ABSTRACT
The role of intraoperative intravenous lidocaine infusion has been previously evaluated for pain relief, inflammatory response, and post-operative recovery, particularly in abdominal surgery. The present study is a randomized double-blinded trial in which we evaluated whether IV lidocaine infusion reduces isoflurane requirement, intraoperative remifentanil consumption and time to post-operative recovery in non-laparoscopic renal surgery. Sixty patients scheduled to undergo elective non-laparoscopic renal surgery under general anesthesia were enrolled to receive either systemic lidocaine infusion (group L: bolus 1.5 mg/kg followed by a continuous infusion at the rate of 2 mg/kg/hr until skin closure) or normal saline (0.9% NaCl solution) (Group C). The depth of anesthesia was monitored using the Bispectral Index Scale (BIS), which is based on measurement of the patient’s cerebral electrical activity. Primary outcome of the study was End-tidal of isoflurane concentration (Et-Iso) at BIS values of 40–60. Secondary outcomes include remifentanil consumption during the operation and time to extubation. Et-Iso was significantly lower in group L than in group C (0.63% ± 0.10% vs 0.92% ± 0.11%, p < 10–3). Mean remifentanil consumption of was significantly lower in group L than in group C (0.13 ± 0.04 µg/kg/min vs 0.18 ± 0.04 µg/kg/min, p < 10–3). Thus, IV lidocaine infusion permits a reduction of 31% in isoflurane concentration requirement and 27% in the intraoperative remifentanil need. In addition, recovery from anesthesia and extubation time was shorter in group L (5.8 ± 1.8 min vs 7.9 ± 2.0 min, p < 10–3). By reducing significantly isoflurane and remifentanil requirements during renal surgery, intravenous lidocaine could provide effective strategy to limit volatile agent and intraoperative opioids consumption especially in low and middle income countries.
Responsible Editor Amin Bredan, Ghent University, Belgium Alsemberg, Belgium
1. Introduction
Post-operative nausea or vomiting and pain are the main causes of discomfort and dissatisfaction of patients following general anesthesia. They are increased by volatile agents and high doses of opioids [Citation1]. By reducing the requirement for anesthetic agents, we suppose to decrease these side effects, shorten recovery and extubation time, and reduce anesthetic procedure costs. Several approaches were then proposed, such as intraoperative systemic lidocaine infusion. Perioperative effects of intravenous lidocaine infusion were evaluated in several animal studies showing a reduction of minimum alveolar concentration (MAC) of volatile anesthetics and intraoperative opioids consumption [Citation2,Citation3]. The mechanism of action of lidocaine involves its binding to sodium channels and its interaction with the general anesthetic agents resulting in a synergic effect [Citation4]. Lidocaine has also been shown to possess an anti-inflammatory action, and to prevent central hyperalgesia [Citation5]. In humans, the role of intraoperative systemic lidocaine [AQ] infusion was evaluated in pain relief, cytokine response, bowel function recovery, post-operative nausea and vomiting, and length of hospital stay, particularly in abdominal surgery [Citation6–Citation9]. However, the benefit of systemic lidocaine infusion is still controversial when used in others fields such as orthopedic and cardiovascular surgeries reflecting a possible organ dependent effects [Citation10]. To the best of our knowledge, there is no clinical study assessing the effect of intravenous lidocaine infusion during general anesthesia on the total dose of anesthetic agents required for lumbotomy. The aim of this study is to evaluate the effect of intravenous lidocaine on isoflurane and remifentanil requirements during general anesthesia for renal surgery by lumbotomy as monitored by Bispectral Index Scale (BIS).
2. Methods
The study protocol was approved by the institutional ethic committee of Sahloul teaching hospital. Written informed consent was obtained. Sixty patients aged 18–80 years () with American society of anaesthesiologists (ASA) physical status 1 and 2 were scheduled for elective lumbotomy under general anesthesia and enrolled prospectively in this randomized double-blinded study, which was conducted between November 2015 and December 2016. Exclusion criteria were: ASA physical status ≥ 3, history of hepatic, renal or cardiac failure, prior lumbotomy, morbid obesity (BMI > 40), pregnancy, chronic use of opioids or benzodiazepines or anti-inflammatory drugs, allergy to local anesthetics and nephrectomy for renal transplantation. Patients with a history of psychiatric disorder, arrhythmia or seizures were also excluded. All the procedures were performed by the same team of anesthesiologists and surgeons. The study was conducted in the urology operating room in Sahloul teaching hospital (Sousse, Tunisia). Patients were randomized into two groups using computer-generated randomization tables to receive either intraoperative systemic lidocaine infusion (group L) or normal saline (0.9% NaCl) infusion (group C). Approximately 30 minutes before skin incision, patients in group L (n = 30) received an IV bolus (1.5 mg/kg) of 1% lidocaine HCl without exceeding 100 mg, this was then followed by a continuous IV infusion of lidocaine at the rate of 2 mg/kg/hr until skin closure. The control group (n = 30) received the same volume of bolus and continuous infusion of normal saline. Standard monitor was used including ECG, end-tidal carbon dioxide level (EtCO2), non invasive arterial pressure and pulse oximetry. The BIS (BIS Vista®, USA) was also used to assess the depth of narcosis. The end-tidal of isoflurane (Et-Iso) concentration was monitored continuously every 10 minutes until the end of the surgical procedure using a Draeger Primus monitor (Draeger Primus, Draeger Medical Austria GmbH, Vienna, Austria). The anesthesiologist who executed the study protocol was not blinded to the group allocation, however the anesthesiologist in charge of the patient in the operating room was. All patients received the same anesthetic protocol without premedication. Anesthesia was induced with intravenous remifentanil 1 µg/kg during one minute, propofol 3 mg/kg and cisatracurium 0.15 mg/kg. After tracheal intubation, anesthesia was maintained with isoflurane in a mixture of oxygen (50%) and air (50%). Remifentanil infusion was adjusted between 0.1 and 0.5 µg/kg/min to maintain systolic blood pressure within 20% of the base line. To ensure similar anesthetic depth in all patients during the surgical procedure, the Et-Iso concentration was adjusted to maintain BIS in a range of 40–60. No supplemental neuromuscular blocking agent was given during maintenance of anesthesia. The lungs were ventilated with a tidal volume of 6–8 ml/kg, and the respiratory rate was adjusted to achieve normocapnia (EtCO2 30–35 mmHg). Intravenous infusion of crystalloids was given to patients at a rate of 5 ml/kg/hr during surgery. Hemodynamic parameters, Et-Iso concentration and its MAC Iso were monitored and recorded by the anesthesiologist in charge every 10 minutes until the end of the procedure. Surgery was performed by one of the surgeons of the urology team, with patient in lumbotomy position and with a retroperitoneal approach. Post-operative analgesia was started with paracetamol 1g and nefopam 20 mg given 20 minutes before the end of surgery. Isoflurane was stopped at the end of skin closure as well as the remifentanil. The endotracheal tube was removed when the patient met all criteria for extubation. The total dose of remifentanil, the duration of anesthesia and the extubation delay were recorded. Primary endpoint of our study was the Et-Iso concentrations at BIS 40–60. According to a previous study [Citation11], a sample of 30 patients per group was sufficient to permit a reduction of 20% in Et-Iso concentration in the lidocaine group, with an alpha error of 0.01 and beta error of 0.1. Secondary endpoints included total dose of remifentanil (µg/kg/min) infused and the time to extubation (min).
Table 1. Patients characteristics.
Statistical analysis was conducted using SPSS software for windows (Version 20.0 SPSS, Inc, Chicago, Il). The Kolmogorov-Smirnov test was used to assess variables normality distribution. Categorical variables were analyzed by chi square test. Continuous variables were compared by student’s t test. p value less than 0.05 was regarded as statically significant.
3. Results
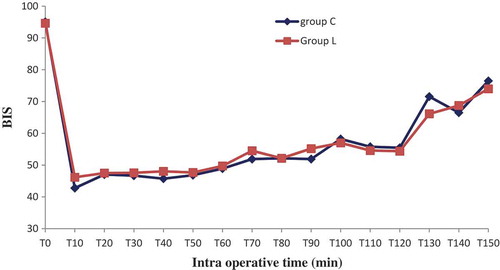
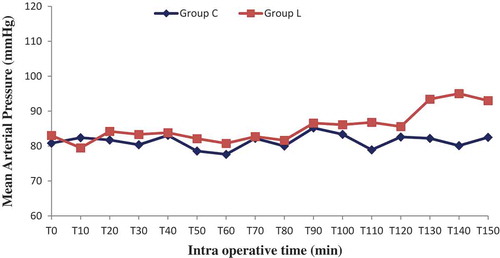
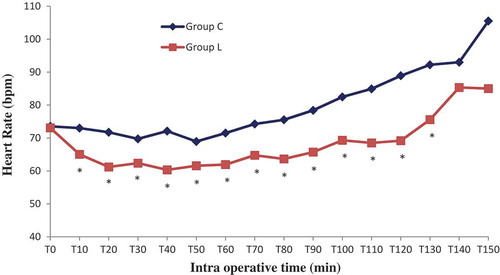
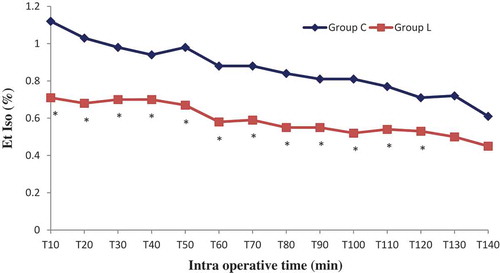
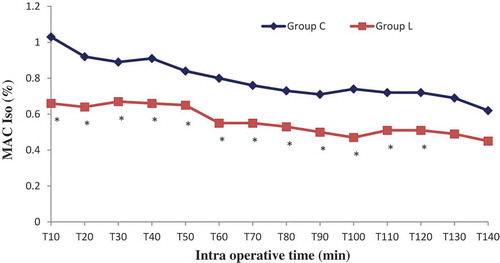
Sixty patients were randomized into two groups: Lidocaine (L) group (n = 30) and control (C) group (n = 30). All patients completed the study successfully without waivers. Baseline data of the two groups were similar. There were no significant differences in the patient’s characteristics. The numbers of pyelotomy and nephrectomy surgeries were similar, surgery and anesthesia duration was comparable (). No patient experienced a serious adverse event related to the bolus or continuous perfusion of lidocaine, except one occasional ventricular extrasystole without change in vital signs. No patient reported signs of lidocaine toxicity (metallic taste, paresthesias, dizziness, blurred vision, tinnitus, perioral numbness, drowsiness, restlessness) in the post anesthesia unit care. Regarding the BIS which was measured every 10 minutes, its value was comparable in both groups in the different measurement points, p > 0.05 (). Mean blood pressure was similar throughout anesthesia between group C and L, with p > 0.05. However, heart rate was significantly lower in group L, p < 0.05 ( and ). In the lidocaine group, it was possible to lower the end tidal and the minimum alveolar isoflurane concentrations (Et-Iso and MAC, respectively) that were required to maintain the BIS value between 40 and 60 ( and ). Average Et-Iso concentration was 0.63% ± 0.10% and 0.92% ± 0.11% in group L and C respectively (p < 10–3). The mean difference between Et-Iso concentration in the lidocaine group and the control group was 0.29%, which is a relative reduction of approximately 31% in the isoflurane concentration requirement. Moreover, the total dose of remifentanil given to patients in the group L was lower than the one administered to group C: 0.13 ± 0.04 µg/kg/min vs 0.18 ± 0.04 µg/kg/min (p < 10–3). Thus, IV lidocaine allowed a 27% reduction in the total dose of remifentanil used during the operation. At the end of surgery, the time to extubation was 5.8 ± 1.8 min for group L and 7.9 ± 2.0 min for group C (p < 10–3), a 26% reduction in recovery time. No patient needed a reversal of curare-induced neuromuscular blockade at the end of the surgical procedure, there were no differences in ephedrine and atropine use between groups.
4. Discussion
In humans, systemic lidocaine infusion was used for the first time in 1951 by Gilbert et al. in different situations: pancreatitis pain, analgesia for labour and metastatic pain [Citation12]. Three years later, De Clive-Lowe et al. used intravenous lidocaine for the first time as adjuvant during general anesthesia. They showed that lidocaine provides post-operative analgesia with low incidence of post-operative nausea and vomiting [Citation13]. Other studies showed that the use of lignocaine with thiopentone nitrous oxide-oxygen provide post-operative analgesia, low incidence of nausea and vomiting, and short recovery time without changes in pulse rate or blood pressure [Citation14,Citation15]. In our study, we assessed the intraoperative analgesic effects of a constant IV infusion of lidocaine during lumbotomy in adult patients under general anesthesia. We demonstrated, in this type of urologic surgery, that IV infusion of lidocaine during general anesthesia decreases isoflurane requirement, reduces intraoperative remifentanil doses, and shortens time to extubation. Many animal studies showed that systemic lidocaine decreases MAC of volatile anesthetic agents in several species [Citation3,Citation16]. Lidocaine infusion seems to decrease isoflurane MAC in anesthetized cats in a dose-dependent manner [Citation17]. In dogs, a bolus of 2 mg/kg followed by 100 µg/kg/min of lidocaine allowed the investigators to reduce isoflurane MAC by 27.3% [Citation18]. Although the analgesic effects of systemic lidocaine have been demonstrated for chronic pain especially the neuropathic type, the results for acute pain have been contradictory [Citation19]. Recent meta-analysis [Citation9] has found a significant difference in pain control at rest, during cough, or during movement with the use of intravenous lidocaine infusion in surgical patients under general anesthesia. Perioperative lidocaine infusion was associated with a shorter hospital stay, decreased incidence of nausea or vomiting, and faster return of bowel function, with no impact on in-hospital mortality. These differences were noticeable mainly in abdominal surgery [Citation9,Citation20]. The effect of systemic lidocaine infusion in urological surgery is poorly documented in the literature. Gourdine et al. [Citation21] confirmed the benefit of a continuous infusion of a small dose of lidocaine during radical retropubic prostatectomy. First bowel movement and flatus occurred faster in the lidocaine group than the control group (17% vs 33%). Lidocaine-treated patients had a two-thirds reduction in total pain score compared with the control group. But in this work, the authors did not study the effect of lidocaine on the consumption of volatile anesthetic agents and opioids. Lauwick et al. [Citation22] assessed the effect of systemic lidocaine infusion on post-operative functional walking capacity as a measure of postsurgical recovery after laparoscopic prostatectomy. Forty patients were enrolled to receive either lidocaine infusion (bolus of 1.5 mg/kg followed by a continuous infusion 2 mg/kg/h during surgery and 1 mg/kg/h in the PACU for 24h) or same volume of normal saline (0.9% NaCl). In the lidocaine group, patients were able to walk a longer distance over a short period of time (56 vs 43.5 m). The authors attribute these results to the anti-inflammatory action of lidocaine and its opioids-sparing effect in the post-operative period. In this same study, the intraoperative consumption of desflurane dropped by 12% (5.6 vs 6.3) in the lidocaine group while the BIS value was similar in the two groups. However, the intraoperative fentanyl use was not reduced in the lidocaine group (250 µg vs 254 µg). Only one study had evaluated the effect of IV lidocaine infusion during laparoscopic renal surgery [Citation23], and the authors did not find any significant influence of lidocaine on the length of hospital stay, post-operative pain, return of bowel function, or stress responses. Also, fentanyl dosage did not differ significantly between the two groups (lidocaine group: 810 µg vs control group: 680 µg, p = 0.47). Similarly, there was no volatile agent-sparing effect as assessed by isoflurane MAC (lidocaine group: 0.86% vs control group: 0.93%). BIS monitoring was not performed in this study unlike our trial. In this study, the investigators used transperitoneal approach for the laparoscopic renal surgery, with mobilization of the colon to access the retroperitoneal space. In our study, the patients are installed in the lumbotomy position and the retroperitoneal approach was used exclusively. To reach the kidney, surgeons must swerve the latissimus dorsi, the large external oblique, the large internal oblique and the transverse abdominal muscles [Citation24]. So we assume that pain and inflammatory reactions are more pronounced in the surgical compared to the laparoscopic approach, although we have not measured inflammatory markers during surgery. This idea was suggested by Wuethrich et al. In fact, there were no significant changes in Procalcitonin and C-Reactive Protein values observed in their patients. It may reflect the lack of systemic inflammatory response following this minimally invasive surgery [Citation23]. In an observational study Tauzin-Fin et al. showed that intravenous lidocaine improves post-operative analgesia, reduces post-operative opioids requirement, accelerates post operative recovery of bowel function and facilitates acute rehabilitation in patients undergoing laparoscopic nephrectomy. Target-controlled infusion of sufentanil (Gepts pharmacokinetic model) was used during anesthesia with sevoflurane to maintain the BIS value between 40 and 50. Intraoperative sufentanil consumption was similar for both groups (139 µg vs 121 µg), and no difference in time to tracheal extubation was noted [Citation25]. Butterworth et al. suggested inhibition of brain cell excitability as a mechanism for analgesia from infused lidocaine by increasing the current threshold of rat hippocampal pyramidal cells. The lidocaine concentration used was below those needed to block peripheral nerve conduction. This may underlie the analgesia and supplementation of general anesthesia produced by systemic lidocaine infusion [Citation26]. IV lidocaine also depresses spike activity, amplitude and conduction time in both myelinated A and unmyelinated C fibres [Citation5,Citation27]. Lidocaine anti-inflammatory activity may be another potential mechanism in improving perioperative pain by reducing cytokines release and neutrophil activation [Citation8]. It reduces spinal cord neurons activity and decreases postsynaptic depolarization mediated by N-methyl-D-aspartate (NMDA) [Citation5]. Lidocaine infused intravenously has analgesic, anti-hyperalgesic, anti-inflammatory properties, and it also reduces intra- and post-operative analgesic requirements [Citation5]. The effect of systemic lidocaine infusion on the MAC of volatile anesthetics is partially elucidated. The anesthetic agents suppress CNS Na+ channels in a voltage-dependent manner [Citation28]. Likewise, lidocaine action on both peripheral and central nervous systems involves blockade of Na+ channels [Citation5]. So both inhalant anesthetics and lidocaine act on voltage-gated Na+ channels in the central nervous system [Citation28] and thus their effects during general anesthesia could be additive. Lidocaine ensures pain relief on the spinal level [Citation29], which presupposes a reduction in MAC and decreases the volatile agents demand. Finally, lidocaine is capable of blocking brain cell excitability, and this could explain both its analgesic and MAC-reducing properties [Citation17,Citation26]. These observations have been made in several animal studies [Citation4,Citation17,Citation18]. Our results are consistent with previous reports in which IV lidocaine was found to reduce intraoperative opioid use. We found an opioid sparing effect in the intraoperative period in the lidocaine group allowing us to decrease remifentanil dosage by 27%. A literature search yielded only a few studies in which remifentanil was used as the opioid in conjunction with intraoperative systemic lidocaine infusion. Uzun et al. report that perioperative IV lidocaine infusion had no significant effect on remifentanil use during hypotensive anesthesia for transsphenoidal endoscopic hypophyseal adenoma excision [Citation30]. Kaba et al. found that the total dose of sufentanil given to patients in the lidocaine group during laparoscopic colectomy was significantly lower than in the control group (13.0 ± 3.7 µg versus 16.3 ± 3.6 µg) [Citation7]. A similar result was obtained in ambulatory surgery where lidocaine use yielded a 30% reduction in intraoperative opioid use [Citation31]. In another study, in patients undergoing laparoscopic cholecystectomy, the total fentanyl consumption was found to be significantly lower in the lidocaine group (242 ± 48.5 µg vs 323 ± 70.8 µg) [Citation32]. It should be pointed out the evidence for an opioid sparing effect of systemic lidocaine is not consistently clear in the published clinical literature regardless of whether lidocaine is used with volatile or intravenous anesthesia [Citation11,Citation22,Citation23,Citation25,Citation30,Citation33]. However, the evidence is strong in support of the effect of systemic lidocaine in reducing the total dose of volatile anesthetic required for general anesthesia. Overall, these observations appear are consistent with those made in animal studies. Sevoflurane requirement was significantly reduced during abdominal surgery as monitored by BIS at all intraoperative time points [Citation11]. The same result was found in different types of surgeries using sevoflurane [Citation7,Citation11,Citation32,Citation34] and desflurane [Citation6,Citation22,Citation27]. Similarly, systemic lidocaine allowed Altermatt et al. to reduce propofol requirement during the maintenance of total intravenous anesthesia for laparoscopic cholecystectomy as measured by BIS. However, Wuethrich et al. does not find a decrease in isoflurane MAC during laparoscopic renal surgery when using systemic lidocaine administration.
The metabolic and the endocrine responses as measured by serum cortisol, C-reactive protein (CRP and procalcitonin did not differ in the lidocaine and control group [Citation23]. These findings can be explained by the lack of a major inflammatory reaction following laparoscopic renal surgery unlike colonic surgery. In the present study we demonstrated that systemic lidocaine administration during renal surgery in the lumbotomy position has a synergistic effect on narcosis by sparing isoflurane requirement in adults, as monitored by BIS. We also showed improved analgesia resulting in reduced remifentanil dosage. Another important result of our study is that lidocaine significantly shortens the time to extubation. With respect to hemodynamics, the MAP values were comparable in the two groups, while the mean heart rate was lower in the lidocaine group. This significant negative chronotropic effect is likely the result of the direct depression of the sinus node and sympathetic tone blockade [Citation35]. This observation is particularly important for patients with severe coronary artery disease. The protocol of administering a loading dose followed by a continuous IV infusion of lidocaine during general anesthesia has been used in several previous investigations [Citation7,Citation10,Citation11,Citation31,Citation33]. The doses used are calculated to achieve a plasma lidocaine concentration well below the toxic levels (> 5 µg/ml) [Citation7,Citation21]. Plasma levels of lidocaine were not determined in our patients because this service is not available in our institution.
Lidocaine has neurological and cardiac toxicity. Perioral paresthesia, metallic taste, dizziness, slurred speech, diplopia, tinnitus, confusion, agitation and muscular spasms occur with high lidocaine serum level (> 5 µg/ml). Seizures indicate severe intoxication, which is due to inhibition of inhibitory neurons by gamma-aminobutyric acid receptors in the cerebral amygdala. Bradycardia with an increase in PR interval and widening QRS complex is seen in cardiovascular toxicity [Citation5].
There are some limitations in our study. We focused on lidocaine effect only during intraoperative period. We have not evaluated its effects after the surgery especially on pain relief, nausea and vomiting and hospital stay. Another limitation is the heterogeneity of the patients’ ages, which mean that MAC and requirement in isoflurane are not uniform especially in elderly patients. Also, the pharmacoeconomic impact of lidocaine was not addressed in our study. Nevertheless, reducing isoflurane needs by 31% and remifentanil consumption by 27% seems to be a key factor in minimizing anesthesia costs for renal surgery.
5. Conclusion
Intravenous lidocaine infusion during general anesthesia for renal surgery decreases BIS-guided isoflurane needs and remifentanil consumption. The low cost of this drug and its attractive profile regarding side effects could provide effective strategies to limit volatile agent and intra-operative opioids consumption, especially in low- and middle-income countries.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:1–7.
- Suarez MA, Seddighi R, Egger CM, et al. Effect of fentanyl and lidocaine on the end-tidal sevoflurane concentration preventing motor movement in dogs. Am J Vet Res. 2017 Jan;78(1):12–16.
- Ortega M, Cruz I. Evaluation of a constant rate infusion of lidocaine for balanced anesthesia in dogs undergoing surgery. Can Vet J. 2011;52:856–860.
- Zhang Y, Laster MJ, Eger EI 2nd, et al. Lidocaine, MK-801, and MAC. Anesth Analg. 2007;104:1098–1102.
- De Oliveira CM, Issy AM, Sakata RK. Intraoperative intravenous lidocaine. Rev Bras Anestesiol. 2010;60:325–333.
- Kuo CP, Jao SW, Chen KM, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. 2006;97:640–646.
- Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–18.
- Herroeder S, Pecher S, Schönherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200.
- Vigneault L, Turgeon AF, Côté D, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58:22–37.
- Kim KT, Cho DC, Sung JK, et al. Intraoperative systemic infusion of lidocaine reduces postoperative pain after lumbar surgery: a double-blinded, randomized, placebo-controlled clinical trial. Spine J. 2014;14:1559–1566.
- Omar AM, Aboushanab OH. Effect of intravenous lidocaine infusion on sevoflurane requirements as monitored by bispectral index: A randomized double-blinded controlled study. Egypt J Anaesth. 2013;29:235–239.
- Gilbert CR, Hanson IR, Brown AB, et al. Intravenous use of xylocaine. Curr Res Anesth Analg. 1951;30:301–313.
- De Clive-Lowe SG, Gray PWS, North J. Succinyldicholine and lignocaine by continuous intravenous drip; report of 1000 administrations. Anaesthesia. 1954;9:96–104.
- De Clive-Lowe SG, Desmond J, North J. Intravenous lignocaine anaesthesia. Anaesthesia. 1958;13:138–146.
- Phillips OC, Lyons WB, Harris LC, et al. Intravenous lidocaine as an adjunct to general anesthesia: a clinical evaluation. Anesth Analg. 1960;39:317–322.
- Schnellbacher RW, Carpenter JW, Mason DE, et al. Effects of lidocaine administration via continuous rate infusion on the minimum alveolar concentration of isoflurane in New Zealand White rabbits (Oryctolagus cuniculus). Am J Vet Res. 2013;74:1377–1384.
- Pypendop BH, Ilkiw JE. The effects of intravenous lidocaine administration on the minimum alveolar concentration of isoflurane in cats. Anesth Analg. 2005;100:97–101.
- Acevedo-Arcique CM, Ibancovichi JA, Chavez JR, et al. Lidocaine, dexmedetomidine and their combination reduce isoflurane minimum alveolar concentration in dogs. PLoS One. 2014;9:e106620.
- Koppert W, Weigand M, Neumann F, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98:1050–1055.
- Marret E, Rolin M, Beaussier M, et al. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95:1331–1338.
- Groudine SB, Fisher HA, Kaufman RP Jr, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86:235–239.
- Lauwick S, Kim DJ, Mistraletti G, et al. Functional walking capacity as an outcome measure of laparoscopic prostatectomy: the effect of lidocaine infusion. Br J Anaesth. 2009;103:213–219.
- Wuethrich PY, Romero J, Burkhard FC, et al. No benefit from perioperative intravenous lidocaine in laparoscopic renal surgery: a randomised, placebo-controlled study. Eur J Anaesthesiol. 2012;29:537–543.
- Descotes JL. Open partial nephrectomy techniques for cancer of the kidney. Prog Urol. 2009;19:234–237.
- Tauzin-Fin P, Bernard O, Sesay M, et al. Benefits of intravenous lidocaine on post-operative pain and acute rehabilitation after laparoscopic nephrectomy. J Anaesthesiol Clin Pharmacol. 2014;30:366–372.
- Butterworth J, Cole L, Marlow G. Inhibition of brain cell excitability by lidocaine, QX314, and tetrodotoxin: a mechanism for analgesia from infused local anesthetics? Acta Anaesthesiol Scand. 1993;37:516–523.
- Wu CT, Borel CO, Lee MS, et al. The interaction effect of perioperative cotreatment with dextromethorphan and intravenous lidocaine on pain relief and recovery of bowel function after laparoscopic cholecystectomy. Anesth Analg. 2005;100:448–453.
- Rehberg B, Xiao YH, Duch DS. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 1996;84:1223–1233.
- Bach FW, Jensen TS, Kastrup J, et al. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. 1990;40:29–34.
- Uzun S, Yuce Y, Erden A, et al. Impact of perioperative lidocaine infusion and bis monitorization on remifentanil dosage in hypotensive anesthesia. Eur Rev Med Pharmacol Sci. 2014;18:559–565.
- McKay A, Gottschalk A, Ploppa A, et al. Systemic lidocaine decreased the perioperative opioid analgesic requirements but failed to reduce discharge time after ambulatory surgery. Anesth Analg. 2009;109:1805–1808.
- Saadawy IM, Kaki AM, Abd El Latif AA, et al. Lidocaine vs. magnesium: effect on analgesia after a laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2010;54:549–556.
- Altermatt FR, Bugedo DA, Delfino AE, et al. Evaluation of the effect of intravenous lidocaine on propofol requirements during total intravenous anaesthesia as measured by bispectral index. Br J Anaesth. 2012;108:979–983.
- Hamp T, Krammel M, Weber U, et al. The effect of a bolus dose of intravenous lidocaine on the minimum alveolar concentration of sevoflurane: a prospective, randomized, double-blinded, placebo-controlled trial. Anesth Analg. 2013;117:323–328.
- Satoh H. Comparison of the chronotropic responses to local anesthetics (procaine, lidocaine, prilocaine, mepivacaine and bupivacaine) of the canine sinus node in situ. Jpn J Pharmacol. 1981;31(1):85–93.