ABSTRACT
Background Zika virus (ZIKV) has been associated with a variety of neuropathologies, including microcephaly. We hypothesize that ZIKV genes activate host microRNAs (miRNAs) causing dysfunctional development of human fetal brains.
Objectives/methods A bioinformatics search for miRNA genome-wide binding sites in the prototypic ZIKV (strain MR766) was undertaken to hunt for miRNAs with significant similarities with MCPH genetic sequences responsible for inducing MCHP in human fetal brains.
Results Six ZIKV miRNAs were found to share mutual homology with 12 MCPH genetic sequences responsible for inducing MCPH. Noteworthy was miR-1304, which expressed 100% identity to six different MCPH genes.
Conclusions We suggest that following infection of fetal neurons ZIKV may modulate the action of various miRNAs, and miR-1304 in particular, resulting in microcephaly.
KEYWORDS:
Responsible Editor Omran Bakoush, Sweden
Zika virus (ZIKV) is of emerging public health concern. It is a positive-sense, single-stranded RNA member of the Spondweni serocomplex within the genus Flavivirus, family Flaviviridae [Citation1]. The natural transmission cycle of ZIKV predominantly involves vectors from the Aedes genus and monkeys [Citation2], although ZIKV antibodies have been discovered in a number of other animals [Citation3,Citation4], including rodents [Citation5]. Humans generally act as infrequent hosts [Citation6], but may represent a primary amplification and reservoir species [Citation7]. While first isolated in 1947, ZIKV was initially described in 1952 [Citation8].The original ZIKV isolate was derived from a captive fevered rhesus macaque, Macaca mulatta, that had been platform caged and held in the canopy of Zika Forest, near Lake Victoria, Uganda, as part of a yellow fever sentinel programme. The macaque’s serum was inoculated intracerebrally into mice which, after 10 days, became ill. A filterable communicable agent was subsequently isolated from the mice brains [Citation9]. Dick [Citation9] and MacNamara [Citation10] also established the presence of ZIKV antibodies in human sera derived from West Nile and Bwamba provinces, Uganda and from Nigeria respectively. Transmission of ZIKV from A. aegypti to laboratory mice and a monkey was established by Boorman and Porterfield in 1956 [Citation11], while the first report of humans infected with ZIKV was from Nigeria in 1954, following an outbreak of jaundice in Afikpo [Citation12]. ZIKV causes an acute febrile illness, symptomatically similar to Dengue, and is characterized by mild headache, fever, maculopapular rash, joint and back pain, and general malaise, sometimes accompanied by conjunctival hyperemia, anorexia, dizziness, diarrhea, and constipation [Citation13–Citation17].The incubation period is between 3 and12 days and symptoms may last for 2–7 days. Only ~20% of patients infected with ZIKV exhibit any symptoms, and ZIKV has never been reported to cause hemorrhagic fever or death and is often misdiagnosed as Dengue [Citation13–Citation15].
The natural range of ZIKV appears to be restricted to equatorial Africa and South-east Asia. Molecular isolate evaluations of 43 strains of ZIKV from 9 countries indicate that the virus likely emerged in Uganda between 1892 and 1943 [Citation6,Citation15] and undertook two westward migrations, firstly in the mid-1930s and again in 1940, and an easterly immigration during 1945. Until recently, other than occurrences in Uganda and Nigeria, ZIKV outbreaks had been reported only sporadically in Burkina Faso, the Cameroon, Cape Verde Islands, Côte d’Ivoire, Gabon, Senegal, Sierra Leone, and the Central African Republic [Citation7,Citation15]. Cases and or serological evidence place ZIKV in Pakistan, India, Bangladesh, Malaysia, the Philippines, Thailand, Vietnam, and Indonesia [Citation2]. In 2007 the first occurrence of ZIKV outside of its apparent natural range was recorded on the island of Yap, Federated States of Micronesia [Citation13,Citation16]. This outbreak was initially thought to be Dengue, but serological analyses identified RNA of ZIKV. Subsequently, ZIKV occurrences were reported for French Polynesia, Easter Island, the Cook Islands, and New Caledonia [Citation14,Citation15,Citation17]; with the initiating strain most likely being derived from South-east Asia [Citation7]. The French Polynesian outbreak of ZIKV was remarkable because 74 infected individuals presented with neurological symptoms, of which 47 were later diagnosed with Guillain-Barré Syndrome (GBS; [Citation18]).
In early 2015, a serious ZIKV outbreak was recorded in Camaçari, Bahia, Brazil, [Citation19] and it has been suggested [Citation20] that this resulted from viral transmission from French Polynesia, as later confirmed by RT-PCR [Citation21].The Camaçari, Bahia ZIKV outbreak was followed by similar illnesses in five neighbouring states, which led the Brazilian Ministry of Health to issue a ZIKV alert in April 2015 [Citation22]. Similar to the French Polynesian outbreak, patients presented with neurologic disorders consistent with GBS. More troubling, however, was the sharp increase in the number of children being born with microcephaly [Citation12–Citation15,Citation23]. In certain instances, impacted regions registered a more than tenfold increased occurrence; a magnitude surge that cannot be explained by random clustering. Because the microcephaly amplification was registered within nine months of the ZIKV outbreak, and since materno-fetal transmissions of other Flaviviruses are known to occur, it has been suggested that a link may exist for ZIKV and microcephaly [Citation14,Citation18]. There is mounting evidence that ZIKV is associated with a variety of neurological syndromes, including GBS, meningitis, myelitis, and meningocephalitis [Citation15]. The existence of a link between fetal defects, microcephaly, and ZIKV infection during pregnancy is strong [Citation15]. Furthermore, increased CNS deformities have been reported for newborns in French Polynesia following their ZIKV epidemic, [Citation18] and new reports linking microcephaly and ZIKV have appeared [Citation14,Citation15].
Since the report of Brazilian ZIKV outbreaks, the virus has migrated throughout the region and illnesses have been described for most other South and Central American countries [Citation19]. The pandemic has also swept into the Caribbean, with autochthonous transmission in at least six island states (Barbados, Guadeloupe, Haiti, Martinique, Puerto Rico, and Saint Martin). The rapidity of movement of ZIKV, and its potential association with microcephaly, has prompted some governments of the region to go as far as to advise women against becoming pregnant [Citation14]. On 1 February 2016, the World Health Organization (WHO) announced that the ZIKV outbreak was an ‘international health emergency’; WHO expects up to four million cases in the Americas. ZIKV has also been imported from infected regions into the USA, Canada, and some EU member states (Finland, Germany, and the Netherlands).
As is the case for other viruses that pose significant health threats to humans (e.g. Dengue and Chikungunya), it is now evident that ZIKV can be transmitted by the Asian tiger mosquito, Aedes albopictus [Citation17]. From a public health perspective, appreciating the current and future ranges of vectors will be critical to developing effective planning for disease control [Citation20]. An essential component of risk analysis must, therefore, be changes in prevailing climate, since climate change has been associated with range expansion. A. albopictus is already established in Italy, southern France, and Spain, and its range extends along the Adriatic to Greece and thence to Bulgaria, Romania, Russia, and Palestine. Transient occurrences for A. albopictus have been reported in Belgium, the Netherlands, Germany, Serbia, and Hungary. Taking into account climate change and associated rising temperatures, including modifications in precipitation and vegetation, various models reveal that the distribution of A. albopictus will inevitably broaden substantially across Europe and around the Mediterranean [Citation19–Citation21]. Sustained drought, however, may lessen local range in Sardinia and parts Spain.
As in Europe, the Asian tiger mosquito is also well established in the United States, being resident in at least 36 states, including New Jersey [Citation22]. It has been associated with the transmission of West Nile Virus, eastern equine encephalitis, and La Crosse viruses and, mimicking the models of Europe, climate-based evaluations of its spread indicate significant range extension [Citation21,Citation22]. A. albopictus could provide a vector for the onward transmission of ZIKV across both continents. At present, no specific vaccines or treatment for ZIKV are available and, although the possibility exists that present flavivirus vaccine technologies could be adapted to ZIKV [Citation21,Citation22], it is important to investigate the molecular pathogenesis of the virus.
There are number of questions that remain to be answered before an effective strategy toward a ZIKV vaccine can be mapped out. It is still unclear how and at what time of gestation ZIKV infects fetal brain cells, and what types of progenitor cells are infected by the virus. Data from Tang et al. [Citation23] and our laboratory suggest that human progenitor neurons are highly permissive to ZIKV, whereas mature neurons are relatively resistant to the virus ([Citation23], and unpublished data). Equally important is to establish whether the virus targets specific types of progenitor neurons (i.e. cerebral cortex, ocular neuron, or other neuronal types) that express specific receptors, or whether the virus infection causes global dysregulation in fetal brain development [Citation16]. It is also unknown if certain genotypes of ZIKV are more neurotropic than others [Citation24].
We hypothesize that ZIKV infection of the fetal brain modulates microRNAs (miRNAs) involved in regulating microcephaly genes and explore whether ZIKV can activate intracellular pathways leading to this condition [Citation25]. Autosomal recessive primary microcephaly (MCPH) is a neurogenic mitotic disorder present at birth, and is characterized by non-progressive mental retardation [Citation26–Citation28]. MCPH is the outcome of a smaller but architecturally normal brain, with patients exhibiting a significant decrease in size of the cerebral cortex. The head circumference (HC) shows ≥3 standard deviations from the norm when compared to age and sex matched controls (typical HC: [Citation29,Citation30]). Twelve MCPH loci (MCPH1-MCPH12) have been mapped that contain the following genes: Microcephalin, WDR62; CDK5RAP2; CASC5; ASPM; CENPJ; STIL; CEP135; CEP152; ZNF335; PHC1; and CDK6. It is predicted that MCPH gene modulations may lead to the disease phenotype due to a disturbed mitotic spindle orientation, premature chromosomal condensation, altered signaling response as a result of damaged DNA, deranged microtubule dynamics, transcriptional control, or other hidden centrosomal mechanisms that regulate the number of neurons produced by neuronal precursor cells [Citation27].
In order to explore ZIKV-mediated modulations of host genetic pathways, we hypothesized that ZIKV genes activate numerous host miRNAs that results in miRNA-based modifications of neuronal genetic pathways that share mutual homologies to MCHP genes. miRNAs are small noncoding RNAs that act as gene regulators. They are directly or indirectly involved in many cellular functions owing to their ability to target mRNAs for degradation or translational repression [Citation25]. Experimental evidence shows that miRNAs have a direct role in different cellular processes, such as immune function, apoptosis, and tumorigenesis. In a viral infection context, miRNAs have been connected with the interplay between host and pathogen, occupying a major role in pathogenesis. While numerous viral miRNAs from DNA viruses have been identified, characterization of functional RNA virus-encoded miRNAs and their potential targets is still ongoing [Citation31]. How cellular miRNAs are regulated and their functions controlled during ZIKV infection are largely unknown. miRNAs are critical regulators of gene expression that utilize sequence complementarity to bind to and modulate the stability or translation efficiency of target mRNAs. In the case of GBV-C, a nonpathogenic flavivirus, profound modulations of miRNAs have been reported [Citation32]. Accumulating data suggest that miRNAs regulate a wide variety of molecular mechanisms in host cells during viral infections [Citation31,Citation32].
In the present study, we have conducted a bioinformatics search for miRNA genome-wide binding sites in the prototypic ZIKV MR766 whole genome, and discovered six miRNAs that share significant mutual homology with the 12 MCPH genetic sequences which may be responsible for inducing MCPH in the developing human fetal brain. The six miRNAs – miR-627–5, miR-4646, miR-1304, miR-6771, miR-4528, and miR-3198 – shared high degrees of mutual homologies to the ZIKV genome and the 12 MCPH genes ( and ). In all cases, these miRNAs shared 100% identity at the seed sequences (3ʹ-2–8 base pairs) and between 75–100% homologies at the genetic level, suggesting that the genetic targets we identified most likely play a significant role in inducing MCPH [Citation32]. To our knowledge, this is the first report of the comparative analyses of mutually homologous miRNAs in ZIKV and MCPH genes. Our findings will underpin further studies of miRNAs’ roles in ZIKV replication and identify potential candidates for antiviral therapies against ZIKV, as well as future studies to prevent MCPH [Citation12–Citation14,Citation30].
Table 1. MCPH genes, their location, and homologous miRNA.
Figure 1. Locations of miRNA to ZIKV and MCPH. The upper panel illustrates the 10.79KB genomic map of ZIKV while the lower panel presents the 12 MCPH genes involved in microcephaly primary heredity (MCPH) or autosomal recessive primary microcephaly. Six miRNAs aligned perfectly at the seed sequences (shown in the middle panel) with the ZIKV and 12 MCPH genomes. Of note, miR-1304 targets six out of 12 MCPH genes.
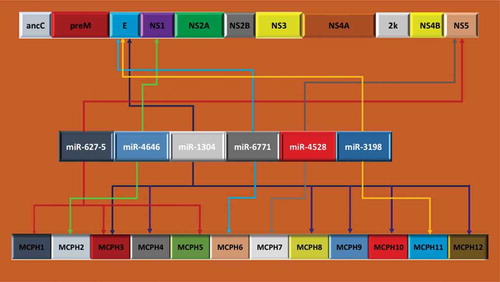
Each of the MCPH genes listed and various functions associated with each of the MCPH genes linked to various molecular pathways that subsequently result in MCPH, either alone or in combination with other genetic mutations or MCPH gene modulations, are depicted in . For example, MCPH1 (Microcephalin) is involved in chromosomal condensation, and reduced MCPH1 enhances the production of early born neurons, which comprise deep layers (IV–VI), and reduces late-born neurons, which produce the thinner outer cortex layer (II–III). MCPH2 (WDR62) is involved in cerebral cortical development, proliferation and migration of neuronal precursors, and mutation in WDR62 affects its role in proliferating and migrating neural precursors and causes severe brain malformations. MCPH3 (CDK5RAP2) regulates microtubule function, and mutation in CDK5RAP2 reduces the progenitor pool/decreases the number of neurons and reduces cell survival. MCPH4 (CASC5) is vital for the spindle checkpoint of the mitotic cycle. CASC5 underscores the role of kinetochore integrity in the proper volumetric development of the human brain. MCPH5 (ASPM) plays an essential role in orientation of mitotic spindles during embryonic neurogenesis, and ASPM mutations are known to decrease the size of the brain by influencing the orientation of the mitotic spindle. MCPH6 (CENPJ) controls centriole length/microtubule function; its deletion causes an increased incidence of multiple spindle poles, apoptosis and mitosis arrest. MCPH7 (STIL) is important in apoptosis regulator/cell cycle progression; its mutation in zebrafish, Danio rerio, causes an embryonic lethal defect, and STIL knockout mice (Sil-/-) exhibit numerous developmental abnormalities/decreased size/defective midline of the neural tube. MCPH8 (CEP135) maintains organization/structure of the centrosome, and CEP135 knockdown showed decreased growth rate/disorganized microtubules. MCPH9 (CEP152) is involved in centriole duplication/shape to cell/polarity/motility; conversion of glutamine into proline disturbs potential coiled-coiled protein domain/reduced head size. MCPH10 (ZNF335) is essential in progenitor cell division/differentiation; mutated ZNF335 gene causes degeneration of neurons, and knockdown of ZNF335 caused a small brain size with an absent cortex/disrupted proliferation and differentiation of neuronal cells. MCPH11 (PHC1) regulates the cell cycle, and its mutation highlights the role of chromatin remodeling in the pathogenesis of primary microcephaly. MCPH12 (CDK6) controls the cell cycle and differentiation of various cell types, and is engaged in microtubule organization [Citation27]. Its mutation affects apical neuronal precursor cell proliferation, reduces the progenitor pool, and decreases neuronal production. We were surprised to observe that one particular microRNA, hsa-miR-1304, showed 100% identify at the seed sequences of six different MCPH genes, namely MCPH 3, 4, 8, 9, 10, and 12, and the ZIKV genome. Therefore, it would not be illogical to hypothesize that this particular miRNA appears to play a pivotal role in development of the cerebral cortex of a developing fetus, and perturbation in this miRNA may impart some kind of neurodevelopmental error. In addition, hsa-miR-627–5 also showed a 100% identity to three different MCPH genes – MCPH1, MCPH3, and MCPH5 – and the ZIKV genome, again, pointing to a possible role in neurodevelopment. In addition, hsa-miR-4646 showed 100% identity for MCPH2, hsa-miR-4528 with MCPH7, hsa-miR-3198 with MCPH11, and the ZIKV genome [Citation19–Citation21]. The locations of each of the miRNAs to ZIKV and each of the MCPH is illustrated in .
Table 2. Summary of neurological disease and behavior genes, their location, and associated role with miRNA-1304.
We have particularly focused on the potential role of miR-1304 that showed 100% identities to six different MCPH genes. Of note, miR-1304 appears to be initiated by the viral envelop gene (E). ZIKV has two non-coding regions and a long reading frame encoding a polyprotein that is cleaved by host cell proteases to release the capsid protein C, the precursor of membrane (prM) and envelope E protein and 7 non-structural proteins (NS1 to NS7, ). The ZIKV virions contain 180 copies of protein E, unknown amounts of other proteins, and a single molecule of viral RNA [Citation16]. The surface of the virions is formed by E and M proteins. The E protein, which is ∼53 kDa, is glycosylated. Since the E protein is the main viral antigenic determinant, with membrane binding and fusion capacity when the virus enters the infected cell, any modulation by miR-1304 would be an important factor to investigate [Citation16,Citation32]. Upon release of viral RNA into the cytoplasm of infected cells, it is replicated and translated by the cellular machinery, leading to the formation of new viral particles.
Neurodevelopment is a complex, dynamic process that involves a precisely orchestrated sequence of genetic, environmental, biochemical, and physical events. A human brain contains approximately 120 billion neurons, of which 20% are located in the cerebral cortex [Citation33]. Each cortical neuron has on average 7,000 synaptic connections to other neurons, resulting in a total of 0.15 quadrillion synapses. This whole sophisticated human brain system is caged into a skull in which the brain floats in cerebrospinal fluid with a volume of 1,350 cm3 and a total surface area of 1,820 cm2 [Citation33]. Malformations of human fetal brain can result due to interrupted cortical development, and are common causes of mental disorders, including developmental delay and epilepsy. However, the clinical features are quite ambiguous: similar structural abnormalities can cause diverse symptoms. For example, the same brain regions are affected by grey matter loss in patients with different psychiatric conditions (i.e. schizophrenia, major depression, and addiction). The severity of cognitive abnormalities scales with the degree of cortical dysplasia. This suggests that brain function is closely correlated to brain structure and that it is essential to understand how and why structural abnormalities form. Several pathologies can originate from in utero viral infection that may disrupt neurodevelopment [Citation34].
Microcephaly is a rare developmental disorder associated with an abnormally small brain. In microcephalic brains, an altered cleavage plane during progenitor cell division reduces vertical, symmetric cell division and promotes horizontal, asymmetric cell division. This reduces the overall number of progenitor cells and neurons and decreases cortical volume. In mild cases, the decrease in the total number of neurons is not accompanied by a commensurate loss of cortical folding. On the other hand, in more severe cases, an additional increase of apoptosis results in thinned cortices and abnormally small convolutions [Citation33,Citation34]. It is well documented that the rubella virus (RV) induces microcephaly, where numerous fetal progenitor cells are affected (reviewed in [Citation35]). Congenital Rubella Syndrome (CRS) generally displays meningoencephalitis, retinopathy, cataract, deafness, and various other non-neuropathic manifestations [Citation33]. CRS was first described in 1938; however, the explicit pathway leading to teratogenicity still remains to be elucidated. Many of the steps leading to CRS and the consequences of CRS are well documented, but exactly how the virus causes this dramatic effect has been the subject of much speculation. Rubella virus entry, tropism, and receptor-related research still remain inconclusive [Citation35].
It is interesting to note that newborns from ZIKV-infected mothers exhibit ocular abnormities, as well as other conditions that overlap with the symptoms of CRS, suggesting that, similar to RV, an early in utero infection may be the major reason for its neuropathic effects and after, perhaps, late second trimester and early third trimester the fetus may be safe from ZIKV-induced neuropathy [Citation12–Citation14],
Lopez-Valenzuela et al. [Citation36] have carried out an extensive analyses of miRNA-1304 and shown that the predicted targets for derived miR-1304 indicates an association with behaviour and nervous system development and function. Most importantly, they identified various genes that miR-1304 targeted, including 24 gene disorders, 14 neurological diseases, as well as nervous system development and 13 function genes (reviewed in [Citation36]).
miRNA-1304 has also been intimately connected with vacuole structure and function through links with VPS52, and the clathrin heavy chain (CLTC). However, more intriguing, in context with the present research is miRNA-1304’s potential to regulate expression of various genes that influence neurogenesis. It is presently believed that miRNAs orchestrate the epigenetic regulation of neural stem cells and differentiation of lineage populations of neurons, oligodendrocytes, and other brain cells [Citation34]. Down-regulation of SOX5, a transcription factor that regulates oligodendrogenesis and the sequential generation of cortical neurons (reviewed in [Citation36]), may be strongly influenced by miRNA-1304. Other pathologies connected with SOX5 deficiency include developmental and speech delay, and a rare 12p.12.1 microdeletion syndrome that is characterized by microcephaly. miRNA-1304 has also been associated with CAPRIN2, which engages in the expression of proteins involved in synaptic plasticity in neurons. CAPRIN2 has positive activation, stimulation, and up-regulation during dendrite morphogenesis.
From extensive analyses, it is suggested that miR-1304 is a relatively novel primate-specific miRNA. This miRNA has been shown to express at very low levels in human embryonic stem cells and after differentiation of these cells into embryonic bodies [Citation6]. The deep RNA sequencing studies have uncovered low expression of miR-1304, in diverse tissues such as peripheral blood, melanoma, and most importantly in brain cortex [Citation36]. The in silico approach, based on target site predictions performed in this work, has allowed us to gain insights into the putative role of miR-1304 in development of microcephaly.
Interestingly, the analysis performed by Lopez-Valenzuela et al. [Citation36] using the Ingenuity software associated the predicted targets of derived miR-1304 with biological processes and disorders related to central nervous system development and function, which suggests that the evolutionary change of miR-1304 may affect aspects of human brain functioning and cognition, establishing a foundation that miR-1304 appear to play important function in neurodevelopment, particularly of human brain cortex.
The main basis of miRNA action is its sequence complementarity with the target regions of the genes that they regulate, particularly at the seed sequence. Functional SNPs that create or disrupt these miRNA target sites have been shown to have diverse phenotypic implications, perhaps contributing toward microcephaly, among other human diseases [Citation32]. Since one miRNA can have multiple targets, a precise identity between the seed sequence and the target gene(s) would be expected to deliver a biological effect that may disturb the expected gene function. So far, very few functional variants in miRNA genes have been described, and only a small number of studies have been devoted to describing their functional consequences [Citation25,Citation32].
Functional roles and targets of miR-627–5, miR-4646, miR-6771, miR-4528, miR-3198 have not been elucidated. However, there is one report that has shown miR-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells in vitro and growth of xenograft tumors in mice [Citation37]. We propose that these miRNAs have certain roles in neurogenesis or in development of microcephaly and may be inhibited by ZIKV infection. It is also possible that some of these miRNAs may be dysregulated indirectly due to ZIKV-induced modulations of miRNAs that have been reported by others in the case of Flaviviral infection [37].
SUPPLEMENTAL_TABLE_1.docx
Download MS Word (60.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
The supplemental material for this article can be accessed here.
References
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15:1–7.
- Henderson BE, Hewitt LE, Lule M. Serology of wild mammals. Virus Research Institute Annual Report. 1968;pp. 48–51. Nairobi, Kenya: East African Printer.
- Kaddumukasa AM, Kayondo KJ, Masiga D, et al. High proportion of mosquito vectors in Zika forest, Uganda, feeding on humans has implications for the spread of new arbovirus pathogens. Afr J Biotech. 2015;14:1418–1426.
- Darwish MA, Hoogstraal H, Roberts TJ, et al. Sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans Roy Soc Trop Med Hyg. 1983;77:442–445.
- Faye O, Freire CCM, Faye O, et al. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl Trop Dis. 2014;8:e2636.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Neg Trop Dis. 2012;6:e1477.
- Dick GWA, Kitchen SF, Haddow AJ, et al. Zika virus. I Isolations and serological specificity. Trans Roy Soc Trop Med Hyg. 1952;46:509–520.
- MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50:442–448.
- Duffy MR, Chen T, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543.
- Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50(3):238–242.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. 1964;58:335–338.
- Plourde AR, Bloch EM. A literature review of zika virus. Emerg Infect Dis. 2016;22(7):1185–1192.
- Cerbino-Neto J, Mesquita EC, Souza TM, et al. Clinical manifestations of zika virus infection, Rio de Janeiro, Brazil. Emerg Infect Dis. 2016;22(6):124–126.
- Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika virus and birth defects - reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Chan JF, Choi GK, Yip CC, et al. Zika fever and congenital Zika syndrome: an unexpected emerging arboviral disease. J Infect. 2016;72(5):507–524.
- Mlera L, Melik W, Bloom ME. The role of viral persistence in flavivirus biology. Pathog Dis. 2014;71(2):137–163.
- European Centre for Disease Prevention and Control (ECDC). Rapid risk assessment on Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barre´ syndrome – 10 December 2015. Stockholm: ECDC; 2015.
- Caminade C, Medlock JM, Ducheyne E, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J Royal Soc Interface. 2012;9:2708–2717.
- Kraemer MUG, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. elife. 2015;4:e08347.
- Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil [letter]. Emerg Infect Dis. 2015;21:1885–1886.
- Rochlin I, Ninivaggi DV, Hutchinson ML, et al. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in northeastern USA: implications for public health practitioners. PLoS One. 2013;8(4):e60874.
- Duong V, Dussart P, Buchy P. Zika virus in Asia. Int J Infect Dis. 2017;54:121–128.
- Tang H, Hammack C, Ogden S, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:1–4.
- Hughes BW, Addanki KC, Sriskanda A, et al. Infectivity of immature neurons to Zika virus: a link to congenital Zika syndrome. Ebiomedicine. 2016;10:66–70.
- Zhu Z, Chan JF, Tee KM, et al. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect. 2016;5:e22.
- Bagasra O, Prilliman KP. RNA interference: the molecular immune system. J Molecular Histology. 2004;35:545–553.
- Woods CG. Human microcephaly. Curr Opin Neurobiol. 2004;14:112–117.
- Faheem M, Naseer MI, Rasool M, et al. Molecular genetics of human primary microcephaly: an overview. BMC Med Genomics. 2015;8:S4.
- Hussain MS, Baig SM, Neumann S, et al. CDK6 associates with the centrosome during mitosis and is mutated in a large Pakistani family with primary microcephaly. Hum Mol Genet. 2013;22:5199–5214.
- Faria NR, Azevedo RDSDS, Kraemer MUG, et al. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352(6283):345–349.
- Nunes ML, Carlini CR, Marinowic D, et al. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. Pediatr. 2016;S0021–7557(16):30001–30008.
- Wakabayashi T, Hidaka R, Fujimaki S, et al. MicroRNAs and epigenetics in adult neurogenesis. Adv Genet. 2014;86:27–44.
- Budday S, Steinmann P, Kuhl E. Physical biology of human brain development. Front Cell Neurosci. 2015;9:257.
- Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev. 2000;13(4):571–587.
- Lopez-Valenzuela M, Ramírez O, Rosas A, et al. An ancestral miR-1304 allele present in Neanderthals regulates genes involved in enamel formation and could explain dental differences with modern humans. Mol Biol Evol. 2012;29(7):1797–1806.
- Bagasra O, Bagasra AU, Sheraz M, et al. Potential utility of GB virus type C as a preventive vaccine for HIV-1. Expert Rev Vaccines. 2012;11(3):335–347.
