ABSTRACT
The pandemic spread of multidrug-resistant (MDR) bacteria (i.e., methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum b-lactamase-producing Enterobacteriaceae (ESBLPE), vancomycin-resistant enterococci, carbapenemase-producing Enterobacteriaceae (CPE), multiresistant Pseudomonas aeruginosa and multiresistant Acinetobacter baumannii) pose a threat to healthcare Worldwide. We found limited data of MDR bacteria in pediatric patients hospitalized in Tunisian tertiary healthcare.The aim of the study is to evaluate the acquisition rate of MDR acquisition during hospitalization and to explore some of the associated risk factors for both carriage and acquisition at the pediatric department, Sahloul University Hospital. During September and October 2016, newly admitted patients were screened, at admission, during care and at discharge. Risk factors for colonization were explored by multivariate analysis. Of 112 newly admitted patients, 8.92% were colonized with at least one MDR. No risk factor was identified at admission. During hospitalization, five newly acquisition MDR (4.9%) were detected and eight (7.84%) at discharge. The specie most frequently detected on admission was Escherichia coli (50%), whereas, on discharge, Escherichia coli and K. pneumoniae were the species most frequently detected (52.7%). The pediatric intensive care unit (PICU) hospitalization, the length of hospital stay (more than 3days) and age under 2 years were identified as risk factor for acquisition of MDR during hospitalization. We identified several independent risk factors for contracting MDR bacteria during hospitalization in a tertiary pediatric department. The incidence of symptomatic MDR Infection among those colonized should be under close surveillance and long-term screening for those children is required. An institutional screening program for MDR especially in PICU might be discussed in regards to cost effectiveness.
1. Background
The problem of antimicrobial resistance (AMR) is worldwide one of the foremost issues that we face in the coming decades [Citation1]. The incidence of infection and colonization due to multi-drug resistant (MDR) bacteria is increasing in hospitals worldwide [Citation2]. Antimicrobial resistance is rapidly increasing in regions with poor hygiene and uncontrolled use of antimicrobials [Citation1,Citation3]. The epidemiology of MDR bacteria varies across countries and institutions. A group of international experts came together through a joint initiative by the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC), to create a standardized international terminology with which to describe acquired resistance profiles in Staphylococcus aureus, Enterococcus spp., Enterobacteriaceae (other than Salmonella and Shigella), Pseudomonas aeruginosa and Acinetobacter spp., all bacteria often responsible for healthcare-associated infections and prone to multidrug resistance [Citation4]. A recent study emphasized the importance of identifying individuals carrying antimicrobial resistant bacteria in both patient and healthy populations [Citation5]. Hospitalization per se is known to predispose to colonization, and those heading to poor regions are more likely to be hospitalized than those opting for high-income countries [Citation6]. The limited number of studies with a broad scope and the lack of surveillance systems hamper any attempts to estimate the burden of MDR acquisition and its impact on nosocomial infections in healthcare at country or regional level in low-and middle-income countries. To our knowledge, in Tunisia, little is known about the MDR colonization rate in the general population or during hospitalization.
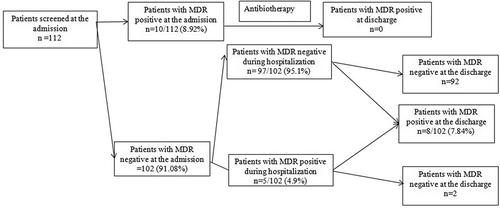
Figure 1. Patients’ distribution according to MDR carriage status on admission, during hospitalization and on discharge.
The aim of this study is to evaluate the rate of rectal and nasal carriage of MDR especially extended-spectrum B lactamase (ESBL)-producing Enterobacteriaceae, vancomycin-resistant enterococci (VRE) and meticillin-resistant Staphylococcus aureus (MRSA) in newly admitted children at our pediatric department and to determine the rate of acquisition of such organisms during their ensuing hospitalization, and risk factors for acquisition.
2. Study design and population
Our study was conducted at the pediatric department in Sahloul Hospital, a university-affiliated hospital.
Sahloul’s pediatric department is a 49-bed, care referral centre with multiple pediatric subspecialties. It serves as both a primary and tertiary care center with a six-bed pediatric intensive care unit (PICU). The nurse patient ratio is intended to be 1:2 only in PICU and 1:6 in the other units. Single room was provided in PICU, a room for two patients under the age of 2 years. In the other cases, at least three children are hospitalized in the same room.
In 2016, the annual number of admissions was 2410 with 215 admissions in PICU. Our study was a single-center prospective, observational and non interventional. It was conducted during a 2-month period (September 1–30 October 2016). During the study period, every patient admitted to the hospital had culture samples obtained at admission, at the conclusion of their hospitalization (defined as either hospital discharge or death) and every third, seventh, fourteenth day depending on length stay in hospital. For each patient enrolled in our study, we intended to collect demographic, clinical and laboratory data to determine risk factors for the acquisition of MDR. Any patient with previous documented infections with MRSA, VRE, or ESBL-producing Enterobacteriaceae was excluded from the study.
3. Microbiological techniques
Each child was tested at admission, one nasal swab and two rectal swabs were taken.
The native samples were transported in sterile tubes without transport medium and were processed immediately upon arrival at the laboratory.
Rectal swabs were immediately plated onto Maconkay agar supplemented with cefotaxime (1mg/l) and on to blood agar suppemented with vancomycin [Citation3]. Nasal swabs were plated onto blood agar with oxacillin disk (30µg).
All Gram Negative Bacilli (GNB) isolates were identified by Gram staining, oxydase production and by Api 20 E system (bioMérieux, Marcy l’Etoile, France). The isolates were then screened for ESBL production using both the resistance phenotype and the double-disk synergy test by the use of conventional combination [Citation5].
Colonies grown on blood agar with vancomycin were identified by conventional tests and by Api strept system (bioMérieux, Marcy l’Etoile, France).
All isolated strains were subjected to susceptibility testing by the disk diffusion method according to EUCAST (the European Committee on Antimicrobial Susceptibility Testing) protocols and evaluated according to EUCAST criteria [Citation7].
4. Statistical analysis
The data collection was done on a predesigned proforma including patient risk-related factors (age, gender, prior hospitalization), co-morbidities, prior exposure to antimicrobials (was defined as the administration of antibiotics for more than 48 h within 3 months preceding current hospitalization), healthcare contact within the last 6 months; admission diagnosis, immunosuppression, infection upon admission, length of stay in PICU, use of invasive devices (intubation and mechanical ventilation, central venous catheterization (CVC), urinary catheterization), use of antibiotics during hospitalization (date of onset and antibiotic therapy).
Statistical analyses were performed using SPSS 10. Continuous variables are presented as mean ± standard deviation and categorical variables as percentages. Correlation of the risk factors with laboratory findings was obtained by using the Pearson Chi-square test and Fisher’s exact test depending on the type of variable. P < 0.05 was considered to indicate a statistically significant difference.
5. Results
Out of the total 112 patients enrolled for the study, 48 (42.9%) were females and 64 (57.1%) were males. The median age of the patients was 2 years. Antimicrobial treatment had been prescribed to 6 (5.35%) of 112 patients within 3 months before admission. 37 (33%) had a history of hospitalization, 16 patients (14.5%)with hospitalization during the last 3 months and 56 patients (50%) have a prior medical history.
Of 112 newly admitted patients, 10 (8.92%) patients were colonized with at least one community-acquired MDR. Risk factors such as age, gender, infection upon admission, previous hospitalization and antibiotic use did not show any significant correlation with carriage of MDR. The spectrum of micro-organisms isolated at the admission is shown in .
Table 1. Characteristic of MDR bacteria isolated at the admission.
The median length of Pediatric department stay was 6 days [range: 2–136 days]. Antibiotic therapy was administrated during hospitalization in 31,3% (35/112).
We found no positive MDR screening nasal swabs. During hospitalization, five patients were screened MDR positive. For those patients, screening at discharge was negative in two cases.
MDR prevalence at discharge was 7.14% (8/112). depicts a flowchart of the patient carriage of MDR during hospitalization. The median time between admission and acquisition of carriage was 6 days [range: 3–15 days].
The PICU hospitalization, the length of hospital stay (more than 3days) and under 2 years were significantly associated with patients’ carriage of MDR with P value respectively 10–3, 0.03 and 0.012. However risk factors such as invasive procedure, antibiotics intake, length of hospital stay did not show any significant correlation with carriage of MDR (). A single type of MDR bacteria was recovered from 8 patients (7.14%) at discharge. The spectrum of micro-organisms at discharge is shown in .
Table 2. Risk factors for MDR acquisition at the hospital discharge.
Table 3. Characteristic of MDR bacteria isolated at discharge.
6. Discussion
The situation of MDR emergence in Africa and especially in North Africa is still unclear because of the lack of data [Citation8]. One of the methods used by various authors and authorities to characterize organisms as MDR is based on in vitro antimicrobial susceptibility test results, when they test ‘resistant to multiple antimicrobial agents, classes or subclasses of antimicrobial agents’ [Citation9,Citation10]. The definition most frequently used for multiresistant Gram-positive [Citation11] and Gram-negative [Citation12–Citation14] bacteria is ‘resistant to three or more antimicrobial classes’The screening is a tool taking part of a strategy to prevent the spread of MDR organisms which needs to take into account the local epidemiology with different strategies function of sporadic of endemic circumstances [Citation15]. However, systematic screening isn’t implanted in developing countries. In Tunisia, surveillance of antimicrobial resistance is hampered by political and financial constraints. To the authors’ knowledge, this is the first study highlighting the screening of MDR at admission and during hospitalization in a Tunisian pediatric department.
The screening of MDR organisms should focus on high risk units such as intensive care units, in association with contact precautions [Citation16]. In our study, we identified the PICU stay as a risk factor predisposing to acquisition of MDR during hospitalization. While, large studies [Citation17,Citation18] conducted have shed some light on this risk factor in intensive care units, the situation in PICU needs to be more thorough.
Most studies have shown that a long hospital stay increases the risk for colonization or infection with MDR bacteria [Citation2,Citation5]. In fact, Day 30 to screen patients was chosen, on the basis of those studies. However, R Friedmann et al. [Citation19] proved that the rate of nosocomial acquisition with MDR (especially ESBL–producing Enterobacteriaceae) increased with the length of hospitalization, doubling to 17% by day 4 or 5 after admission and gradually increasing to 33% after 10 or more days of hospitalization [Citation19]. Hospitalization for longer than 14 days in PUCI is identified as the strongest independent predictors of ESBL-KP colonization [Citation20]. In our study, we identified also this risk factor (p = 10–3) and moreover, the length of hospital stay (more than 3 days) is significantly associated to a high risk of MDR acquisition regardless of the hospitalization unit. This can be explained by the fact that, in our pediatric department there isn’t a single room, then two or three patients were hospitalized in the same room for a period of time. All the more, Huang et al. [Citation21] found that prior room contamination, whether measured via environmental cultures or prior room occupancy by MDR-colonized patients more precisely vancomycin-resistant enterococci (VRE), was highly predictive of VRE acquisition. Moreover, Arcilla et al. [Citation22] supposed a possible MDR transmission among individual who lives in the same house and share the same space.
This data obtained through screening patients during their hospital stay is useful in order to determine and devise at the local strategy of intervention to decrease MDR acquisition.
The optimal ways and means to achieve this goal are still controversial [Citation16,Citation23]. However, the condition of hospitalization in Tunisia and healthcare in developing countries lead probably to a different risk factor for MDR emergence. The carriage rate of MDR at discharge 7.14% in our study, is low comparing to other [Citation1,Citation20]. In fact, a previous prospective study of children in Turkey revealed that 40 (18.5%) of 216 patients became colonized with ESBL-KP during hospitalization [Citation20], while, Andriatahina T et al report carriage rate exceeding 50% of the 154 patients sampled on discharge after more than 48 hours of hospitalization [Citation1].
We presume that our result is the consequences of major efforts made to promote a rational use of antibiotics and strict personal hygiene to prevent the selection and the spread of these strains in our hospital for several years despite the laborious working conditions for our staff.
Worldwide, a higher prevalence of MDR GNB colonization compared with MRSA and VRE was noted, particularly ESBL [Citation24]. The same result is objective in our study.
The main result of our study is that being under 2 years of age is a risk factor of MDR acquisition. This result might reflect an inherent risk to acquire MDR by environmental contamination and hospital stay conditions in our department. Another potential explanation of the finding is that the staff members transmitted the organism from one patient to another especially for patients needing more care such as infants. Those hypothesis’ need to be proofed by screening MDR acquisition among our staff and hospitalized children simultanuously.
The incidence of symptomatic MDR infection among those colonized in a Tunisian pediatric population remains to be examined and further attests to the usefulness of screening high-risk patients. Finally, improving hospitalization conditions in Tunisian pediatric department should be a priority but remains dependent on economic resources.
7. Conclusion
The identification of MDR bacteria colonization is a tool to implement contact precautions appropriately during hospitalization in high-income countries [Citation2,Citation25]. In our country, it seems to be laborious to apply such measures. The current study focuses on the extent of MDR colonization among patients hospitalized not only at the admission, but also during and at discharge of hospitalization.
In clinical practice, these discrepancies together with organizational and economic constraints can lead to establish a systematic screening of MDR in Tunisian pediatric departments and release recommendations for better results especially highlighting the necessity of strict contact precautions [Citation26].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Miniar Tfifha
Conceptualization of the study, study design and data collection: M.Tfifha, A.Ferjani and N.Mlika. Data analysis: M.Mallouli. Draft of article and critical review of final manuscript: M.Tfifha, J.Boukadida and S.abroug. All authors have read and agreed to the final manuscript
References
- Andriatahina T, Randrianirina F, Hariniana ER, et al. High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect Dis. 2010;10:1.
- Buke C, Armand-Lefevre L, Lolom I, et al. Epidemiology of multidrug-resistant bacteria in patients with long hospital stays. Infect Control Hosp Epidemiol. 2007 Nov;28(11):1255–5.
- Khawaja T, Kirveskari J, Johansson S, et al. Patients hospitalized abroad as importers of multiresistant bacteria-a cross-sectional study. Clin Microbiol Infect Dis. 2017 Sep;23(9):673.e1-673.e8.
- Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect Dis. 2012 mars;18(3):268–281.
- Maamar E, Ferjani S, Jendoubi A, et al. High prevalence of gut microbiota colonization with broad-spectrum cephalosporin resistant enterobacteriaceae in a tunisian intensive care unit. Front Microbiol. 2016;7:1859.
- Siikamaki H, Kivela P, Fotopoulos M, et al. Illness and injury of travellers abroad: finnish nationwide data from 2010 to 2012, with incidences in various regions of the world. Euro Surveill. 2015 mai 14;20(19):15–26.
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters [Internet]. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf
- Molton JS, Tambyah PA, Ang BSP, et al. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis. 2013 mai;56(9):1310–1318.
- Kooijman MN, Kruithof CJ, Van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016 déc;31(12):1243–1264.
- Bernards AT, Bonten MJM, Cohen Stuart J et al. Laboratory detection of highly resistant microorganisms (HRMO). Available from: http://www.medinfo.nl/Richtlijnen/Infectieziekten/Laboratory%20detection%20of%20highly%20resistant%20microorganisms%20(HRMO).
- Holländer R, Ebke M, Barck H, et al. Asymptomatic carriage of Klebsiella pneumoniae producing extended-spectrum beta-lactamase by patients in a neurological early rehabilitation unit: management of an outbreak. J Hosp Infect. 2001 juill;48(3):207–213.
- Pop-Vicas A, Strom J, Stanley K, et al. Multidrug-resistant gram-negative bacteria among patients who require chronic hemodialysis. Clin J Am Soc Nephrol. 2008 mai;3(3):752–758.
- O’Fallon E, Schreiber R, Kandel R, et al. Multidrug-resistant gram-negative bacteria at a long-term care facility: assessment of residents, healthcare workers, and inanimate surfaces. Infect Control Hosp Epidemiol. 2009 déc;30(12):1172–1179.
- Vonberg R-P, Wolter A, Chaberny IF, et al. Epidemiology of multi-drug-resistant gram-negative bacteria: data from an university hospital over a 36-month period. Int J Hyg Environ Health. 2008 juill;211(34):251–257.
- Henard S, Lozniewski A, Aissa N, et al. Evaluation of the duration of vanA vancomycin-resistant Enterococcus faecium carriage and clearance during a large-scale outbreak in a region of eastern France. Am J Infect Control. 2011 mars;39(2):169–171.
- Venier AG, Zaro-Goni D, Pefau M, et al. Performance of hand hygiene in 214 healthcare facilities in South-Western France. J Hosp Infect. 2009 mars;71(3):280–282.
- Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013 Oct 16;310(15):1571–1580.
- Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis. 2008 Sep 15;47(6):760–767.
- Friedmann R, Raveh D, Zartzer E, et al. Prospective evaluation of colonization with extended-spectrum beta-lactamase (ESBL)-producing enterobacteriaceae among patients at hospital admission and of subsequent colonization with ESBL-producing enterobacteriaceae among patients during hospitalization. Infect Control Hosp Epidemiol. 2009 juin;30(6):534–542.
- Nseir S, Blazejewski C, Lubret R, et al. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect Dis. 2011 août;17(8):1201–1208.
- Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006 Oct 9;166(18):1945–1951.
- Arcilla MS, Van Hattem JM, Bootsma MCJ, et al. The Carriage Of Multiresistant Bacteria After Travel (COMBAT) prospective cohort study: methodology and design. BMC Public Health. 2014 avr 28;14:410.
- Demir S, Soysal A, Bakir M, et al. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in paediatric wards: a nested case-control study. J Paediatr Child Health. 2008 Oct;44(10):548–553.
- Lim CJ, Cheng AC, Kennon J, et al. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case-control study. J Antimicrob Chemother. 2014 juill;69(7):1972–1980.
- Harbarth S, Sax H, Fankhauser-Rodriguez C, et al. Evaluating the probability of previously unknown carriage of MRSA at hospital admission. Am J Med. 2006;119(3):275.e15-e23.
- Biehl LM, Bertz H, Bogner J, et al. Screening and contact precautions - A survey on infection control measures for multidrug-resistant bacteria in German university hospitals. Antimicrob Resist Infect Control. 2017;6:37.
