEIS1 expression has been noted to be correlated to poor prognosis of various cancers (Schulte and Geerts Citation2019). Our findings show that MEIS1 is highly expressed in high-grade glioma and was significantly associated with decreased overall survival in glioma datasets. Univariate and multivariate analyses revealed MEIS1 expression to be an independent prognosticator in gliomas. These findings suggest for the first time that MEIS1 could be a prognostic biomarker for gliomas.
MEIS1 is upregulated in the GBM classical subtype. The GBM classical subtype is characterized by amplification of Chr.7, loss of Chr.10, EGFR amplification, inactivation of RB pathway, homozygous deletion of focal 9p21.3, and upregulation of Sonic Hedgehog and Notch signaling pathway genes (Zhang et al. Citation2020). Meis1 is reported to upregulate Notch pathway genes (Isogai et al. Citation2022). Notch plays a role in the maintaining of neural stem cells and controlling cell fate decisions in both the embryonic and adult brain, and it is connected with the aggressive characteristics of GBM (Tulip et al. Citation2021). On the other hand, MEIS1 is upregulated in cellular tumors. A recent study shows that the progression of the tumor originates from the tumor core to the leading edge of the tumor through the intermediate state or toward a proliferating state close to the vasculature or necrotic state (Puchalski et al. Citation2018; Celiku et al. Citation2019). Further studies are needed to elucidate the role of MEIS1 in the aggressive characteristics of GBM classical subtype and cellular tumors.
Integrated transcriptomics analysis reveals the role of MEIS1 in gliomas. We show that MEIS1 expression is positively correlated with DNA replication, DNA repair, cell cycle progression, mitosis, and cell division genes. Our in vitro and in vivo experiments show that MEIS1 expression is linked to increased GSC cell proliferation and progression. Moreover, MEIS1 knockdown influenced cell cycle-related pathways in GSC cells. In neuroblastoma, MEIS2 is important for preserving the expression of late cell-cycle genes, including those required for M-phase progression, G2-M checkpoint regulation, and DNA replication (Zha et al. Citation2014). Recently, it was found that MEIS1 is activated by TF-ELF1, then regulates the GFI1/FBW7 axis to promote gliomagenesis (Cheng et al. Citation2021). MEIS1 expression is increased in malignant peripheral nerve sheath tumors, where it inhibits the p27 cell cycle suppressor through TF inhibitor of DNA binding 1 (ID1), thereby promoting cell proliferation and sustaining cell survival (Patel et al. Citation2016). Additional study is necessary to understand the mechanism by which MEIS1 regulates the cell cycle to promote GSC progression.
We also employed single-cell transcriptomics analysis to elucidate the importance of MEIS1 in the tumor microenvironment (TME). The results reveal that MEIS1 is preferentially expressed in gliomas, particularly in the NPC- and OPC-like glioma clusters which corresponds to classical and proneural GBM subtypes. Moreover, MEIS1 is enriched in the highly proliferative region of the cell cluster based on the G1/S and G2/M scores. FGSEA analysis further proves that MEIS1 co-expressed with cell cycle, DNA repair, and DNA metabolic process genes. A study revealed a high correlation between MEIS1 expression and the majority of immune cells in various cancers (Li et al. Citation2023). For example, the FTO/RP5-991G20.1/hsa-miR-1976/MEIS1 regulatory axis is necessary for regulating the infiltration of immune cells and prognosis in ovarian cancer patients (Chen et al. Citation2023). Additionally, in early-stage ovarian cancer, MEIS1 contributes to CD8+ T-lymphocyte infiltration and stimulates the synthesis of chemokines (Karapetsas et al. Citation2018). A more in-depth understanding of how MEIS1 impacts the TME, particularly in GBM, is required in the future.
The oncogenic role of MEIS1 is affected by multiple factors, and the complex role of MEIS1 in proliferation may largely depend on the TME (Meng et al. Citation2021). MEIS-HOX proteins interaction may transactivate HIFs to promote aberrant cell growth in the hypoxic TME, hence contributing to the Warburg effect (Girgin et al. Citation2020). Conversely, MEIS1 deletion decreases HIF1A and HIF2A expression in HSCs, leading to mitochondrial metabolism increased reactive oxygen species generation, and apoptosis (Kocabas et al. Citation2012). Our scRNA sequencing analysis shows that MEIS1 expression is negatively correlated with apoptosis and oxidative phosphorylation genes. Meanwhile, RNA sequencing analysis shows knockdown GSCs show that apoptosis genes are upregulated. Studies show that a decrease in MEIS1 in perinatal cardiomyocytes promotes the maturation of oxidative phosphorylation (Lindgren et al. Citation2019). Also, MEIS1-HOXA9 interaction prevents apoptosis and protects cells against the effect of apoptosis-inducing factors, leading to leukemogenesis (Wermuth and Buchberg Citation2005). A recent study shows that inhibition of MEIS1 in primary leukemia cells reduced cell viability and stem cell property by inducing apoptosis (Meriç et al. Citation2024). Hence, further investigation is needed to elucidate the role of MEIS1 in oxidative phosphorylation and apoptosis, particularly in GSC.
A study shows that MEIS1 inhibition can enhance apoptosis and hampered self-renewal in adult HSCs via downregulating HIF1A and HIF2A (Jiang et al. Citation2021). Accordingly, stabilizing HIF2A increases the expression of stem cell factors such as OCT4, SOX2, and NANOG. Conversely, HIF2A knockdown inhibits GSC self-renewal and tumor formation in vitro and in vivo (Boyd et al. Citation2021). MEIS1-SOX2 interaction maintains the stem cell status and cell proliferation in esophageal squamous cell carcinoma cells (Zargari et al. Citation2020). In our study, there was no significant effect in the expression of stemness markers after MEIS1 knockdown in GSCs. Therefore, more research is required to understand the mechanism of how MEIS1 expression could influence the stemness or self-renewal property of GSCs.
We also observed the downregulation of the ABC transporters pathway as MEIS1 is inhibited. The RNA sequencing data revealed that ABCC6 was substantially downregulated gene upon MEIS1 knockdown. Recent studies have consistently indicated that the ABCC6 gene is a significant factor in developing resistance to pharmacological treatments. In breast cancer cells, the overexpression of the ABCC6P1 pseudogene enhances drug resistance, likely through the activation of its ancestral gene, ABCC6 (Hashemi and Golalipour Citation2022). In another study, the efficacy of Subtrifloralactone G extracted from Deprea subtriflora in targeting ABCC6 was demonstrated, highlighting its promise as a therapeutic agent for drug-resistant triple-negative breast cancer (Pandya et al. Citation2020). ABCC6 expression, enhanced by ID4-mediated SOX2 induction, plays a pivotal role in imparting chemoresistance to GSCs, highlighting a key mechanism in glioma cell drug resistance (Jeon et al. Citation2011). However, there is yet to be research demonstrating that MEIS1 regulates drug resistance through ABCC6. Future investigations into the role of GSCs in mediating drug resistance via MEIS1-ABCC6 interactions might offer novel therapeutic targets for GBM treatment.
In conclusion, MEIS1 is highly expressed in GSCs, and its expression influences GSC proliferation and development. We propose that MEIS1 is a novel biomarker for GBM and a potential therapeutic target. Therefore, there is need for a continued efforts to study the role of MEIS1 in aggressive GSC behavior, as this may represent potentially novel targets for future therapies. Specifically, MEIS1 direct targeting in GSC may open up new possibilities for inhibiting its molecular interactions with other oncogenic transcription factors, which in turn enables the development of novel treatments such as small peptide inhibitors.
Author contributions
Conceptualization: H-JK, S-HK; Acquisition of data: H-JK, DRB; Analysis and interpretation of data: H-JK, DRB, Y-JJ, SSL, SB, S-HK; Writing and editing of the manuscript: DRB, H-JK, Y-JJ, SSL, SB, S-HK; Funding acquisition: SB, S-HK; Study supervision: S-HK.
Supplemental Material
Download Zip (4.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdelfattah N, Kumar P, Wang C, Leu J-S, Flynn WF, Gao R, Baskin DS, Pichumani K, Ijare OB, Wood SL, et al. 2022. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat Commun. 13(1):767. doi:10.1038/s41467-022-28372-y.
- Akoglu H. 2018. User’s guide to correlation coefficients. Turk J Emerg Med. 18(3):91–93. doi:10.1016/j.tjem.2018.08.001.
- Alves ALV, Gomes INF, Carloni AC, Rosa MN, da Silva LS, Evangelista AF, Reis RM, Silva VAO. 2021. Role of glioblastoma stem cells in cancer therapeutic resistance: a perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res Ther [Internet]. 12(1):206. doi:10.1186/s13287-021-02231-x.
- Arunachalam E, Rogers W, Simpson GR, Möller-Levet C, Bolton G, Ismael M, Smith C, Keegen K, Bagwan I, Brend T, et al. 2022. HOX and PBX gene dysregulation as a therapeutic target in glioblastoma multiforme. BMC Cancer. 22(1). doi:10.1186/s12885-022-09466-8.
- Berdasco M, Ropero S, Setien F, Fraga MF, Lapunzina P, Losson R, Alaminos M, Cheung N-K, Rahman N, Esteller M. 2009. Epigenetic inactivation of the Sotos overgrowth syndrome gene histone methyltransferase NSD1 in human neuroblastoma and glioma. Proc Natl Acad Sci USA. 106(51):21830–21835. doi:10.1073/pnas.0906831106.
- Bhanvadia RR, VanOpstall C, Brechka H, Barashi NS, Gillard M, McAuley EM, Vasquez JM, Paner G, Chan W-C, Andrade J, et al. 2018. MEIS1 and MEIS2 expression and prostate cancer progression: a role for HOXB13 binding partners in metastatic disease. Clin Cancer Res [Internet]. 24(15):3668–3680. doi:10.1158/1078-0432.CCR-17-3673.
- Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, et al. 2013. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 24(3):331–346. doi:10.1016/j.ccr.2013.08.001.
- Blasi F, Bruckmann C. 2021. MEIS1 in hematopoiesis and cancer. How MEIS1-PBX interaction can be used in therapy. J Dev Biol. 9(4):44. doi:10.3390/jdb9040044.
- Boyd NH, Tran AN, Bernstock JD, Etminan T, Jones AB, Gillespie GY, Friedman GK, Hjelmeland AB. 2021. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics. 11(2):665–683. doi:10.7150/thno.41692.
- Brotto DB, Siena ÁDD, de Barros II, Carvalho S da CES, Muys BR, Goedert L, Cardoso C, Plaça JR, Ramão A, Squire JA, et al. 2020. Contributions of HOX genes to cancer hallmarks: enrichment pathway analysis and review. Tumour Biol [Internet]. 42(5):1010428320918050. doi:10.1177/1010428320918050.
- Celiku O, Gilbert MR, Lavi O. 2019. Computational modeling demonstrates that glioblastoma cells can survive spatial environmental challenges through exploratory adaptation. Nat Commun. 10(1):5704. doi:10.1038/s41467-019-13726-w.
- Chen L, Gao W, Lin L, Sha C, Li T, Chen Q, Wei H, Yang M, Xing J, Zhang M, et al. 2023. A methylation- and immune-related lncRNA signature to predict ovarian cancer outcome and uncover mechanisms of chemoresistance. J Ovarian Res. 16(1):186. doi:10.1186/s13048-023-01260-9.
- Cheng M, Zeng Y, Zhang T, Xu M, Li Z, Wu Y. 2021. Transcription factor ELF1 activates MEIS1 transcription and then regulates the GFI1/FBW7 axis to promote the development of glioma. Mol Ther Nucleic Acids. 23:418–430. doi:10.1016/j.omtn.2020.10.015.
- Davis M. 2016. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs [Internet]. 20(5):S2–S8. doi:10.1188/16.CJON.S1.2-8.
- Feng Y, Zhang T, Wang Y, Xie M, Ji X, Luo X, Huang W, Xia L. 2021. Homeobox genes in cancers: from carcinogenesis to recent therapeutic intervention. Front Oncol [Internet]. 11. doi:10.3389/fonc.2021.770428.
- Girgin B, Karadağ-Alpaslan M, Kocabaş F. 2020. Oncogenic and tumor suppressor function of meis and associated factors. Turk J Biol. 44(6):328–355. doi:10.3906/biy-2006-25.
- Gonçalves CS, Le Boiteux E, Arnaud P, Costa BM. 2020. HOX gene cluster (de)regulation in brain: from neurodevelopment to malignant glial tumours. Cell Mol Life Sci [Internet]. 77(19):3797–3821. doi:10.1007/s00018-020-03508-9.
- Hashemi M, Golalipour M. 2022. ABCC6P1 pseudogene induces ABCC6 upregulation and multidrug resistance in breast cancer. Mol Biol Rep. 49(10):9633–9639. doi:10.1007/s11033-022-07872-6.
- He Z-C, Liu Q, Yang K-D, Chen C, Zhang X-N, Wang W-Y, Zeng H, Wang B, Liu Y-Q, Luo M, et al. 2022. HOXA5 is amplified in glioblastoma stem cells and promotes tumor progression by transcriptionally activating PTPRZ1. Cancer Lett [Internet]. 533:215605. doi:10.1016/j.canlet.2022.215605.
- Hu Y, Smyth GK. 2009. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 347(1–2):70–78. doi:10.1016/j.jim.2009.06.008.
- Isogai E, Okumura K, Saito M, Tokunaga Y, Wakabayashi Y. 2022. Meis1 plays a roles in cortical development through regulation of cellular proliferative capacity in the embryonic cerebrum. Biomed Res [Internet]. 43(3):91–97. doi:10.2220/biomedres.43.91.
- Jeon H-M, Sohn Y-W, Oh S-Y, Kim S-H, Beck S, Kim S, Kim H. 2011. ID4 imparts Chemoresistance and cancer stemness to glioma cells by derepressing miR-9*–Mediated suppression of SOX2. Cancer Res. 71(9):3410–3421. doi:10.1158/0008-5472.CAN-10-3340.
- Jiang M, Xu S, Bai M, Zhang A. 2021. The emerging role of MEIS1 in cell proliferation and differentiation. Am J Physiol-Cell Physiol. 320(3):C264–C269. doi:10.1152/ajpcell.00422.2020.
- Johng D, Torga G, Ewing CM, Norris JK, McDonnell JD, Isaacs DP, B W. 2019. HOXB13 interaction with MEIS1 modifies proliferation and gene expression in prostate cancer. Prostate [Internet]. 79(4):414–424. doi:10.1002/pros.23747.
- Karapetsas A, Tokamani M, Evangelou C, Sandaltzopoulos R. 2018. The homeodomain transcription factor MEIS1 triggers chemokine expression and is involved in CD8+ T-lymphocyte infiltration in early stage ovarian cancer. Mol Carcinog. 57(9):1251–1263. doi:10.1002/mc.22840.
- Kocabas F, Zheng J, Thet S, Copeland NG, Jenkins NA, DeBerardinis RJ, Zhang C, Sadek HA. 2012. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood. 120(25):4963–4972. doi:10.1182/blood-2012-05-432260.
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. 2006. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 9(5):391–403. doi:10.1016/j.ccr.2006.03.030.
- Li H, Tang Y, Hua L, Wang Z, Du G, Wang S, Lu S, Li W. 2023. A systematic pan-Cancer analysis of MEIS1 in human tumors as prognostic biomarker and immunotherapy target. J Clin Med. 12(4):1646. doi:10.3390/jcm12041646.
- Li W, Huang K, Guo H, Cui G. 2014. Meis1 regulates proliferation of non-small-cell lung cancer cells. J Thorac Dis. 6(6):850–855. doi:10.3978/j.issn.2072-1439.2014.06.03.
- Li Y, Gan Y, Liu J, Li J, Zhou Z, Tian R, Sun R, Liu J, Xiao Q, Li Y, et al. 2022. Downregulation of MEIS1 mediated by ELFN1-AS1/EZH2/DNMT3a axis promotes tumorigenesis and oxaliplatin resistance in colorectal cancer. Signal Transduct Target Ther. 7(1). doi:10.1038/s41392-022-00902-6.
- Li Z, Chen P, Su R, Hu C, Li Y, Elkahloun AG, Zuo Z, Gurbuxani S, Arnovitz S, Weng H, et al. 2016. PBX3 and MEIS1 cooperate in hematopoietic cells to drive acute myeloid leukemias characterized by a core transcriptome of the MLL-rearranged disease. Cancer Res. 76(3):619–629. doi:10.1158/0008-5472.CAN-15-1566.
- Lindgren IM, Drake RR, Chattergoon NN, Thornburg KL. 2019. Down-regulation of MEIS1 promotes the maturation of oxidative phosphorylation in perinatal cardiomyocytes. FASEB J. 33(6):7417–7426. doi:10.1096/fj.201801330RR.
- Liu Z, Shen F, Wang H, Li A, Wang J, Du L, Liu B, Zhang B, Lian X, Pang B, et al. 2020. Abnormally high expression of HOXA2 as an independent factor for poor prognosis in glioma patients. Cell Cycle. 19(13):1632–1640. doi:10.1080/15384101.2020.1762038.
- Mahmoudian RA, Bahadori B, Rad A, Abbaszadegan MR, Forghanifard MM. 2019. MEIS1 knockdown may promote differentiation of esophageal squamous carcinoma cell line KYSE-30. Mol Genet Genomic Med. 7(7). doi:10.1002/mgg3.746.
- Mallo M, Wellik DM, Deschamps J. 2010. Hox genes and regional patterning of the vertebrate body plan. Dev Biol [Internet]. 344(1):7–15. doi:10.1016/j.ydbio.2010.04.024.
- Meng L, Tian Z, Wang J, Liu X, Zhang W, Hu M, Wang M, Zhang Y. 2021. Effect of myeloid ecotropic viral integration site (MEIS) family genes on tumor microenvironment remodeling and its potential therapeutic effect. Transl Androl Urol. 10(2):594–608. doi:10.21037/tau-20-1163.
- Meriç N, Albayrak E, Gülbaş Z, Kocabaş F. 2024. MEIS inhibitors reduce the viability of primary leukemia cells and stem cells by inducing apoptosis. Leuk Lymphoma. 65(2):187–198. doi:10.1080/10428194.2023.2275532.
- Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, et al. 2019. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 178(4):835–849.e21. doi:10.1016/j.cell.2019.06.024.
- Pandya H, Patel CN, Bhavsar M, Pandya PN, Patel SK, Rawal RM. 2020. Analyzing the role of phytochemicals in targeting drug transporter protein ABCC6 using molecular docking and molecular dynamics simulations. Int J Pharm Sci Drug Res. 275–281. doi:10.25004/IJPSDR.2020.120310.
- Park DM, Rich JN. 2009. Biology of glioma cancer stem cells. Mol Cells. 28(1):7–12. doi:10.1007/s10059-009-0111-2.
- Patel AV, Chaney KE, Choi K, Largaespada DA, Kumar AR, Ratner N. 2016. An ShRNA screen identifies MEIS1 as a driver of malignant peripheral nerve sheath tumors. EBioMedicine. 9:110–119. doi:10.1016/j.ebiom.2016.06.007.
- Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. 2020. Glioblastoma stem cells: driving resilience through Chaos. Trends Cancer [Internet]. 6(3):223–235. doi:10.1016/j.trecan.2020.01.009.
- Puchalski RB, Shah N, Miller J, Dalley R, Nomura SR, Yoon J-G, Smith KA, Lankerovich M, Bertagnolli D, Bickley K, et al. 2018. An anatomic transcriptional atlas of human glioblastoma. Science (1979). 360(6389):660–663. doi:10.1126/science.aaf2666.
- Rad A, Farshchian M, Forghanifard MM, Matin MM, Bahrami AR, Geerts D, A’rabi A, Memar B, Abbaszadegan MR. 2016. Predicting the molecular role of MEIS1 in esophageal squamous cell carcinoma. Tumor Biology [Internet]. 37(2):1715–1725. doi:10.1007/s13277-015-3780-9.
- Roh J, Im M, Kang J, Youn B, Kim W. 2023. Long non-coding RNA in glioma: novel genetic players in temozolomide resistance. Anim Cells Syst (Seoul). 27(1):19–28. doi:10.1080/19768354.2023.2175497.
- Schober P, Boer C, Schwarte LA. 2018. Correlation Coefficients: Appropriate Use and Interpretation. [place unknown]. doi:10.1213/ANE.0000000000002864.
- Schulte D, Geerts D. 2019. MEIS transcription factors in development and disease. Development (Cambridge). 146(16). doi:10.1242/dev.174706.
- Suvà ML, Tirosh I. 2020. The glioma stem cell model in the era of single-cell genomics. Cancer Cell [Internet]. 37(5):630–636. doi:10.1016/j.ccell.2020.04.001.
- Taylor OG, Brzozowski JS, Skelding KA. 2019. Glioblastoma Multiforme: an overview of emerging therapeutic targets. Front Oncol [Internet]. 9(SEP). doi:10.3389/fonc.2019.00963.
- Tulip IJ, Kim S-O, Kim E-J, Kim J, Lee JY, Kim H, Kim S-C. 2021. Combined inhibition of STAT and notch signalling effectively suppresses tumourigenesis by inducing apoptosis and inhibiting proliferation, migration and invasion in glioblastoma cells. Anim Cells Syst (Seoul). 25(3):161–170. doi:10.1080/19768354.2021.1942983.
- VanOpstall C, Perike S, Brechka H, Gillard M, Lamperis S, Zhu B, Brown R, Bhanvadia R, Vander Griend DJ. 2020. MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans. Elife [Internet]. 9:1–64. doi:10.7554/eLife.53600.
- Vastrad B, Vastrad C, Godavarthi A, Chandrashekar R. 2017. Molecular mechanisms underlying gliomas and glioblastoma pathogenesis revealed by bioinformatics analysis of microarray data. Med Oncol. 34(11):182. doi:10.1007/s12032-017-1043-x.
- von Burstin J, Bachhuber F, Paul M, Schmid RM, Rustgi AK. 2017. The TALE homeodomain transcription factor MEIS1 activates the pro-metastatic melanoma cell adhesion molecule Mcam to promote migration of pancreatic cancer cells. Mol Carcinog [Internet]. 56(3):936–944. doi:10.1002/mc.22547.
- Wermuth PJ, Buchberg AM. 2005. Meis1-mediated apoptosis is caspase dependent and can be suppressed by coexpression of HoxA9 in murine and human cell lines. Blood. 105(3):1222–1230. doi:10.1182/blood-2004-03-0802.
- Whitlock NC, Trostel SY, Wilkinson S, Terrigino NT, Hennigan ST, Lake R, Carrabba NV, Atway R, Walton ED, Gryder BE, et al. 2020. MEIS1 down-regulation by MYC mediates prostate cancer development through elevated HOXB13 expression and AR activity. Oncogene [Internet]. 39(34):5663–5674. doi:10.1038/s41388-020-01389-7.
- Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L, Wu Y, Daldrup-Link HE. 2021. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol Res [Internet]. 171:105780. doi:10.1016/j.phrs.2021.105780.
- Xiang P, Yang X, Escano L, Dhillon I, Schneider E, Clemans-Gibbon J, Wei W, Wong J, Wang SX, Tam D, et al. 2022. Elucidating the importance and regulation of key enhancers for human MEIS1 expression. Leukemia. 36(8):1980–1989. doi:10.1038/s41375-022-01602-4.
- Yao M, Gu Y, Yang Z, Zhong K, Chen Z. 2021. MEIS1 and its potential as a cancer therapeutic target (Review). Int J Mol Med [Internet]. 48(3). doi:10.3892/ijmm.2021.5014.
- Yuan S, Lu Y, Yang J, Chen G, Kim S, Feng L, Ogasawara M, Hammoudi N, Lu W, Zhang H, et al. 2015. Metabolic activation of mitochondria in glioma stem cells promotes cancer development through a reactive oxygen species-mediated mechanism. Stem Cell Res Ther. 6(1):198. doi:10.1186/s13287-015-0174-2.
- Zargari S, Negahban Khameneh S, Rad A, Forghanifard MM. 2020. MEIS1 promotes expression of stem cell markers in esophageal squamous cell carcinoma. BMC Cancer. 20(1). doi:10.1186/s12885-020-07307-0.
- Zha Y, Xia Y, Ding J, Choi JH, Yang L, Dong Z, Yan C, Huang S, Ding HF. 2014. MEIS2 is essential for neuroblastoma cell survival and proliferation by transcriptional control of M-phase progression. Cell Death Dis. 5(9). doi:10.1038/cddis.2014.370.
- Zhang P, Xia Q, Liu L, Li S, Dong L. 2020. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 7. doi:10.3389/fmolb.2020.562798.
