ABSTRACT
Cirrhotic cardiomyopathy is associated with poor prognosis and risk of acute heart failure after liver transplantation or interventional procedures. We aimed to assess the relationship between the severity of cardiac impairment and hepatic disease. Eighty patients and eighty controls underwent echocardiography, tissue Doppler imaging and speckle tracking measures. We assess the correlation between echocardiographic parameters and Child and MELD scores. Systolic parameters function (s wave, p < 0.001) and global longitudinal strain (p < 0.001) as well as diastolic parameters were significantly more impaired in cirrhotic patients compared to controls. There were no differences among the different groups in ‘Child score’ regarding systolic function as well as diastolic function. Paradoxically, the left atrium size correlated positively to both Child (p = 0.01, r = 0.26) and MELD scores (p = 0.02, r = 0.24). Left ventricular ejection fraction was significantly lower in decompensated patients as compared to compensated patients(p = 0.02).. We did not identify any association between severity of liver disease and cardiac dysfunction. Therefore, a transthoracic echocardiography should be performed in all cirrhotic patients before interventional and surgical procedures regardless of the severity of liver disease.
Responsible Editor Omran Bakoush, University of Lund, Sweden
1. Introduction
In 1953, cirrhotic cardiomyopathy (CC) was described for the first time in patients with alcoholic cirrhosis, in the absence of concomitant cardiac disease, as a hyperdynamic circulation with high cardiac output and peripheric vessel vasodilatation.[Citation1] This entity has poor prognosis, especially in patients undergoing invasive procedures such as transjugular intrahepatic portosystemic shunt insertion (TIPS), hepatic surgery, and hepatic transplantation.[Citation2,Citation3] Recently, diastolic dysfunction (DD) has been shown to be a predictor of mortality in cirrhotic patients.[Citation4] Although some authors suggest an increasing prevalence of DD in the advanced stages of liver disease (decompensated cirrhosis with large ascites), a clear correlation between the severity of liver cirrhosis and the stages of cardiac dysfunction has not yet been established. Identifying this correlation is difficult given the perpetual changes in the definition of CC. In 2005, the World Congress of Gastroenterology in Montreal Working Group defined CC as ‘a chronic cardiac dysfunction in patients with cirrhosis, characterized by blunted contractile responsiveness to stress, and/or altered diastolic relaxation with electrophysiological abnormalities, in the absence of known cardiac disease’.[Citation5,Citation6,Citation7] Then, several studies, using new tools to assess CC, reported widely different prevalence rates.
The hypotheses of circulatory dysfunction in cirrhosis are evolving substantially. Initially, it was explained by a generalized peripheral arterial vasodilation with low peripheral vascular resistance. Subsequent studies on cirrhotic patients with ascites indicated that arterial vasodilation occurs predominantly in the splanchnic circulation with compensatory vasoconstriction in extra splanchnic organs, including brain, kidneys and liver.[Citation6,Citation7] Therefore, the hemodynamic hypothesis is not the only proposed mechanism of systolic dysfunction. The physiopathology is more complex and many mechanisms are involved, such as impairment of beta adrenergic receptors, alteration of the ion channels function in myocardial cells, and the toxic effect of NO overproduction.[Citation8–Citation10] The correlation of these mechanisms with the severity of liver damage is difficult to assess.
We aimed in this study to check in a selected group of cirrhotic patients whether there is a relationship between the stage of liver impairment assessed by conventional scores (CHILD and MELD) and the severity of cardiac impairment assessed by the new ultrasound tools and according to the guidelines of the American Society of Echocardiography.[Citation11,Citation12]
2. Patients and methods
This was a cross-sectional study. We included all the patients who came to the department of gastroenterology between January 2013 and December 2015.
Cirrhosis was diagnosed according to clinical, laboratory, ultrasonographic and histological findings.
We excluded all the conditions that could potentially affect cardiac function: diabetes, hypertension, tabagism, pulmonary diseases, suspected coronary disease, age > 75 years or < 20 years, hepatocellular carcinoma, renal disease, anemia (hemoglobin<10 g dl–1), thyroid disease, alcoholic cirrhosis, and abnormal electrocardiogram (repolarization disorders and rhythm anomalies). We also excluded patients with bad echogenicity.
A12-lead surface EGG was obtained from all subjects in the supine position immediately before echocardiography. All measurements were made by one observer who was blinded to the patients’ characteristics.
All patients were examined at rest in the left lateral decubitus position using a Vivid-9 ultrasound system (GE Healthcare, Chicago, IL, USA). The echocardiographic measurements and analyses were performed by the same physician blinded to the findings of the other physician. Left ventricle (LV) diameters, LV mass and grading of LV hypertrophy, left ventricle ejection fraction (LVEF) (Simpson method), left atrium area and LV diastolic function were assessed as recently recommended by the American and European societies of echocardiography.[Citation11,Citation12] The analyses by two-dimensional speckle-tracking echocardiography were performed offline using EchoPac version 110.1.0 workstation, Chicago,IL,USA with blinding to the clinical characteristics of the patients. The measurements of LV longitudinal systolic strain was performed at basal, mid, and apical levels in the apical four-chamber, two-chamber, and long-axis views.
The LV mass was calculated according to the ASE convention: LVM (g) = 0.8 (1.04 (DIVGd + SIVd + PPd)3 – DIVGd3) + 0.6 g, and then indexed to the body area.[Citation6] In that regard, severe LV hypertrophy was defined as LV mass ≥ 122 g m–2 in women and ≥ 149 g m–2 in men.
LV diastolic function was assessed in terms of LV inflow diastolic velocities (in the E/A ratio, E is the velocity of the mitral flow at the beginning of diastole and A the velocity of the mitral flow during the late phase of diastole, just after atrial contraction), and lateral mitral annulus motion (s wave). Early (Ea) peak diastolic velocities of the LV inflow were determined by pulsed wave Doppler with the sample volume placed at the tips of the mitral valve.
In all patients, the diastolic function pattern was classified by using LV inflow and mitral annulus diastolic velocities as well as left atrial size according to the current recommendations.[13,14]
Normal diastolic function was defined as mitral E/A ratio < 0.8, E/Ea < 10, peak tricuspid regurgitation velocity < 2.8 and normal left atrium size.
Grade I diastolic dysfunction was defined as mitral E/A ratio < 0.8, E/Ea < 10, peak tricuspid regurgitation velocity < 2.8 m s–1 and increased left atrium size.
Grade II diastolic dysfunction was defined as mitral E/A between 0.8 and 2, E/Ea between 10 and 14, peak tricuspid regurgitation velocity > 2.8 m s–1 and increased left atrium size.
Grade III diastolic dysfunction was defined asmitral E/A between 0.8 and 2, E/Ea > 14, peak tricuspid regurgitation velocity > 2.8 m s–1 and increased left atrium size.
The assessment of liver disease severity was based on calculating Child and MELD scores.
The MELD score was calculated according to the formula MELD = 9.57 (log creatinine) + 3.78 (log bilirubine) + 11.2 (log INR) + 6.43.
2.1. Statistical analysis
All calculations were performed with the PASW statistical package (SPSS version 20.0, Chicago, IL, USA). Chi-square and Fisher exact tests were used to compare dichotomous variables where appropriate. Mann–Whitney and Wilcoxon tests were used to compare nonparametric continuous variables. P-values < 0.05 were considered significant. Spearman rank correlation was used to assess the association between echocardiographic parameters and MELD and Child scores. The results are expressed as the mean ± standard deviation and 95% confidence interval when indicated.
3. Results
Between January 2013 and December 2015, 274 patients with liver cirrhosis presented consecutively to the department of hepatogastroenterology of our hospital. Based on the selection criteria, only 80 patients were included in our study. The etiology of the cirrhosis was post-viral in most cases (). Many patients (77.5%) were under beta blockers when undergoing the echocardiography. Systolic parameters function (s wave, p < 0.001) and global longitudinal strain (p < 0.001)) as well as diastolic parameters were significantly more impaired in cirrhotic patients compared to controls ().
Table 1. Clinical data of cirrhotic patients.
Table 2. Comparison of echocardiographic data between patients and controls and among the three groups of Child score.
3.1. Systolic function
According to the above-mentioned definition (LVEF < 55%), systolic dysfunction was found in 14 patients (17.5%). Using the new echocardiographic techniques, s wave on the lateral LV wall < 8 cm s–1 was found in 33 patients (41.3%) and global longitudinal strain (GLS) > −18% was found in 18 patients (22.5%). The basal strain was significantly lower than the apical strain (−18 ± 3 vs. −22 ± 5, p < 0.001). In fact, when considering only the basal strain, we found that 40% of the patients had a low basal strain > −18%. But there was no difference among the different stages of Child scores in the prevalence of systolic parameters impairment (). Moreover, there was no correlation between Child score or MELD score and LVEF, s wave, global longitudinal strain or the Tei index ().
Table 3. Correlation of ultrasound parameters with liver disease scores.
Table 4. Comparison of echocardiographic data between compensated and decompensated patients.
Paradoxically, the ejection fraction was significantly lower in patients with decompensated cirrhosis compared with compensated patients (), and among decompensated patients there was a negative correlation between the Child score and cardiac output.
3.2. Diastolic function
Diastolic dysfunction was found in 61.2% of our patients, and it was mild (stage I or II) in most cases. No diastolic function parameter was correlated to the severity of cirrhosis (). Besides, there was no difference in the severity of diastolic function among the different groups of Child A, B and C ().
3.3. Morphological changes
The left atrium was more enlarged in the advanced stage of liver disease; there was a positive correlation between the size of the left atrium and Child and MELD scores (). The LV mass was larger in patients with ascites than in compensated patients, but there was no statistical correlation between LV mass and Child or MELD score.
3.4. Electrophysiological abnormalities
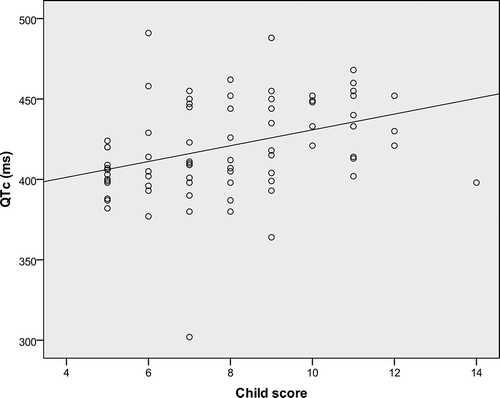
We found a longer QTc interval in patients with a more compromised liver function (patients with Child C) (), and we concluded that there was a positive correlation between the QTc interval and the severity of cirrhosis as assessed by Child score (p < 0.001, r = 0.46) ().
4. Discussion
The prevalence of cirrhotic cardiomyopathy varies widely among studies because of the lack of a clear definition, the variability of the tools used to assess this entity, and the variability of inclusion criteria. According to the World Congress of Gastroenterology, the diagnostic criteria of CC are a systolic function (a resting ejection fraction < 55% or a blunted increase in cardiac output after exercise or pharmacological stress), or a diastolic function (E/A mitral age corrected < 1, or deceleration time > 200 ms, or isovolumetric relaxation (IVRT) > 80 ms). Other supportive criteria were added, such as prolongation of QT interval, abnormal chronotropic response, enlarged left atrium, increased myocardial mass, increased brain natriuretic peptide, and increased troponin I.[Citation3] Using this definition, the prevalence of CC in the literature has been estimated between 50 and 70% in several studies.[Citation15–Citation17]
New ultrasound techniques can detect the early signs of cardiac damage, especially impairment of longitudinal function (the s wave on DTI and the global longitudinal strain on the speckle tracking analysis). According to the recent guidelines of the American Society of Echocardiography and different studies using the Vivid system, longitudinal systolic dysfunction is suspected if the global longitudinal strain (GLS) is superior to −18% and if the s wave is inferior to 8 cm s–1.[Citation11,Citation18] In our study, 17.5% of the patients had a LVEF < 55%, while 22.5% had a GLS > −18% and 41.3% had an s wave < 8 cm s–1. In fact, when considering only the basal longitudinal strain, we found that 40% of the patients had a low basal longitudinal strain > −18%, which is concordant with the degree of s wave impairment; both parameters assess basal segments. Moreover, the basal strain was significantly lower than the apical strain. Therefore, we conclude that in cirrhotic cardiomyopathy, systolic dysfunction concerns first the basal myocardium, and that is why we found a higher prevalence of CC when based on the s wave or the basal longitudinal strain. However, there was no correlation between the stage of cirrhosis and any of the systolic function parameters (LVEF, s wave, Tei index, GLS, basal strain). Disruptions in systolic function are harder to detect, as they are subtle in the resting state and may be detectable only when they are aggravated by physiological stress. Our findings are in agreement with previous studies, as no trial has found a correlation between systolic function and the severity of cirrhosis (). In our patient population, we found a negative correlation between the cardiac output and the severity of cirrhosis in patients with ascites, and the LVEF was also significantly higher in compensated patients than in decompensated patients. Recent studies indicate that although cardiac output is significantly elevated in patients with cirrhosis, it decreases during the course of the disease and it is generally low in patients with hepatorenal syndrome.[Citation19,Citation20] Nazar et al. showed that cardiac output was significantly lower in patients with ascites and in those with a marked increase in plasma renin compared to those with normal or moderately increased renin. One of the proposed mechanisms is the lack of cardiac chronotropic response to the activation of sympathetic nervous activity.[Citation14] We did not observe significant differences in heart rate between patients with compensated cirrhosis and those with ascites or among the different stages of Child. This is in agreement with the findings of Nazar et al., as they did not find an increase in the heart rate of decompensated patients despite arterial hypotension and intense activation of sympathetic nervous activity.[Citation14] A lack of increment in heart rate is also observed in cirrhosis during severe sepsis and following paracentesis-induced circulatory dysfunction.
Table 5. Correlation between liver disease severity and cardiac impairment in the literature.
Li et al. [Citation20] observed that in cirrhotic patients, increases in MELD score were accompanied by an increase in LV end systolic diameter, LV end diastolic diameter and interventricular septum thickness, with no significant changes in LV posterior wall thickness and LVEF regardless of MELD score classification; correlation analysis showed that the LV end diastolic diameter was positively correlated with MELD score. In our patient population, the size of the left atrium was positively correlated to the severity of the liver disease. In the publication of Sylvestre et al. [Citation17], left-atrial diameter, left-ventricular diastolic diameter and systolic pulmonary artery pressure were significantly correlated with the MELD score. However, diastolic function did not correlate with the severity of liver disease. Conversely, many studies did not find any differences in left atrium and left ventricle size among the different Child or MELD groups.[Citation14,Citation18,Citation21,Citation24,Citation25,Citation26] The physiopathology of morphological changes is not well understood. In cardiovascular diseases, as well as diabetes and arterial hypertension, the mechanism of these changes is the severity of diastolic dysfunction, but there was no correlation between the severity of the liver disease and the stage of diastolic dysfunction in our patients. The hepatopulmonary syndrome, defined as an oxygenation arterial defect secondary to intra-pulmonary vascular dilatation, seems to be the main cause of left atrium enlargement in liver disease. Moreover, the selection of patients is very important. Unlike previous studies, in our study we excluded all the conditions that could change the size of the left atrium, such as diabetes and hypertension. This strengthens and gives more value to our findings.
The use of complex algorithms results in some variability in the classification of DD among echocardiographers and leads to variability of the reported prevalence of DD among more recent studies on cirrhosis. Initially, the definition of DD in cirrhotic people was based on the E/A ratio, but this parameter is strongly dependent on preload and often requires age correction. Unlike transmitral valve Doppler flow, TDI directly measures the velocity of myocardial displacement during the expansion of the left ventricle in the diastole and is therefore independent of volume status and left atrial pressure. Most of the papers have included TDI parameters in the definition of DD. The prevalence of DD in our study was 61.2%, but in most cases it was mild and not related to the etiology of cirrhosis. A recent study based on TDI parameters found a DD prevalence of about 46% in cirrhotic patients and concluded that DD was a sensitive marker of advanced cirrhosis type 1 hepatorenal syndrome development, and a predictor of mortality.[Citation27]
Some authors found a lower mean E/A ratio in the ascitic subgroup as compared to non-ascitic subgroup.[Citation21,Citation28] Pozzi et al. [Citation28] showed that removal of ascitic fluid by total paracentesis reduced the A wave velocity and increased the E/A ratio to values similar to those of cirrhotic patients without ascites, but they remained abnormal compared to healthy controls. The mechanism of DD in cirrhotic patients is multifactorial. Some authors suggest that diastolic dysfunction is secondary to the increased preload status, while others consider that it is related to the increased ventricular stiffness seen in DD, including a combination of myocardial hypertrophy, fibrosis, and subendothelial edema; 75% of patients with LVDD and cirrhosis have cardiac hypertrophy.[Citation4,Citation5,Citation29] However, overt structural changes in the left ventricle are not a prerequisite for diastolic dysfunction. Indeed, a normal left ventricular morphology in patients with cirrhosis does not exclude cardiac dysfunction. Interestingly, alternative pathogenic mechanisms such as a decrease in cardiomyocyte metabolism have been recently proposed to explain DD and its reversibility after liver transplantation. Alterations in membrane physical properties play an important role in the impaired β-adrenoceptor and ion channel function in the hearts of cirrhotic rats.[Citation30] Moreover, the intake of β-blockers could have aggravated the diastolic dysfunction. Nazar et al. [Citation14] compared three groups of cirrhotic patients: those with compensated cirrhosis and, therefore, without effective arterial hypovolemia, those with ascites without hepatorenal syndrome (HRS) (this group represents patients with moderately decreased arterial blood volume), and those with ascites and HRS. There was a progressive decrease in mean arterial pressure and a progressive increase in plasma renin activity and plasma norepinephrine from the first group to the third group, reflecting a progressive deterioration in circulatory function from compensated cirrhosis to development of HRS. But there were no differences between the three groups in relation to the presence of DD or LV ejection fraction. In addition, there were no differences if we consider each individual parameter used to assess diastolic dysfunction.
Finally, repolarization abnormalities represent supportive diagnostic criteria of cirrhotic cardiomyopathy. QT prolongation is frequent in patients with cirrhosis, and in previous studies it correlated well with the severity of the liver disease. Accordingly, at least 60% of patients with end-stage liver disease have this electrocardiographic abnormality. In a prospective study, Zambruni et al. [Citation31] found a longer QTc interval in patients with a more compromised liver function. Patients with a QTc interval > 440 ms were 27% of those with Child A vs. 56% of those with Child B/C (p = 0.02), 36% of patients with MELD < 15 vs. 63% of MELD > 15 (p = 0.03), and 35% in patients without ascites vs. 62% in patients with ascites (p = 0.03).[Citation31] Conversely, the prevalence of sudden cardiac death and torsades des pointes is reported to be low in cirrhotic people.[Citation31] In our study, the QT interval correlated well to Child and MELD scores and was significantly more prolonged in patients with ascites as compared to those without ascites. But we did not find any correlation between the QT interval and the other parameters of diastolic or systolic function.
Prolongation of the QT interval appeared to be a result of a combination of ion-channel dysfunction, plasma membrane abnormalities and receptor pathway defects and may also worsen after interventional procedures and hepatic transplantation. However, the administration of β-blockers is effective for reducing the QT interval only in patients with a prolonged baseline value.[Citation32]
4.1. Limitations of the study
The limitation of our study is the unavailability of brain natriuretic peptide analysis, which is recognized as important in the evaluation of DD.[Citation21] Moreover, we did not perform a stress test (physical activity or pharmacological stress), which could have better revealed a subclinical cardiac dysfunction.
5. Conclusion
The mechanism of cardiac involvement in patients with cirrhosis seems to be complex and multifactorial, and there is no correlation between the severity of cirrhosis and the stage of cardiomyopathy. Therefore, in order to prepare the best post-procedural management of cirrhotic patients, echocardiography is required for all patients before any interventional procedure or liver transplantation regardless of the stage of cirrhosis.
Conflict of interest
The authors declare no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32(10):1–8.
- Rabie RN, Cazzaniga M, Salerno F, et al. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104(10):2458–2466.
- Cazzaniga M, Salerno F, Pagnozzi G, et al. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56(6):869–875.
- Ruíz-del-Árbol L, Achécar L, Serradilla R, et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatolbaltim Md. 2013;58(5):1732–1741.
- Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57(2):268–278.
- Guevara M, Bru C, Ginès P, et al. Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatolbaltim Md. 1998;28(1):39–44.
- Maroto A, Ginès P, Arroyo V, et al. Brachial and femoral artery blood flow in cirrhosis: relationship to kidney dysfunction. Hepatolbaltim Md. 1993;17(5):788–793.
- Herring N, Danson EJF, Paterson DJ. Cholinergic control of heart rate by nitric oxide is site specific. News Physiolsciint J Physiol Prod Jointly Int Union Physiolsci Am Physiol Soc. 2002;17:202–206.
- Anselmi A, Gaudino M, Baldi A, et al. Role of apoptosis in pressure-overload cardiomyopathy. J Cardiovasc Med Hagerstown Md. 2008;9(3):227–232.
- Szabó Z, Harangi M, Lörincz I, et al. Effect of hyperlipidemia on QT dispersion in patients without ischemic heart disease. Can J Cardiol. 2005;21(10):847–850.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Socechocardiogr off Publ Am Socechocardiogr. 2016;29(4):277–314.
- Nazar A, Guevara M, Sitges M, et al. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013;58(1):51–57.
- Sampaio F, Pimenta J, Bettencourt N, et al. Systolic and diastolic dysfunction in cirrhosis: a tissue-Doppler and speckle tracking echocardiography study. Liver Intoff J Intassoc Study Liver. 2013;33(8):1158–1165.
- Morris DA, Boldt L-H, Eichstädt H, et al. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5(5):610–620.
- Silvestre OM, Bacal F, De Souza Ramos D, et al. Impact of the severity of end-stage liver disease in cardiac structure and function. Ann Hepatol. 2013;12(1):85–91.
- Merli M, Calicchia A, Ruffa A, et al. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med. 2013;24(2):172–176.
- Fattouh AM, El-Shabrawi MH, Mahmoud EH, et al. Evaluation of cardiac functions of cirrhotic children using serum brain natriuretic peptide and tissue Doppler imaging. Ann Pediatrcardiol. 2016;9(1):22–28.
- Li X, Yu S, Li L, et al. Cirrhosis-related changes in left ventricular function and correlation with the model for end-stage liver disease score. Int J Clinexp Med. 2014;7(12):5751–5757.
- Salari A, Shafaghi A, Ofoghi M, et al. Diastolic dysfunction and severity of cirrhosis in nonalcoholic cirrhotic patients. Int J Hepatol. 2013;2013:1–6.]
- Valeriano V, Funaro S, Lionetti R, et al. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95(11):3200–3205.
- Papastergiou V, Skorda L, Lisgos P, et al. Ultrasonographic prevalence and factors predicting left ventricular diastolic dysfunction in patients with liver cirrhosis: is there a correlation between the grade of diastolic dysfunction and the grade of liver disease? ScientificWorldJournal. 2012;2012:1–6.
- Thomas JD, Badano LP. EACVI-ASE-industry initiative to standardize deformation imaging: a brief update from the co-chairs. Eur Heart J Cardiovasc Imaging. 2013;14(11):1039–1040.
- Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2000;6(4 Suppl 1):S44–S52.
- Krag A, Bendtsen F, Henriksen JH, et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59(1):105–110.
- Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatolbaltim Md. 2005;42(2):439–447.
- Pozzi M, Carugo S, Boari G, et al. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatolbaltim Md. 1997;26(5):1131–1137.
- Torregrosa M, Aguadé S, Dos L, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42(1):68–74.
- Albillos A, De La Hera A, González M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatolbaltim Md. 2003;37(1):208–217.
- Zambruni A, Trevisani F, Di Micoli A, et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008;48(3):415–421.
- Meyer JS, Mehdirad A, Salem BI, et al. Sudden arrhythmia death syndrome: importance of the long QT syndrome. Am Fam Physician. 2003;68(3):483–488.