ABSTRACT
Her2-neu overexpression has a pathogenetic, therapeutic and a controversial prognostic role in gastric cancer. p-53 mutation status and Ki-67 proliferation index are established prognostic markers in many tumors. In this study we evaluated p-53 and Ki-67 in relation to Her2-neu positive and negative gastric adenocarcinoma (GA). This cross-sectional study was carried out at King Fahd Hospital of Imam Abdulrahman bin Faisal University. Fifty cases of GA were retrieved from pathology archives. Clinico-pathological parameters were evaluated. Immunohistochemical protein analysis for Her2-neu, Ki-67 and p-53 was carried out. Fluorescent in situ hybridization (FISH) analysis was done for Her2-neu positive cases showing 2+ immunoexpression. Frequency of Ki-67 and p-53 positivity in Her2-neu positive cases was calculated and compared with those in Her2-neu negative cases. Correlation of clinicopatological parameters with Her2 positive and negative cases, p-53 mutation status and Ki-67 proliferation index was carried out. Her2-neu overexpression was present in 12% (n = 6) cases. A high Ki-67 was seen predominantly in Her2-neu positive cases (83%, n = 5). Her2-neu negative cases (n = 44) showed moderate (31.88%, n = 14) to low (34%, n = 15) Ki-67. Diffuse p-53 positivity was seen predominantly in Her2-neu positive cases (33.33%, n = 2). Focal p-53 was seen mainly in Her2-neu negative cases 56.8% (n = 25). Negative p-53 was seen to be independent of Her2-neu status. Her2-neu positivity is strongly associated with diffuse p-53 mutation status and high Ki-67 proliferation. Her 2-neu negative status is associated with focal p-53 positivity and low to moderate Ki-67 proliferation index. Such stratifications in prognostic markers could not only be predictive in patient’s prognostics but could also form a basis of molecular classification of gastric cancer.
KEYWORDS:
1. Introduction
Gastric cancer is one of the most common and aggressive malignant tumors worldwide with a high mortality rate, preceded only by lung cancer [Citation1]. Globally gastric cancer is the fourth most common cancer and second leading cause of cancer related mortality with a 5-year overall survival rate less than 25% [Citation2].
Her2-neu overexpression has a pathogenetic, therapeutic and prognostic role in gastric cancer. Evaluation of Her2-neu overexpression for targeted therapies is currently the mainstay treatment strategy [Citation3]. Her2-neu as a negative prognostic parameter has established a strong foot holds in breast cancer. The ToGA trial (Trastuzumab for Gastric Cancer) attributed a similar status to Her2-neu in gastric cancer, yet no unanimous consensus has evolved so far in this regards till now [Citation4,Citation5]. Prognostic factors defined for the staging and therapeutic interventions in gastric cancer are imprecise as patients with similar parameters actually prove to be at different levels clinically, attributing a controversial prognostic status to Her2-neu overexpressivity [Citation6].
Ki-67 and p-53 are established prognostic markers in many tumors. Ki-67 is a useful predictive and prognostic marker in cancers with a proliferation index exceeding 10%-14% delineating a high-risk prognostic category [Citation7]. A pre-chemotherapy evaluation of rate of Ki-67 is also a strong predictor of efficacy of the therapy [Citation8]. p-53 is the most commonly mutated gene in human cancer with a critical role in cell cycle regulation and tumor suppression. In gastric lesions, an increasing frequency of p-53 mutations is seen in H.pylori infection, a key player in gastric carcinogenesis, intestinal metaplasia, gastric dysplasia and gastric carcinoma [Citation9]. Its expression is associated with advanced staged gastric carcinomas with a poorer postoperative prognosis.
In this study p-53 and Ki-67 were assessed in Her2-neu positive and negative gastric adenocarcinoma (GA) with an idea to find an association between Her2-neu status and these prognostic markers that could segregate a subset of more aggressive GA, requiring a tailored, separate and aggressive therapeutic approach.
2. Material and methods
This cross sectional study was carried out at Pathology Department of King Fahd Hospital of Imam Abdulrahman bin Faisal University in 2015–2016. Approval of the protocol was granted by the Institutional Review Board.
A sample size of 45 (one sample implies a biopsy specimen from an individual patient) from was calculated for a study population (registered patients) of 10,000, using a confidence level of 95%, margin of error of 5% and response rate of 3%, based on cancer incidence report for gastric cancer of Saudi Arabia-2010 [Citation10]. This sample was drawn from pathology archives of the department, and comprised gastric carcinoma resection specimens and endoscopic biopsies over a period of 10 years preceding the study.
The inclusion criteria were availability of complete patient record, representative paraffin blocks, and sufficient tissue material to perform the required histopatholgical procedures. A total of 55 cases were selected initially on the assumption that some cases might not qualify on selection criteria at the time of tissue processing and staining. However we succeeded to get 50 qualifying samples, therefore we reported out of 50 instead of 45.
GA were histologically classified according to Laurens [Citation11] which divides GA into two types; intestinal (consisting of well-formed tubules) and diffuse (diffuse tumor infiltration without well-formed tubules, frequently with signet ring cells).
Immunohistochemical staining using the labeled streptavidin-biotin method with 3,3ʹ-diaminobenzidine as a chromagen was performed for Her2-neu, p-53 and Ki-67 on 4 µm thick paraffin sections cut from conventional blocks. Prediluted antibodies were used. Her-2 neu(clone CB11), Ki-67(clone MIB-1)) and p-53(clone DO-7) were obtained from Ventanna, Dako and Navacastro respectively. The staining was performed concurrently in a Ventana Benchmark automated immunostainer according to the manufacturer’s instructions (Ventana Medical Systems Inc., Strasbourg, France). The immunostained sections were examined under a light microscope and evaluated manually by both the authors. Any interpretational discrepancies were resolved under a double-headed microscope.
A modified scoring system for GA that has been shown to be predictive in a large Phase III ToGA trial was used for Her2-neu expression in gastric cancer [Citation12]. The 10% cut-off rule was kept in resection specimen, whereas in biopsies any group of at least five tumor cells showing distinct membranous staining (typically lateral at cell-cell junctions) was considered specific. The Microscope Magnification rule was applied to determine Her2-neu expression.
Demonstration of distinct intercellular membranous staining whether linear, basolateral or ring-shaped was required. If a strong intensity could be seen at 2.5×–5× magnification it was designated a score of 3 + . A visible weak to moderate staining at 10×–20× was scored as 2+, barely visible staining at 20×–40× was scored as 1+ and a negative staining was given a score of 0. A score of 2+ and 3+ was considered as Her2-neu positive (overexpression). While the scores of 0 and 1+ were regarded as Her2-neu negative [Citation12]. The unequivocal cases (Her2-neu 2+) were confirmed by FISH analysis.
FISH for HER2 gene amplification was conducted on FFPE of gastric tumor according to the protocol of FDA approved kit from Abbott. The probes consist of a dual color, two probe mixture of DNA sequences on specific regions of chromosome 17, including the centromere of chromosome 17 (CEP17) and the Her2 gene region at chromosome 17q12. Signals were visualized under a Zeiss Axioskop microscope (Zeiss, Germany) using a FITC/Rhodamine dual band filter. We followed the ASCO criteria for analyzing the results. The guidelines stated that Her2/CEP17 ratio <2 is considered a normal result, ≥2 is considered an amplified abnormal result.
Ki-67 and p-53 were done for both Her2-neu positive and negative cases. Frequency of Ki-67 and p-53 were compared between Her2-neu positive and negative cases and statistical significance was determined.
Ki-67 expression was defined as the presence of nuclear staining. The percentage of cells expressing Ki-67 was determined by counting 1000 cells/slide. The percentage of positive cells was scored as follows: less than 10% = low proliferative activity, 10%–40% = moderate proliferative activity and more than 40% = high proliferative activity [Citation13].
p-53 mutation status was defined as positive in presence of nuclear staining. Cytoplasmic staining was considered negative. Tumors were considered focally positive when unequivocal staining was present in 10%–50% of tumor cells and as diffusely positive when more than 50% of the tumor cells were positive [Citation9].
Data was entered into SPSS (version 19). Descriptive statistics were used to calculate frequencies (percentages) for expression of Her2-neu, Ki-67 proliferation index and p-53 mutation status. Frequency of Ki-67 and p-53 positivity was compared between Her-2neu positive and negative cases using Chi square test. A P value of less than 0.05 was considered as statistically significant.
3. Results
Out of a total of 50 cases of gastric cancer retrieved, 43 specimens were biopsies and 7 were partial gastrectomies. Male to female ratio was 37:13 and median age of the patients was 67 years (maximum & minimum 88 & 42 years). Diffuse type gastric cancer was seen in 23 (46%) and intestinal type in 27 (54%) of cases. The clinicopathological parameters are presented in . The relationship of Ki-67 proliferation index and p-53 mutation status with clinico-pathological parameters is presented in and .
Table 1. Cliniopathological parameters in Her2-neu positive and negative gastric adenocarcinoma (n = 50).
Table 2. Relationship between Ki-67 proliferation index in gastric adenocarcinoma (n = 50) to clinico-pathological parameters.
Table 3. Relationship between p-53 mutation status in gastric adenocarcinoma (n = 50) to clinico- pathological parameters.
Her2-neu expression pattern is given in while Ki-67 proliferation index and p-53 mutation status is given in respectively. In Her2-neu positive and negative cases, p-53 mutation status and Ki-67 proliferation index are represented in and respectively.
Table 4. Her2-neu expression in gastric adenocarcinoma (n = 50).
Table 5. Ki-67 proliferation index p-53 mutation status and in gastric adenocarcinoma (n = 50).
Table 6. Crosstab showing distribution of p-53 mutation status according to Her2-neu status of gastric adenocarcinoma cases (n = 50).
Table 7. Crosstab showing distribution of Ki-67 expression according to Her2-neu status of gastric adenocarcinoma cases (n = 50).
Her2-neu positivity was found in six cases ().Diffuse p-53 positivity was seen in 33.33% (n = 2) of Her2-neu positive cases as compared to 15.91% (n = 7) in Her2-neu negative cases. (Diffuse p-53 was significantly high, P value ˂0.01 in Her2-neu positive cases) ().
Focal p-53 positivity was seen in 33.33% (n = 2) of Her2-neu positive cases as compared to 56.8% (n = 25) in Her2-neu negative cases. (Focal p-53 was significantly high, P value ˂0.01 in Her2-neu negative cases). ()
Negative p-53 was observed to be independent of Her2-neu status.
A high Ki-67 was seen in 83% (n = 5) of Her2-neu positive cases as compared to 34% (n = 15) in Her2-neu negative cases. (High Ki-67 was significantly high, P value ˂0.01 in Her2-neu positive cases). ()
A moderate Ki-67 was seen in 16.66% (n = 1) of Her2-neu positive cases as compared 31.88% (n = 14) in Her2-neu negative cases. (Moderate Ki-67 was significantly high P value ˂0.01, in Her2 neu negative cases). ()
A low Ki-67 was seen in 0% (n = 0) of Her2-neu positive cases as compared 34% (n = 15) in Her2-neu negative cases. (Low Ki-67 is significantly high P value ˂0.01, in Her2 neu negative cases). ()
4. Discussion
Gastric cancer is not one disease entity but a complex combination of multiple genetic and epigenetic alterations. Marked variation in the prognosis of patients with GA within a similar pathological stage requires the identification of subgroups of patients with a more aggressive disease.
In this study an overall estimation of Her2-neu overexpression, p-53 mutation status and Ki-67 proliferation index in GA along with evaluation of p-53 and Ki-67 in Her2-neu positive and Her2-neu negative GA was evaluated.
Overall in this study there was a cumulative 12% of Her-2neu over expressivity with 8% showing 2+ and 4% demonstrating 3+ immunoreactivity ((a,b)). Her2-neu over expression in GA has been reported to range from 11.7% [Citation14] to 23% [Citation15] in different studies. Only some studies have documented Her2-neu protein overexpression or gene amplification to be associated with a worse prognosis [Citation16] however larger studies could not confirm this as an independent prognostic factor [Citation17,Citation18]. Her2-neu prognostic significance is further minimized by its reported loss in approximately one third of patients when treated with trastuzumab, leading to drug resistance. Additionally with advanced aggressive Her2-neu positive tumors, other genetic alterations involving specifically p-53 (92%) and other cell-cycle mediators supervene [Citation19]. These data suggest the need for periodic Her2-neu status evaluation during therapy but also incorporation and monitoring of other cell cycle regulators such as p-53 from the start so as to have a baseline level available for further patient evaluation.
Figure 1. Her 2 neu ‘3+’ immunoreactivity with its associated high Ki-67 proliferation index and diffuse p-53 mutation status.
(a) Her2 neu3+ (×20); (b) Her2 neu3+ (×40); (c) High Ki-67 (×20); (d) High Ki-67 (×40); (e) Diffuse p-53 (×20); (f) Diffuse p-53 (×40).
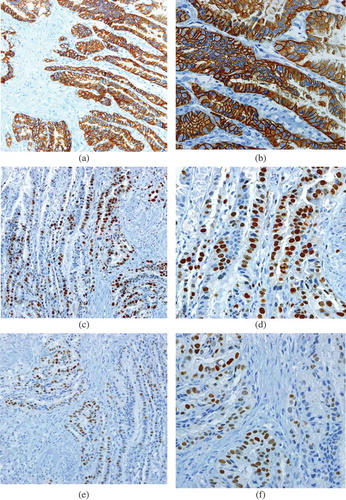
In gastric carcinomas, p-53 expression frequency has been reported to vary from 46% [Citation20] to 61% [Citation21].In our set of cases, with a total of 72% of cases revealing focal to diffuse p-53 expression ((e,f)), a somewhat higher representation is seen. Out of this Her2-neu positive cases predominantly showed diffuse p-53 positivity (33.33%) while Her2-neu negative cases (56.81%) revealed mostly focal p-53 positivity. Negative p-53 expression was seen irrespective of Her2-neu status. This stratification of p-53 and Her2-neu expression pattern has not been reported before. p-53 mutation is the most common genetic alterations in human cancer initiation and progression and has been reported to be an independent prognostic factor in patient’s overall and disease-free survival in gastric cancer [Citation22]. A stratified expression was seen in our cases with increase from focal in Her2-neu negative to diffuse in Her2-neu positive cases. Could this point out toward a direct genetic link between these two entities with both increasing concomitantly and being codependent and not independent factors?
Therapeutically this link could be important as currently anti-p53 therapies [Citation23] are in stages of development. Addition of these to anti-Her2-neu drugs such as traztuzumab might improve patients’ clinical outcome that has been so poor till now.
Based on the above discussion Her2-neu and p-53 cases can be categorized into two groups. One set of Her2-neu positive cases that retain p-53 expression but lose their Her2-neu co- expression and when cancer becomes advanced. This set will be the ones that will be facing traztuzumab resistance. Addition of anti-p53 might be helpful to some extent. The other set can be the ones that have conjoined expression of Her2-neu and diffuse p-53. A double therapy targeting both could be beneficial to these patients. More extensive studies unravelling these facts at genetic and clinical levels need to be carried out.
In our study an overall high to moderate Ki-67 proliferating index was seen in 70% of cases. A range of 70%–76% has been reported in other studies [Citation24,Citation25]. Ki-67 is a nuclear protein that is expressed in proliferating cells throughout the cell cycle. In a meta-analysis comprising a total of 5600 gastric cancer patients from 29 studies, it was concluded that a high Ki-67 expression could serve as a predictive biomarker for poor prognosis in gastric cancer patients [Citation26]. The value of Ki-67 becomes more marked if it is seen in conjunction with Her2-neu positivity. An association between Her2-neu overexpression, Ki-67 proliferation index and a high grade and stage has been reported [Citation27]. In our study 83% of Her2-neu positive cases showed a high Ki-67 proliferation index ((b,c)). Her-2 neu negative cases revealed mainly moderate to low Ki-67. Hence Ki-67 expression increases with Her2-neu overexpression. Could it be that the concept of Her2-neu gene working as an independent prognostic marker needs to be reevaluated? At the genetic level Her2-neu and Ki-67 maybe partners that express collectively in high grade advanced gastric carcinomas. The protein expression of Ki-67 and Her-2neu has overlapping features in the clinical pathologic characteristics of gastric cancer. Ki-67 is strongly linked to gastric cancer differentiation, infiltration and lymphatic spread, whereas Her-2 neu plays a role at the level of tumor differentiation and nodal metastasis [Citation28]. Stratification of cases by Her2-neu status and extent of Ki-67 expression could be a very useful tool for selection of specific therapies as Ki-67 as a molecular target for anticarcinogenic therapies is being explored [Citation29].
A more advanced gastric cancer genetic profiling needs to be determined with formulation of molecular classification of gastric cancer as is currently being done in breast cancer. This will pave the way for individualized targeted treatment options based on gastric cancer molecular characteristics that could improve the patient’s prognostics that have been so dismal till now.
5. Conclusion
Her2-neu positivity is strongly associated with diffuse p-53 mutation status and high Ki-67 proliferation. Her 2-neu negative status is associated with focal p-53 positivity and low to moderate Ki-67 proliferation index. This strong association in Her2-neu positive cases could be the basis of a separate molecular subset of gastric carcinoma, a potential candidate for separate tailored molecular therapeutic regimens.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Son HS, Shin YM, Park KK, et al. Correlation between HER2 overexpression and clinicopathological characteristics in gastric cancer patients who have undergone curative resection. J Gastric Cancer. 2014 Sep;14(3):1–6.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359–E386.
- Ieni A, Barresi V, Rigoli L, et al. HER2 status in premalignant, early, and advanced neoplastic lesions of the stomach. Dis Markers. 2015;2015:234851. doi: 10.1155/2015/234851.
- Bang Y, Chung H, Xu J, et al. Pathological features of advanced gastric cancer (GC): relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol. 2009 May 20;27(15S):4556.
- Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748.
- Wang HB, Liao XF, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: a meta-analysis. Medicine. 2017 Nov 1;96(44):e8437.
- Amrani HJ, Marchoudi N, Sadaoui I, et al. Ki-67 expression in gastric cancer and correlation with clinico-pathological characteristics. Int J Sci Res Publ. 2014;4(6):254–258.
- Shien T, Kinoshita T, Seki K, et al. p53 expression in pretreatment specimen predicts response to neoadjuvant chemotherapy including anthracycline and taxane in patients with primary breast cancer. Acta Med Okayama. 2013;67(3):165–170.
- Busuttil RA, Zapparoli GV, Haupt S, et al. Role of p53 in the progression of gastric cancer. Oncotarget. 2014 Dec;5(23):12016.
- Cancer incidence report Saudi Arabia, Kingdom of Saudi Arabia, Council of health services. Saudi cancer registry; April 2014 [cited 2015 Jun 4]. Available from: www.scr.org.sa
- Hu B, El Hajj N, Sittler S, et al. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012 Sep;3(3):251–261.
- Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial: a phase III study of trastuzumab added to standard chemotherapy in first-line HER2-positive advanced gastric cancer. J Clin Oncol. 2009;27:15s.
- Saricanbaz I, Karahacioglu E, Ekinci O, et al. Prognostic significance of expression of CD133 and Ki-67 in gastric cancer. Asian Pac J Cancer Prev. 2014;15(19):8215–8219.
- Kataoka Y, Okabe H, Yoshizawa A, et al. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2013 Jan 1;16(1):84–93.
- Phillips BE, Tubbs RR, Rice TW, et al. Clinicopathologic features and treatment outcomes of patients with human epidermal growth factor receptor 2‐positive adenocarcinoma of the esophagus and gastroesophageal junction. Dis Esophagus. 2013 Apr 1;26(3):299–304.
- Tanner M, Hollmen M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005 Feb 1;16(2):273–278.
- Aizawa M, Nagatsuma AK, Kitada K, et al. Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer. 2014 Jan 1;17(1):34–42.
- Terashima M, Kitada K, Ochiai A, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012 Sep 12;18:5992–6000. clincanres-1318.
- Janjigian YY, Riches JC, Ku GY, et al. Conference: Gastrointestinal Cancers Symposium. Journal of Clinical Oncology. 2015;33(3): Supplement Meeting Abstract: 63.
- Choi WH, Lee S, Cho S. Microsatellite alterations and protein expression of 5 major tumor suppressor genes in gastric adenocarcinomas. Transl Oncol. 2018 Feb 28;11(1):43–55.
- Babacan NA, Eğilmez HR, Yücel B, et al. The prognostic value of UHRF-1 and p53 in gastric cancer. Saudi J Gastroenterol. 2016 Jan;22(1):25.
- Yıldırım M, Kaya V, Demirpence O, et al. Prognostic significance of p53 in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2015;16(1):327–332.
- Duffy MJ, Synnott NC, McGowan PM, et al. p53 as a target for the treatment of cancer. Cancer Treat Rev. 2014 Dec 31;40(10):1153–1160.
- Zheng Y, Wang L, Zhang JP, et al. Expression of p53, c-erbB-2 and Ki67 in intestinal metaplasia and gastric carcinoma. World J Gastroenterol. 2010 Jan 21;16(3):339.
- Lazăr DA, Tăban SO, Sporea I, et al. Ki-67 expression in gastric cancer. Results from a prospective study with long-term follow-up. Rom J Morphol Embryol. 2010;51(4):655–661.
- Luo G, Hu Y, Zhang Z, et al. Clinicopathologic significance and prognostic value of Ki-67 expression in patients with gastric cancer: a meta-analysis. Oncotarget. 2017 Jul 25;8(30):50273.
- Ieni A, Barresi V, Giuffrè G, et al. HER2 status in advanced gastric carcinoma: a retrospective multicentric analysis from Sicily. Oncol Lett. 2013 Dec 1;6(6):1591–1594.
- El-Gendi S, Talaat I, Abdel-Hadi M. HER-2/Neu status in gastric carcinomas in a series of Egyptian patients and its relation to Ki-67 expression. Open J Pathol. 2015 Aug 17;5(4):101.
- Wang S, HüTtmann G, Zhang Z, et al. Light-controlled delivery of monoclonal antibodies for targeted photoinactivation of Ki-67. Mol Pharm. 2015 Aug 13;12(9):3272–3281.
