ABSTRACT
Background: We evaluated the effects of intravenous dexmedetomidine during spinal anesthesia on hemodynamics, respiratory rate, oxygen saturation, sedpain, and compared them with those of saline infusion. Sixty American Society of Anesthesiologists physical status I and II cases were randomly divided into two groups. Patients were connected to the monitor after premedication, and spinal anesthesia was administered. Sensory and motor blockades were assessed using pinprick test and Bromage scale, respectively. Group I received dexmedetomidine infusion and Group II received saline infusion. Throughout the infusion process, hemodynamic data, respiratory rate, oxygen saturation, sedation, pain, Bromage score, amnesia, bispectral index, and side effects were recorded. Postoperative hemodynamic measurements, oxygen saturation, sedation, pain scores were obtained. Sedation and pain were evaluated using the Ramsay and visual analog scales, respectively. Analgesics were administered in cases with high scores on the visual analog scale. Postoperative analgesic consumption, side effects, treatments were recorded. No significant differences were found between the groups with respect to oxygen saturation, respiratory rate, pain, and side effects in the intraoperative period. Time to onset of sensorial block, maximum sensorial block, onset of motor block, and maximum motor block; bispectral index values; and apex heartbeat until 80 min of infusion, systolic arterial blood pressure until 90 min, and diastolic arterial blood pressure until 50 min were lower, whereas amnesia and sedation levels were higher in dexmedetomidine group. Postoperative pain and analgesic requirement were not different. Apex heartbeat at 15 min and systolic arterial blood pressure at 30 min were lower and sedation scores were higher in the dexmedetomidine infusion group. We demonstrated dexmedetomidine infusion had a hemodynamic depressant effect intraoperatively whereas it had no significant effect on peripheral oxygen saturation, respiratory rate, visual analog scale scores, and side effects. Dexmedetomidine infusion enhanced motor and sensory blockade quality and induced amnesia and sedation.
Responsible Editor Amin Bredan, Ghent University, Belgium Alsemberg, Belgium
1. Introduction
Regional anesthesia has several important advantages: it does not obtund normal reflexes such as coughing and swallowing, it does not affect respiration, its effects continue in the postoperative period, it is associated with lower costs and a shorter length of hospital stay, and it has been administered successfully almost in all surgical branches [Citation1].
Thus, today, low-risk regional anesthesia techniques are widely preferred in the treatment of local lesions in various parts of the body because the techniques are easy to perform, their effects last for shorter periods of time, they minimize the anxiety of the patient, and sufficient anesthesia is adminstered in the surgical field. Conscious sedation is a technique during which the patient cooperates and is able to follow the commands, even if they may be slightly obtunded; however, the patient does not realize the incidents during surgery and postoperatively while the sensory and motor functions are preserved. Administering sedation through local procedures for diagnostic and treatment purposes is recommended both to facilitate surgery at the surgeon’s end and to ensure the patient’s comfort [Citation1,Citation2].
Studies have demonstrated that α2 agonists produce sympatholytic effects and dose-dependent analgesia and sedation, and attenuate neuroendocrine and hemodynamic responses related to surgery and anesthesia. These characteristics suggest that dexmedetomidine, which is an α2 receptor agonist, is theoretically an appropriate agent for use in anesthesia [Citation3]. Moreover, reduction of opioid requirements and lack of respiratory depression are other advantages of dexmedetomidine with regard to its use in monitored anesthesia [Citation2,Citation4].
In the present study, we aimed to investigate the effects of intravenous dexmedetomidine infusion on sedation, analgesia, amnesia, spinal anesthesia (block quality), and hemodynamics in varicocelectomy, orchiectomy, inguinal hernia, and lower-extremity operations, including soft-tissue surgeries, in cases where spinal anesthesia is administered with the purpose of monitored anesthesia care.
2. Materials and methods
After obtaining the approval of the Ethics Committee and consent of the patients, the study was conducted in the Department of Anesthesiology at Trakya University Faculty of Medicine. We included 60 American Society of Anesthesiologists physical status I and II cases aged between 18 and 45 years who were to undergo inguinal hernia, varicocelectomy, orchiectomy, hemorrhoidectomy, anal fissure, or soft-tissue surgery. Patients with a medical history of opioid and warfarin use, abnormalities in coagulation tests, lumbar vertebral anomalies, neurological disorders, or infections and those under the age of 18 years were excluded from the study.
Atropine (0.015 mg kg−1; atropine sulfate ampoule 1 mg mL−1, Galen Pharmaceutical Ind. and Trade Inc., Istanbul,Turkey) plus midazolam (0.07 mg kg−1; Dormicum ampoule 5 mg mL−1, Roche Pharmaceutical Ind. and Trade Inc., Istanbul,Turkey) were administered 45 min prior to procedure for premedication. A peripheral vascular line was inserted in all subjects, lactate infusion of 10 mL kg−1 was initiated, and prophylactic antibiotics were administered through the same vascular line.
The cases were randomly divided into two groups comprising equal numbers of patients. Group I received IV dexmedetomidine (Precedex vial 200 μg mL−1, Abbott Pharmaceutical Ind. and Trade Inc., North Chicago, USA) infusion at a rate of 0.2 μg kg−1 h−1 for sedation simultaneously with spinal anesthesia. Control patients in Group II received IV saline infusion at a rate of 0.5 mg kg−1 h−1 simultaneously with spinal anesthesia.
After patients were placed on the operating table, systolic blood pressure (SAP), diastolic blood pressure (DAP), and apex beat of the heart (ABH), respiratory rate per minute (RR), peripheral oxygen saturation (SpO2), sedation and amnesia scores, and bispectral index (BIS) and visual analog scale (VAS) scores were recorded prior to intrathecal injection. Following skin preparation and covering in line with the rules of antisepsis, all patients were informed about each phase of the procedure. After the cutaneous–subcutaneous injection of 2 mL of 2% lidocaine (Jetosel ampoule 20 mg 2 mL−1, Biosel Pharmaceutical Ind. and Trade Inc., İstanbul), into the subarachnoid space from the L2-3 or L3-4 site via a 22G needle by median approach and upon detecting that cerebrospinal fluid was released from the same space, spinal anesthesia was administered by injecting 3 mL (15 mg) of bupivacaine (Marcaine heavy, 15 mg 5 mL−1, Astra Zeneca Pharmaceutical Ind. and Trade Inc., İstanbul, Turkey) SAP, DAP, ABH values, RR, SpO2, sedation and amnesia scores, as well as BIS and VAS levels were recorded at 1, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, and 90 min of dexmedetomidine infusion. The time to onset of sensory and motor blockade, duration of sensory and motor blockade, level of maximum sensory blockade, and Bromage scores were recorded. Motor blockade was evaluated using the Bromage scale [Citation5], and sensory blockade was evaluated using the pinprick test. Sedation was evaluated using the Ramsay Sedation Scale [Citation6]. Prior to the onset of medication infusion, three images were shown to the subjects. Their amnesia scores were evaluated prior and subsequent to the administration of anesthesia. Remembering the content and subject of each image was rated as 1 point. The scoring comprised 0–6 points. The infusion rate of the medication was set so as to maintain the BIS score at a level of 60–80. The values of 0–40 were regarded as deep hypnotic state, 40–60 as mild hypnotic, 60–80 as lower limit of awareness, and 80–100 as awareness [Citation7]. VAS was used to assess pain. The leftmost value on the 10-cm horizontal line represented no pain, and the rightmost value represented severe pain. The subjects indicated their levels of pain using this line [Citation8].
At the postoperative (when the subjects were taken to the recovery room following the administration of anesthesia) 5, 15, 30, 45, 60, and 120 min and 2, 4, 6, 12, 24 h, pain was assessed using VAS, and SAP, DAP, SpO2, ABH, and sedation score and initial analgesic requirements were recorded. In addition, we documented the side effects and complications such as hypotension and bradycardia induced by the medications as well as additional treatments that were used in the study.
In subjects with postoperative pain scores equal to and higher than 4, 8 mg of lornoxicam (Xefo vial 8 mg, Abdi İbrahim Pharmaceutical Ind. and Trade Inc., İstanbul) was administered intravenously. Additional doses of analgesics were recorded.
The scores were presented as mean ± SD or mean (min–max). The Kolmogorov–Smirnov test was used to test whether the quantitative variables were normally distributed. In the comparison between the two groups, the t-test was used to assess normally distributed variables in independent groups, whereas the Mann–Whitney U-test was used to assess non-normally distributed variables. A p value of <0.05 was considered statistically significant. The STATISTICA 7.0 (License Code: 31N6YUCV38) package program was used for statistical analysis.
3. Results
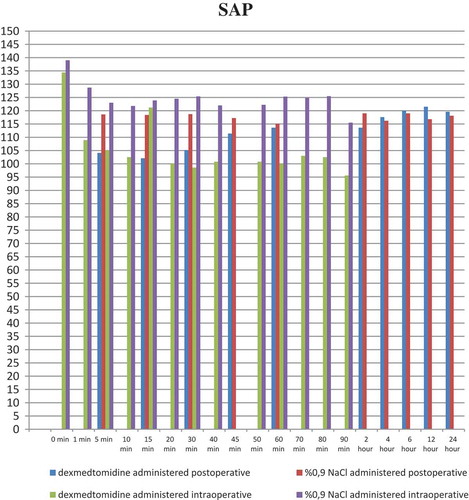
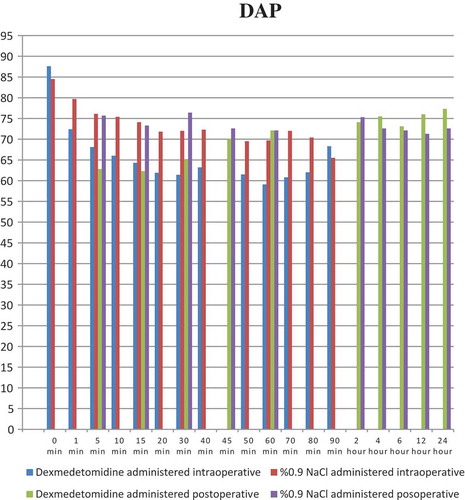
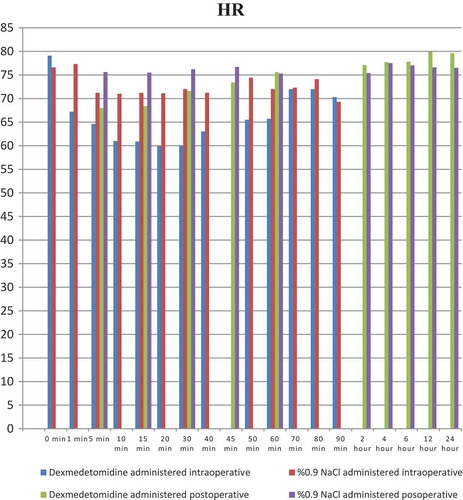
shows demographic and anthropometric data and operating times of the groups. When the intraoperative systolic arterial pressure (SAP) was evaluated between the groups, there was a statistically significant difference at 1, 5, 10, 15, 20, 30, 40, 50, 60, 70, and 80 min of infusion (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.003, p < 0.007, and p < 0.028, respectively). In terms of intraoperative DAP values between the groups, there was a statistically significant difference with respect to values at 1, 5, 10, 15, 20, 30, and 40 min of infusion (p < 0.001, p < 0.024, p < 0.001, p < 0.001, p < 0.001, p < 0.002, and p < 0.034, respectively). In addition, a statistically significant difference was found between the groups at 1, 5, 10, 15, 20, 30, 40, 50, 60, and 70 min with respect to the intraoperative ABH scores (p < 0.031, p < 0.019, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.023, p < 0.006, p < 0.006, and p < 0.028, respectively) (–).
Table 1. Demographic and antropometric data of groups, duration of operations (min–max).
There was no statistically significant difference between the groups in terms of intraoperative RR (p > 0.05). Similarly, no statistically significant difference was found between intraoperative oxygen saturation levels (SpO2) for the two groups (p > 0.05).
Intraoperative Ramsay sedation scores of the groups are shown in . There was a statistically significant difference between the groups at 1, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, and 90 min of dexmedetomidine infusion (p < 0.023, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, and p < 0.033, respectively).
Table 2. Ramsay sedation scores of the groups (min–max).
demonstrates the intraoperative amnesia scores of the groups. There was a statistically significant difference between the groups at 1, 5,10, 15, 20, 30, 40, 50, 60, 70, 80, and 90 min of dexmedetomidine infusion (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, and p < 0.006, respectively).
Table 3. Amnesia scores between the groups (min–max).
Intraoperative BIS scores of the groups are shown in . There was a statistically significant difference between the groups at 1, 5, 10, 15, 20, 30, 40, 50, 60, 70, and 80 min of dexmedetomidine infusion (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, and p < 0.005, respectively).
Table 4. BIS analysis between the groups (min–max).
Comparison of intraoperative VAS values of the groups revealed no statistically significant difference (p > 0.05).
There was a statistically significant difference between the groups at 5 min of dexmedetomidine infusion with respect to Bromage scores (p < 0.003).
In terms of intraoperative side effects, no statistically significant difference was found between the groups (p > 0.05). Nausea occurred in three cases in Group I and 2 cases in Group II, whereas bradycardia occurred in three cases in Group I and none of the cases in Group II. Hypotension was encountered in one case in Group I and none of the cases in Group II. However, there was no statistically significant difference between the groups with respect to intraoperative side effects (p > 0.05).
The mean time to onset of sensory blockade was 2.90 ± 1.47 min in Group I (range 1–8) and 5.00 ± 2.66 min in Group II (range 2–12). Therefore, there was a statistically significant difference between the groups in this aspect (p = 0.001). The mean time of cessation of the sensory blockade was found to be 131.77 ± 27.09 in Group I (105–232) and 124.80 ± 26.77 in Group II (68–180). No statistically significant difference was found between the groups (p = 0.478). The mean time to maximum sensory blockade was 11.30 ± 7.48 in Group I (2–45) and 20.20 ± 37.99 in Group II. There was a statistically significant difference between the groups (p = 0.007) ().
Table 5. Onset of sensory blockage, duration of sensory blockage, and mean time to achieve max. sensory blockage.
The mean time to onset of motor blockade was 5.27 ± 3.50 (1–20) in Group I and 8.83 ± 6.10 in Group II (2–25), and there was a statistically significant difference between the groups (p = 0.016). The mean time of cessation of motor blockade was 106.40 ± 41.02 in Group I (43–215) and 90.50 ± 35.61 in Group II (52–190). Thus, there was no statistically significant difference between the groups in this aspect (p = 0.084).
The mean time to Bromage 3 was 11.03 ± 17.13 in Group I (3–100) and 11.83 ± 5.33 in Group II (5–30). There was a statistically significant difference between the groups (p = 0.004) ().
Table 6. Onset of motor blockage, duration of motor blockage, time to achieve Bromage III.
The mean amnesia score before anesthesia was 6.00 ± 0.0 in Group I (6–6) and 6.00 ± 0.00 in Group II (6–6). Thus, no statistically significant difference was found between the groups in this aspect (p = 1.000).
The mean amnesia score after the administration of anesthesia was 3.30 ± 1.11 in Group I (2–6) and 6.00 ± 0.00 in Group II (6–6). Thus, there was a statistically significant difference between the groups in this aspect (p ≤ 0.001).
When the postoperative SAP values of the groups were compared, there was a statistically significant difference at postoperative 5, 15, and 30 min between the groups (p < 0.001, p < 0.001, and p < 0.001, respectively). The comparison of postoperative DAP values between groups revealed a statistically significant difference at postoperative 5, 15, and 30 min (p < 0.001). With respect to the postoperative ABH values of the two groups, there was a statistically significant difference at postoperative 5 and 15 min (p < 0.006 and p < 0.013, respectively) (–).
There was a statistically significant difference between the groups at postoperative 30, 45, and 60 min and at postoperative 2, 4, 6, 12 and 24 h with respect to postoperative SpO2 values (p < 0.005, p < 0.004, p < 0.008, p < 0.008, p < 0.008, p < 0.008, p < 0.008, and p < 0.011, respectively).
When the postoperative sedation scores of the groups were compared, there was a statistically significant difference at postoperative 5, 15, and 30 min (p < 0.001, p < 0.001, and p < 0.011, respectively).
In terms of postoperative VAS scores of the groups, a statistically significant difference was found at postoperative 12 h (p < 0.045). Eleven patients in Group I and 20 patients in Group II required additional analgesics. There was no statistically significant difference between the groups with respect to postoperative medication requirements (p = 0.092).
4. Discussion
Spinal anesthesia is a regional anesthesia technique wherein local anesthetic is administered into the subarachnoid space. The drug produces sympathetic sensory or motor blockade depending on its dose, concentration, and/or volume. It is widely preferred in urologic interventions [Citation9]. Spinal anesthesia has been used as a popular method since 1970s. Spinal anesthesia has various advantages such as enabling the patient to be awake during the operation, continuation of spontaneous respiration, and protection of reflexes such as swallowing and coughing. Other advantages include early mobilization in the postoperative period, prolonged postoperative analgesia, low rates of respiratory complications, and shorter length of hospital stay. However, patients who remain awake throughout surgery may experience severe stress and anxiety because they are aware of the surgical process and fear that they might feel pain. This undesired outcome is eliminated by sedating the patients during the operation. Sedation is possible through inhalation or bolus or continuous infusion of pharmacological agents injected intravenously [Citation10].
Sedation used to relieve mental and physical anxiety depresses the relevant centers in the brain and produces an altered mental state in a broad spectrum from a fully alert state to unconsciousness depending on the dose of the pharmacological agent administered [Citation11]. The primary risk of sedation is respiratory depression; therefore, ECG and noninvasive blood pressure of the patients should be monitored via devices such as pulse oxymeters, and the anesthesiologist should establish a visual and/or verbal relationship with the patient throughout the operation [Citation12].
Fcarcsi et al. compared the sedative activity of and hemodynamic responses to dexmedetomidine and midazolam administration in patients who underwent cataract surgery under topical anesthesia [Citation13]. They divided the patients into two groups and administered dexmedetomidine to Group I (loading dose of 1 μg kg−1 and maintenance dose of 0.05–0.7 μg kg−1 h−1 after 20 min) and midazolam to Group II (loading dose of 50 μg kg−1 and maintenance dose of 2.5–3.5 μg kg−1 h−1) by adjusting the infusion according to the Ramsay Sedation Scale. In Group I, the mean pulse rate decreased from 68 to 55, whereas in Group II, the mean pulse rate decreased from 68 to 63. No hypotension or desaturation episodes or significant side effects were observed in their patients. On the other hand, dexmedetomidine group demonstrated faster postoperative recovery. As a result, dexmedetomidine group showed better patient compliance and sedation level compared with midazolam group in cataract surgery.
Another study evaluated the effects of dexmedetomidine and midazolam in children aged 1–7 years via MRI with respect to their sedative, hemodynamic, and respiratory effects. Following the loading dose, dexmedetomidine infusion of 0.5 μg kg−1 h−1 was administered to one group, whereas the other group received midazolam infusion at a rate of 6 μg kg−1 min−1 following a loading dose of 0.2 mg kg−1. The level of sedation was more satisfactory and shorter in duration, and the analgesic requirement was also lower in the dexmedetomidine group. Furthermore, it was asserted that dexmedetomidine provided improved sedation without causing hemodynamic and respiratory effects [Citation14].
Coşkuner et al. [Citation15] in a study, following an injection of patients with epidural anesthesia via bupivacaine, administered dexmedetomidine infusion of 0.5 μg kg−1 h−1 throughout surgery, after an initial dose of 1 μg kg−1. The researchers found that BIS scores were lower in this group in the first 7 min compared with those in the control group, and they reported that prolonged duration of recovery from sensory blockade increased atropine consumption related to bradycardia.
A study [Observer Assessment of Alertness/Sedation] comparing the loading dose of dexmedetomidine 1 μg kg−1 followed by a maintenance dose of 0.4–0.7 μg kg−1 h−1 with a loading dose of propofol 75 μg kg−1 min−1 followed by a maintenance dose of 12.5–75 μg kg−1 min−1 evaluated the level of sedation and BIS. The level of sedation was maintained at a BIS value of 70–80. Although sedation was induced faster with propofol and slower with dexmedetomidine, it was indicated that the level of sedation was similar after the first 25 min. It was determined that a dexmedetomidine dose of 0.7 μg kg−1 h−1 was equivalent to a propofol dose of 38 μg kg−1 min−1 [Citation16].
The short half-life of dexmedetomidine ensures a primary advantage in its use in sedation. In addition, other superior characteristics include its analgesic effect and maintenance of cardiorespiratory function.
During dexmedetomidine infusion, hypotension and bradycardia may occur, particularly after the administration of the loading dose. These cardiovascular side effects can be reduced to a negligible level by lowering the initial loading dose. When infusion is discontinued, these levels increase slowly, and no rebound cardiovascular effects and symptoms of withdrawal syndrome are observed [Citation17]. In the present study, we found that systolic and diastolic arterial pressures in the dexmedetomidine group that received infusion at a rate of 0.2 μg kg−1 h−1 were significantly lower than those in the control group (p < 0.05). This result may be explained by the hypotensive effect of dexmedetomidine. On the other hand, despite the fact that there was a statistically significant difference between the groups, no intervention was required for reduced levels of arterial pressure and ABH because they did not affect the clinical conditions in the dexmedetomidine group. In our study, the fact that we did not detect any significant cardiovascular side effects may be related to the lack of administration of loading dose.
Shehabi et al. [Citation18] reported, on average, a 16% decrease in SAP and a 21% decrease in ABH in 20 intensive care unit patients during dexmedetomidine infusion that lasted 71.5 h (0.2–0.7 μg kg−1 h−1).
Santpur et al. [Citation19] divided 60 patients who underwent lower-extremity surgery into two groups, and following spinal anesthesia, they administered dexmedetomidine infusion at an initial dose of 1 μg kg−1 and at a maintenance dose of 0.5 μg kg−1 h−1 after 20 min to Group I. They administered saline infusion to Group II and compared hemodynamic stability and the duration of spinal anesthesia. They concluded that intravenous dexmedetomidine infusion provided hemodynamic stability and prolonged the duration of spinal anesthesia.
In the study by Kang et al. [Citation20], 74 patients undergoing spinal anesthesia for lower-extremity surgery were divided into two groups. Patients in Group I received an initial dexmedetomidine infusion at a rate of 1 μg kg−1 for the first 10 min followed by a maintenance infusion at a rate of 0.2 μg kg−1. Patients in Group II received the same doses over the course of 5 min. Preoperative vital signs, anxiety, and patient comfort were compared between the groups. As a result, no statistically significant difference was found between the groups in terms of bradycardia and oxygen saturation. The level of anxiety and patient comfort were also not different.
In the study by Yoon et al. [Citation21], 90 patients undergoing spinal anesthesia were divided into two groups. Patients in Group I received midazolam infusion with a loading dose of 0.05 mg kg−1 over the course of 10 min, followed by dexmedetomidine infusion at a rate of 0.5 μg kg−1 h−1 within 10 min. Patients in Group II received continuous dexmedetomidine infusion following an initial dexmedetomidine dose of 0.5 μg kg−1 h−1 for 10 min. Consequently, bradycardia was more frequent, and sedation was deeper in the midazolam plus dexmedetomidine group.
Ahn et al. [Citation22] used intravenous atropine injection during premedication to avoid development of bradycardia as a side effect of dexmedetomidine used for sedation purpose in patients administered spinal anesthesia. In a group of 140 patients, they administered dexmedetomidine infusion at a loading dose of 0.6 μg kg−1 over the course of 10 min followed by a maintenance dose of 0.25 μg kg−1 and atropine 0.5 mg following the loading dose in Group I. Patients in Group II received saline solution. There was no statistically significant difference between the two groups with respect to hypotension episodes, whereas bradycardia was frequent in the group that received no IV atropine.
Hall et al. [Citation23] studied seven healthy volunteers and used dexmedetomidine at different infusion rates (0.2 versus 0.6 μg kg−1 h−1); they reported an increase in the rate of ABH and a decrease in mean arterial pressure and cardiac output with increasing infusion doses.
Although cardiovascular effects of dexmedetomidine and morphine were parallel in the study by Arain et al. [Citation16], it was observed that ABH was significantly lower in the dexmedetomidine group that received infusion at a rate of 0.4 μg kg−1 h−1 following a loading dose of 1 μg kg−1. In a different study by the same researchers, dexmedetomidine was administered using a same dosing protocol, and similar results were obtained. These results were attributed to the loading dose of dexmedetomidine [Citation24].
In the present study, the mean time to onset of sensory blockade and motor blockade and the mean time to maximum sensory blockade were shorter in the dexmedetomidine group. As a result, the initiation of surgery was faster, and it was easier to administer sufficient anesthesia. In a similar study, Agrawal et al. [Citation25] demonstrated that IV dexmedetomidine prolonged the duration of blockade in patients administered spinal anesthesia.
Faraj et al. [Citation26] conducted a similar study on 364 patients administered spinal anesthesia and found that intravenous administration of dexmedetomidine prolonged the duration of sensory and motor blockade and delayed the time to first analgesic requirement.
In our study, we monitored the depth of sedation using BIS. The infusion rate was set so as to maintain a BIS score between 60% and 80%. In many studies in the literature [Citation27–Citation29], we observed that infusion rate is adjusted to achieve a desired level of sedation. We found that the infusion rate in dexmedetomidine group was significantly lower than that in the control group in the intraoperative period over the course of 90 min.
In the present study, we observed no excessive sedation in any of the cases (BIS < 50%), and there were no changes in respiratory rate or SpO2. In another study reporting on volunteers who received IV dexmedetomidine infusion for 24 h with a target plasma concentration of 0.3–1.25 μg L−1, no respiratory depression was observed, and oxygen saturation was maintained above 90% in all individuals [Citation30]. One of the limitations of our study was atropine midazolam premedication. We used intramuscular atropine to dry the secretions, help to produce bronchodilatation, and prevent vasovagal reactions. It has superior benefits with the use of dexmedetomidine. Other future studies could be planned with other premedication agents.
Dexmedetomidine is an anxiolytic, hypnotic, sedative, analgesic, and anesthetic agent that has no significant depressive effect on the respiratory system.
5. Conclusions
In conclusion, the present study compared IV administration of dexmedetomidine with saline infusion in urologic cases administered spinal anesthesia and found that dexmedetomidine had intraoperative depressive effects on hemodynamic parameters, whereas we demonstrated that dexmedetomidine improved the quality of motor and sensory blockade and induced amnesia and sedation. Moreover, we found that dexmedetomidine did not have prolonged postoperative effects and did not reduce analgesic requirements.
The authors of the present study conclude that intravenous dexmedetomidine infusion produces quality sedation in patients administered regional anesthesia.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Velickovic I, Pujic B, Baysinger CW, Baysinger CL. Continuous spinal anesthesia for obstetric anesthesia and analgesia. Front Med (Lausanne). 2017;4:1.
- Wang J, Han Z, Zhou H, et al. Effective loading dose of dexmedetomidine to induce adequate sedation in parturients undergoing caesarean section under spinal anaesthesia. Turk J Anaesthesiol Reanim. 2017;45(5):260–263.
- Turan A, Şapolyo Ö, Karamanlıoğlu B, et al. Comparison of propofol infusion versus dexmedetomidine for monitorized anesthesia care. Turk J Anaesthesiol Reanim. 2004;32:100–9.
- Koyuncu O, Alagöl A, Turan A. Comparison of the effects of intravenous dexmedetomidine and ketamine added to epidural anesthesia. Mustafa Kemal Üniversitesi Tıp Dergisi. 2015;6(21). DOI:10.17944/mkutfd.06920
- Bromage PR. Epidural analgesia. Philadelphia (PA): WB Saunders; 1978. p. 301–320.
- Ramsay MA, Savage TM, Simson BR, et al. Controlled sedation with alphaxalone-alphadalone. BMJ. 1974;2:656–659.
- Kearse LA, Rosow C, Zaslavsky A. Bispectral analysis of the electroencephalogram predict conscious processing of information during propofol sedation and hypnosis. Anesthesiology. 1998;88:25–34.
- Önal A. Algoloji. Elazığ: Nobel Tıp Kitabevleri; 2004. p. s.74.
- Brown DL, Wedel DJ. Spinal, epidural and caudal anesthesia. In: Miller RD, editor. Anesthesia. 3rd ed. New York (NY): Churchill-Livingston; 1990. p. 1377–1395.
- Ersoy A, Kara D, Ervatan Z, et al. Sedation in hypoalbuminemic geriatric patients under spinal anesthesia in hip surgery. Midazolam or propofol? Saudi Med J. 2015;36(10):1191–1198.
- Shelly MP, Wang DY. The assessment of sedation. Br J Intensive Care. 1992;82:45–50.
- Eledjam JJ, Bruelle P, Lalourcey L, et al. Sedation and regional anaesthesia. Eur Soc Regional Anaesth. 1995;92:136–143.
- Fcarcsi SM, Frca E, Med P, et al. Comparasion of dexmedetomidine and midazolam sedation for cataract surgery under topikal anesthesia. J Cataract Refract Surg. 2005;31(9):1845–1846.
- Köroglu A, Demirbilek S, Teksan H, et al. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94:821–824.
- Coskuner I, Tekin M, Kati I, et al. Effects of dexmedetomidine on the duration of anaesthesia and wakefulness in bupivacaine epidural block. Eur J Anesthesiol. 2007;23:1–6.
- Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for introperative sedation. Anesth Analg. 2002;95:461–466.
- Doğan Ö, Ünver S, Tunçer Yİ, et al. Comparison of dexmedetomidine versus midazolam/remifentanil combination for monitorized anesthesia care. Turk Anesthesiol Reanim J. 2011;39(6):292–301.
- Shehabi Y, Ruettimann U, Adamson H, et al. Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med. 2004;30:2188–2196.
- Santpur MU, Kahalekar GM, Saraf N, et al. Effect of intravenous dexmedetomidine on spinal anaesthesia with 0.5% hyperbaric bupivacaine in lower abdominal surgeries: a prospective randomized control study. Anesth Essays Res. 2016 Sep–Dec;10(3):497–501.
- Kang E, Lee KH, Jeon SY, et al. The timing of administration of intravenous dexmedetomidine during lower limb surgery: a randomized controlled trial. BMC Anesthesiol. 2016 Nov 21;16(1):116.
- Yoon D-K, Ban J-S, Lee S-G, et al. Dexmedetomidine combined with midazolam vs. dexmedetomidine alone for sedation during spinal anesthesia. Korean J Anesthesiol. 2016 Oct;69(5):446–452.
- Ahn EJ, Park JH, Kim HJ, et al. Anticholinergic premedication to prevent bradycardia in combined spinal anesthesia and dexmedetomidine sedation: a randomized, double-blind, placebo-controlled study. J Clin Anesth. 2016 Dec;35:13–19.
- Hall JE, Uhrich TD, Barney JA, et al. Sedative, amnestic and analgesic properties of small – dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705.
- Arain SR, Ruehlow RM, Uhrich TD, et al. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–158.
- Agrawal A, Agrawal S, Payal YS. Comparison of block characteristics of spinal anesthesia following intravenous dexmedetomidine and clonidine. J Anaesthesiol Clin Pharmacol. 2016 Jul–Sep;32(3):339–343.
- Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg. 2013 Jul;117(1):271–278.
- Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J. 2004;97(5):451–455.
- Parmaksız R, Tüfekçioğlu S, Gümüş T, et al. Spinal anestezi sırasında sedasyon için kullanılan deksmedetomidin ve remifentanilin psikomotor fonksiyonlara etkisi. In: TARK; Turkish Anesthesiology and Reanimation Congress 2005; Antalya. Özet Kitabı. Vol. 33. p. 163.
- Constant I, Leport Y, Ricard P, et al. Agitating and changes of bispectral index and electroencephalograhic-derived variables during sevoflurane induction in children: clonidine premedication reduces agitation compared with midazolam. Br Anesth. 2004;92:504–511.
- Venn M, Newman J, Grounds M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29(2):201–207.