ABSTRACT
The purpose of this study is to prepare and characterize solid lipid nanoparticles (SLN) of N-Acetyl Carnosine (NAC) to treat cataract since surgery necessitates equipments and professional help. Cataract is believed to be formed by the biochemical approach where the crystalline eye proteins lose solubility and forms high molecular weight masses. Added advantages of SLN of NAC (henceforth referred as SLN-NAC) in the study are reduced size, sustained release and better corneal penetration of drug. The method of preparation of SLN-NAC by Mill’s method is unique in itself.
The size of the SLN-NAC was 75 ± 10 nm in the range of ideal for penetration. The in-vitro release study and the SLN-NAC formulations prepared with Mill’s method demonstrated sustained release up to 24 h following an initial burst after 1 h. The zeta potential of the prepared formulation was −22.1 ± 1 mV. Corneal permeation studies using goat corneas indicate that SLN-NAC penetration rate was higher than those from NAC eye drops. Corneal hydration studies indicated that the formulation caused no harm to the corneal cells.
Therefore it may be concluded that SLN-NAC may revolutionize cataract treatment and reversal by improving drug permeation, reducing toxicity and no damage to corneal tissue.
1. Background
Eye is an intricate organ isolated by blood–ocular barriers which includes retinal, aqueous and vitreous humor making it a primary and accessible site for drug delivery investigations [Citation1]. The main way of administering a drug for any eye disorder is ophthalmic application but the conventional formulations ensure only a limited amount of drug penetrating these barriers. Although eye is generally protected, but still problems relating to age like cataract frequently surface and treatment of cataract generally leads to a single outcome – surgery [Citation2] and intravitreal procedures associated with numerous side effects like detachment of retina [Citation3]. Cataract may be the chief cause of blindness in 50% of cases worldwide. The principal cause of cataract is ageing and frequently other associated factors may be exogenous like UV radiation and trauma causing accidents or endogenous like diabetes and genetics [Citation2]. Surgery has been the most established and effective treatment for reversing cataract except for the fact that this requires medical expertise and instrumentation. These two factors are limiting since they are not available everywhere. Hence there is an immediate need for non-invasive techniques for treatment and reversal of cataract.
Cataract is believed to be formed by the biochemical approach where the crystalline proteins lose solubility and forms high molecular weight masses [Citation4,Citation5]. Moreover aggregation of these despite the durable crystalline and chaperone concentration may lead to insoluble proteins. It is also known to have formed from oxidative stress. N-Acetyl Carnosine (NAC) is an ophthalmic drug which shows potential for treatment or even reversal of cataract [Citation6–Citation8]. A known antioxidant effect of the protein, L-carnosine, on cataractuous lens may allow us biochemically for exploring L-carnosine as a cause to converse or even avert development of cataract [Citation9,Citation10]. L-carnosine cannot penetrate the eye as an eye-drop. However, when smeared on the surface of the eye, NAC penetrates the cornea into the front chamber of the eye close to the place where the cataractuous lens is and there is metabolised into L-carnosine [Citation7]. Deterrence and reversal of cataract by carnosine are mediated by various mechanisms like inactivation of lens proprietary antioxidant enzymes induced by free-radical (superoxide dismutase); inhibition of cross-linking glycation reactions to the lens proteins induced by auto-oxidation of ascorbic acid which is in turn catalyzed by carbohydrate and metal; carnosine having transglycation properties which permits it to contend for the glycating agent, shielding proteins (lens crystallins) alongside alteration; common antioxidant and scavenging properties towards lipid hydroperoxides, aldehydes and oxygen radicals; L-carnosine ingredient of proteasome activity within the lens; NAC and its bioactivated primary component carnosine also causes disintegration of lens crystalline activity.
It has been observed in numerous studies that nanoparticulate systems are capable of penetrating the cornea of the eye more efficiently as when compared with the free drug [Citation11–Citation17]. Nano-biotechnology in clinical research has led to abundant pathbreaking therapeutics. The unique possibilities of nanoparticles like reducing toxicity of a drug and biocompatibility make them excellent nanocarriers for therapeutic purposes [Citation18]. Nanoparticulates also help in achieving the most important feature of ocular drug delivery that is equal bioavailability due to their reduced size [Citation19]. Moreover, nanoparticulates are not easily removed from the ocular area because of blinking or tear mechanism as compared to free drug [Citation16,Citation20].
Therefore in our study, we prepared the solid lipid nanoparticles (SLN) of NAC for prevention of cataract. Henceforth we will be referring to them as SLN-NAC. SLNs are microscopic colloidal exporters alternating between 50 and 1000 nm, made of biological lipid, distributed in water or in aqueous surfactant solution [Citation21]. These are appropriate for the assimilation of lipophobic, hydrophobic, and low water solvable drugs [Citation22]. It has been concluded that for ocular drug delivery SLNs are extreme stable, maximum efficiency of entrapment and are promising carriers of ocular drugs like meloxicam [Citation23]. The purpose of the study was to formulate NAC loaded-SLN preparations as constant eye drug delivery systems with the aim of improving obtainability and sustainability of NAC at the corneal level. The current study was based on the lines of a similar study of encapsulating NAC in PLGA polymer for increased drug availability for cataract treatment [Citation24]. However, in our study, preparation of SLN of NAC was carried out to ensure decreased toxicity and relatively more stability. Another novel achievement was the development of human corneal cell construct from human endothelial cell line for the drug permeation studies.
1.1. Design considerations for SLN-NAC
The size of the SLN-NAC will determine its functionality like uptake of the nanoparticles by ocular tissues, adherence to cell layer, sustainability and clearance from the ocular region. It was approximated from various studies that size of 10–400 nm will allow better passage of nanoparticulates into the deeper layers of ocular structure for prevention of cataract [Citation17,Citation18]. The preparation technique of the SLN-NAC will ensure that the nanoparticles will be transformed from lipophilic to hydrophilic and also may reduce the irritation of the eye. This was designed to be an ideal ocular drug delivery system which may possess the essential features mentioned above.
2. Methodology
2.1. Material
Raw material of NAC, precisely the L-isomeric form of was produced at the cGMP facility. Zirconia beads and Bead Smash 12 (a bead mill) was acquired from Wakenyaku Co. Ltd, Kyoto, Japan.
HENC, elaborated as Human corneal endothelial cells, Endothelium stroma equivalent and Epithelial cells (CEPI 17 CL4) were procured from Thermo Fisher Scientific, Beijing, China. Ham’s F12 and Medium F99, FCS an antibiotic and antimycotic were procured from Sigma, China. All other chemicals were procured from Sigma Beijing, China.
Sosium Selenite for inducing cataract in HENC for impedance studies was also procured from Sigma, China.
2.2. Cell culture
HENC are normal human corneal endothelial cells isolated elsewhere and more than 5 × 105 viable cells are contained in each vial of this product which was cryopreserved at the culmination of the secondary culture level. The cells were thawed and seeded and cultured in Medium F99, which is the amalgamation of Ham’s F12 and Medium F99 in the ratio of 1:1 and 5% FCS and 1% antibiotic/antimycotic solution was added to above media according to the instructions provided [Citation25–Citation28].
25 cm2 tissue culture flasks (Costar) were utilized for conservation of the cultures in regular conditions at 37 °C in a moistened atmosphere having 5% CO2. Replacements of growth medium were carried out thrice every week.
2.2.1. Human corneal construct
A step by step in-vitro corneal cell construct was prepared from the HENC as described by Reichl et al and Kawazu et al [Citation25,Citation29]. 2 × 106 cells were seeded onto a 4.7 cm2 surface area containing polycarbonate filter with pore size of 3.0 mm. These were concealed with a layer of type I collagen and matured to confluence in a time span of 7 days in medium F99. A type I collagen gel matrix comprising of stromal fibroblasts was layered over the endothelial cell layer which was already confluent. Equivalent of endothelium stroma was cultured after 4 days of submergence in DMEM media. Epithelial cells (CEPI 17 CL4) at a concentration of 1.5 × 105 were cultured onto the contracted lattice of collagen and developed in culture medium DMEM/F12 for further 7 days until confluence was achieved. Following the confluence of the epithelium, tissue construct was elevated to the air–liquid interface was carried out for further 12 days and cultured in DMEM/F12 media with the serum content lessened to 2%. In a span of 12 days, a multilayered epithelium was designed. An incubator which was humidified at 37 °C containing 5% CO2 was used to sustain the cultures with replacement of medium three times per week.
2.3. Preparation of SLN-NAC
The conventional preparation of SLN-NAC was done following the Mill method described by Nagai and Ito [Citation17] with suitable modifications. The zirconia beads (diameter: 2 mm) were supplemented with NAC microparticles (solid) containing methylcellulose (MC) and with the bead mill for 30 s @ 3,000 rpm, the mixtures were smashed at 4°C. The mixes were disseminated in saline with 5% 2-Hydroxypropyl-β-cyclodextrin (HPβCD), and smashed over again with the bead mill @ 5,500 rpm, 30 s ×15 times at 4 °C utilizing lesser sized zirconia beads which were of diameter of 0.1 mm. SLN-NAC were obtained by the bead mill method and alongside there was no precipitation of nanoparticles in the dispersion mixes after 7 days of preparation.
2.3.1. Lyophilization of the nanoparticles
Khare et al [Citation18] and Huang et al [Citation30] described a simple protocol of lyophilization of nanoparticles which was followed with suitable modifications. 2.5% w/v mannitol was mixed as a cryoprotectant to the above prepared dispersion followed by lyophilization executed for 24 h to check physical stability and redispersibility of the mix. To begin with, pre-freezing was completed by freezing the mixture at −74 °C, at 0.02 mmHg pressure, in vials which were then transferred into the adapter. The adapter was then fit into freeze-dryer Lyophilizer FD-5–3, and lyophilized powder of SLN-NAC was obtained
2.4. SLN-NAC characterization
The morphological investigation of the SLN-NAC was done with the help of a high-resolution transmission electron microscopy (TEM, JEM-2010HR). Briefly, the liquid solution was positioned on the copper grid and then staining was carried out with Phosphotungstic acid. Further sample were air dried and incubated for 3 h.
2.5. Size distribution and stability of formulation
The average size distribution of SLN-NAC was determined using Malvern zeta sizer (Malvern Instrument Inc,). The samples were diluted with PBS and uniformly dispersed with ultra-purified water and the analysis was performed. The SLN-NAC was characterized with Zeta potential. The measurements were executed using an aqueous dip cell in an automatic manner by insertion of diluted samples in the capillary measurement cell and position of cell was attuned.
The prepared formulation was studied for particle size distribution and electrical properties by zeta potential measurement. Zeta potential is a significant procedure for characterization of surface morphology that supports defining the probable stability and charge on the surface of the nanoparticulate structure. Usually for colloidal dispersion stability, complete large negative or positive zeta potential values are necessary as aggregation of nanoparticles may be avoided by electrostatic repulsion amongst particles with identical charges.
The experiments were performed thrice and the results were discussed as mean ± SD.
2.6. Determination of % of drug trapped in SLN-NAC
This was carried out by a protocol described by Khare et al [Citation18]. Entrapment efficiency is described as the percentage of the actual drug mass captured in the polymeric carrier, comparative to the original amount of drug loaded, and was calculated using the following equation:
Calculation of theoretical drug loading was performed with the following equation from the quantity of drug taken comparative to the quantity of total drug and excipients utilized for preparation of SLN-NAC:
Actual drug loading was done utilizing the following procedure: a SLN-NAC dispersion was set by dissolving 25 mg of the lyophilized powder of SLN in 5mL mixture of methanol: distilled water (1:4) and was centrifuged for 20 min @ 13,000 rpm. The clear supernatant was noted for free NAC residue by assessing absorbance at 255 nm in an UV-visible spectrophotometer. The entire quantity of drug existing in the SLN-NAC was calculated by dissolving the lyophilized 25 mg of SLN-NAC powder in 10 mL dichloromethane by sonication and thereafter straining it through a microsyringe filter (0.2 µm) and evaluating the remains for NAC by calculating absorbance at 255 nm using UV visible spectrophotometer [Citation31]. The succeeding formula calculated the actual drug loading percentage:
2.7. In-vitro drug release from the SLN-NAC
This is an extremely important experiment since the release of the NAC from the nanoparticles will determine whether this technique can be effectively implied in ocular drug delivery. 1 mL of phosphate buffered saline (PBS; pH 6.8) was utilized to dissolve 5 mg of incubating SLN-NAC lyophilized powder with horizontal shaking at 37 °C. The release investigation began by insertion of the end-sealed dialysis bag into 50.0 mL of simulated tear fluid (pH – 7.4) at 37 °C with persistent pulsating. At particular time interims, 3.0 mL of simulated tear fluid was drawn and reloaded with an identical quantity of freshly prepared tear fluid. Data were obtained in triplicate and analyzed through graph; that is, % drug release versus time graph was plotted.
2.8. FTIR studies
The infra-red (IR) spectra provides description regarding the functional groups and their location in a novel drug formulation. Henceforth, IR spectra of NAC and SLN-NAC were analyzed utilizing Fourier transform infrared (FT-IR) spectrophotometer with total reflectance (ATR) mode (Shimadzu, Kyoto, Japan) for detailed analyses of the functional groups. The peaks noted in the ATR spectrum were interpreted and equated with known values.
2.9. Impedance measurement
The impedance sensing devices were purchased from Applied Biophysics and used to measure impedance of cultured adherent HENC on its electrode surfaces. The HENC cells were treated with 100 µm sodium selenite, an oxidative stress inducing agent for starting a cataract infection [Citation32]. The cataract induced cells were treated with 10 µl vol/wt NAC, PBS (control) and SLN-NAC. Impedances of treated and control samples were measured after 48 h of inoculation within a frequency limit from 100 Hz to 1 MHz in a logarithmic scale. The ECIS device consisted of eight wells and each well possessed 10 gold microelectrodes (250 μm diameter) which sense flow of current within the solution as described by Benson et al [Citation33]. The substrate area was 0.8 cm2 and the volume of each well was 600 μl. The ECIS devices were equilibrated overnight with cataract induced HENC in a tissue culture incubator. Then more cataract induced cells were added to the well in 300 μl media for impedance measurement.
Subsequently the impedance data was incorporated into ZsimpWin (Ver. 3.10) for fitting it. The comparable circuit was attained from investigations by Dou et al [Citation34] and Gong et al [Citation35].
2.9.1. Statistical data analysis
All the experiments including impedimetric analysis and cell assay were carried in triplicate and the data were represented with their corresponding relative standard deviations (RSD).
2.10. Drug permeation studies
Three aqueous solutions containing NAC and SLN-NAC were utilized for studies for diffusion for the assessment of cell permeability at 37 °C using revised Franz diffusion cells. The drug permeation studies were done over the human corneal construct. The protocol was adapted from Reichl et al [Citation25] with some necessary modifications. The donor drug formulation had NAC 2% and SLN NAC 2%. The receiver solution comprised of isotonic PBS with pH 7.4 and was agitated with utilizing a magnetic stirrer at 400 rpm throughout the study. At a regular gap of 60 min, from the receiver chamber the samples were collected and quantitatively investigated by high-performance liquid chromatography (HPLC). Calculation of the amounts (mg/cm2) of drug infused through the corneal construct versus the time (minutes) revealed the permeation parameters of SLN-NAC and NAC. Calculation of permeation coefficient KP was conceded as drug concentration from the rectilinear gradient of a permeation curve. Apart from this, drug permeation was also seen by staining the corneal constructs with H&E as per the protocol described by Reichl et al [Citation25].
2.10.1. Statistical analysis
Data investigation and quantification were achieved by Waters Millenium 32 Chromatography Manager software (Waters) and also by Origin 8. The data was represented as mean ± SD (standard deviation) of five determinations.
2.11. Corneal hydration studies
The whole corneal planning technique was adopted from Khare et al [Citation18] with suitable modifications and had to be completed within 1 h of the goat sacrifice. Proper institute ethical clearance for large animals was obtained for the studies. Entire eyeballs of freshly sacrificed goats were acquired from a local dealer and sent to the research laboratory ice-cold in normal saline (4 °C). Cautious excision of the cornea was then performed together with 2–4 mm of adjacent scleral tissue and was readied using normal saline until the sample was protein free. Subsequently, each cornea was unencumbered from adhered sclera and treated with SLN-NAC and NAC. After the respective treatments, 1 ml methanol was used to soak the cornea which was then dried overnight at 80 °C, and weighed again. Calculation of corneal hydration was done after noting the differences in the weights.
2.12. Cytotoxicity test
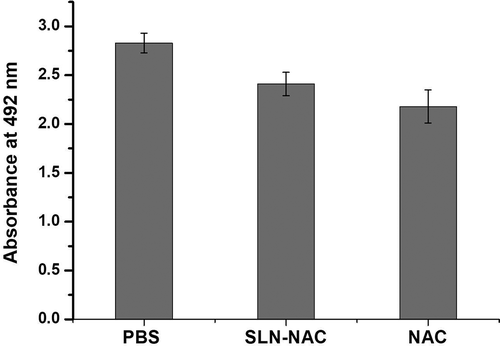
Cytotoxicity of NAC and SLN-NAC evaluated according to Banerjee et al [Citation36] and Dou et al [Citation34] with appropriate modifications. Confluent HENC cultures were obtained as mentioned in procedure above. For the estimation of in-vitro cytotoxicity, MTT assay was being done. Cells were harvested at 5 × 103 cells/well in 96-well plates and incubated for 72 h ensuring that cells are viable on addition of the following; 0.1 mL of PBS (control), NAC nanoparticles (0.1 mg/ml) and SLN-NAC (0.1 mg/ml). These were incubated for 24 h. 0.1 mg/ml was selected as the dose since it is regularly used for ophthalmic formulations. Then, 20 μL of MTT solution (5 mg/mL) was added in each well and the plates were incubated at 37°C for 4 h. The absorbance was measured at 492 nm after the incubation using a microplate reader.
3. Results
3.1. Human corneal cell constructs
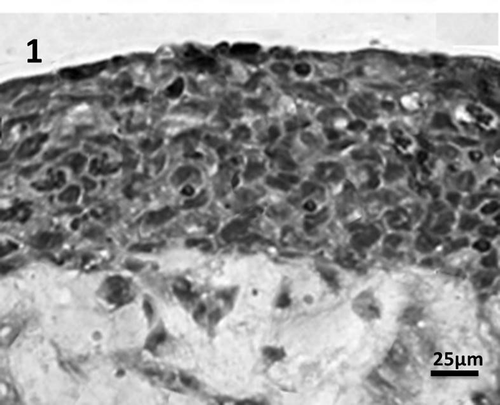
The novel bioengineered corneal construct consists of a cell layer comparable to stroma encompassing inherent fibroblasts, endothelium lying underneath, topped with stratified epithelium which was derived from HENC and CEPI 17 CL4 respectively. A mosaic like pattern was observed when stained with haematoxylin and eosin (H&E) and viewed in phase contrast micrography as depicted in . In order to express the multilayer epithlium, the construct was lifted to air–liquid interface and from the , it can be clearly seen. The basal cell layers appear flattened. This agrees with other corneal construct studies done before [Citation25–Citation28] and also in corneal in-vivo.
3.2. Size distribution and stability of formulation
Particle size distribution is a significant parameter towards progress of suitable nanoparticulates for therapeutic resolutions. It determines in-vivo drug release behaviour, biological fate, toxicity and the specific targeting of SLN-NAC after administration. Also it can also affect the loading, release and stability of drug (NAC) inside SLN. The diameter distribution of the SLN-NAC varied from 65 to 85 nm. The particle size distribution is shown in . The measurements were done thrice and results given as mean ± SD. The zeta potential ranges from −22.1 to ± 1 is also shown in . The zeta potential can greatly influence the stability of the nanoparticles.
Table 1. Size distribution and Zeta Potential measurement of SLN-NAC.
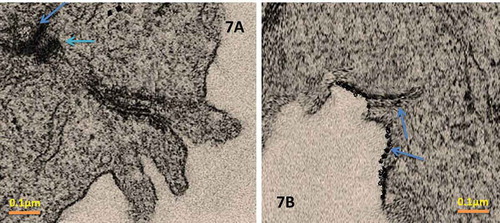
3.3. Transmission electron microscopy of SLN NAC
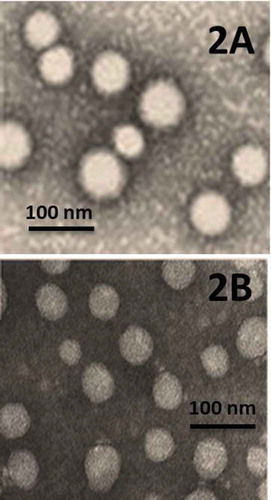
Morphological examinations of the SLN-NAC were done utilizing TEM. This study depicted that all the SLN-NAC prepared were spherical in shape albeit a little obtuse towards the circumference without any rough or sharp edges as seen in . The particles were sufficiently small enough to avoid sedimentation.
3.3.1. Determination of % of drug trapped in SLN-NAC
summarizes the entrapment efficiency of the SLN-NAC. It is clearly calculated that the entrapment efficiency is 86% i.e. entrapment efficiency is determined that if the % occurs to be 86, then it is assumed that in 1 mg of nanoparticles there is 0.86 ml of drug. Actual drug loading was calculated to be 8% which is a comparatively high drug loading.
Table 2. Percentage of drug loading SLN and the percentage of entrapment of drug in the SLN-NAC.
3.4. In-vitro release of SLN-NAC
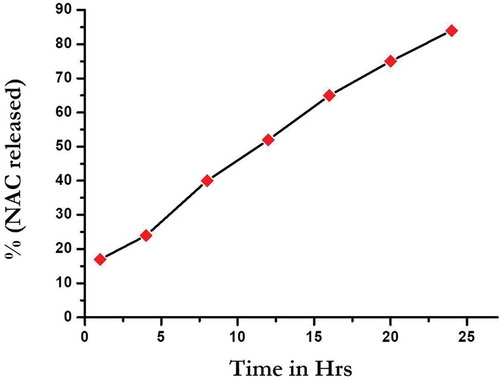
In-vitro release of the drug from SLN-NAC is depicted in . The graph shows a steady release of drug from the solid lipid nanoparticles. The release of NAC at the end of 1 h was 17.89 ± 0.23% and after 24 h it was 88 ± 0.23%. Therefore, after an initial burst of drug, there was a steady release of NAC which is important for treatment of cataract since NAC eye drops will tend to wash away immediately after application. With SLN-NAC aside from an initial burst, there is a steady sustained release of the drug for quite a long time before drug renewal. The corneal residence time for SLN-NAC is much longer than free NAC.
3.5. FTIR studies
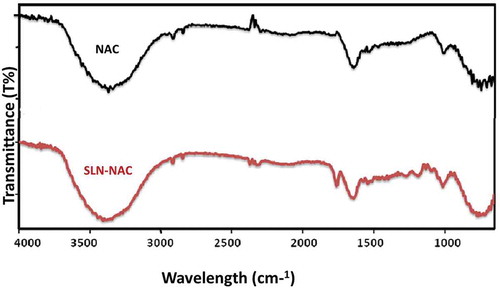
The ATR spectra were recorded from 4000 to 650 cm−1 as depicted in . The NAC molecule exhibited the chief peaks contributed by the functional groups of molecules, such as carbonyl – C = O stretching (1649 cm−1), – CH, – CH2, and – CH3 stretching (2920–3850 cm−1), – O–H and – N–H stretching (3564–3343 cm−1), and – C–N stretching (1000–1339 cm−1). The SLN-NAC also exhibited similar types of functional groups only the difference lies in the absence of some peaks in the region especially in 1000–1339 cm−1. This may be due to the fact that concentration of NAC found in pure drug may not be present in the SLN-NAC.
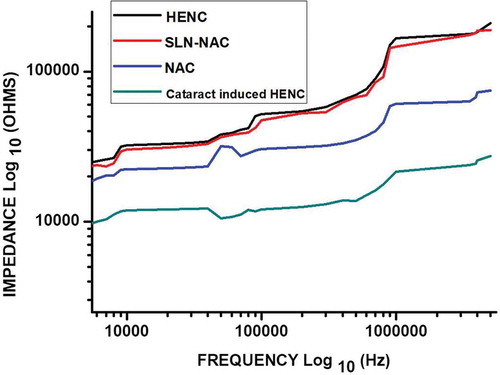
3.6. Impedance measurement
Bode plot for the effect of NAC and SLN-NAC on cataract induced HENC line is presented in . A normal HENC line is also plotted as control. The investigational data are fitted quite well with the used equivalent circuit as represented in . It is evident from the that the impedance increases for SLN-NAC treated sample as compared to free NAC treated and control samples. The impedance curve for SLN-NAC treated cataract induced sample moves towards the curve for normal HENC sample. Thus it can be interpreted that SLN-NAC may treat the cataract infection and cause its reversal. It is also verified from the results that the SLN-NAC are adept at initial burst release and subsequent sustained release of the incorporated drug in order to achieve the desired results. The relative standard deviations (RSD) for SLN-NAC, NAC treated and control are found to be below 10% that proves the data of the experiments is reproducible.
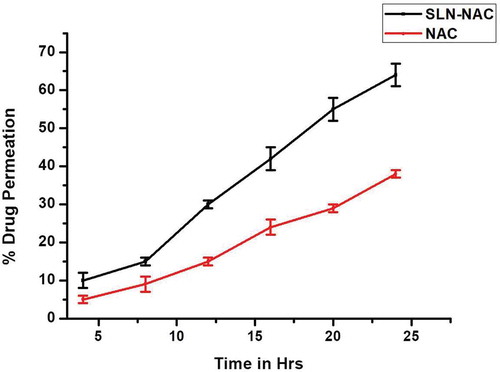
3.7. Drug permeation studies
The permeation profiles of SLN-NAC and NAC are represented in and . Graphically presented depicts the drug permeation in corneal construct for a period of 24 h considering the release of drug from SLN-NAC over the same period. It is seen that about 64 ± 3% of SLN-NAC permeates through the corneal membrane as against 38 ± 1% of free NAC. Therefore a higher permeability of SLN-NAC as compared to free NAC is demonstrated which is corroborated by the microscopic images in . The human corneal construct shows good permeability in both SLN-NAC and a little less permeability with free NAC although it is to be noted that corneal construct acts as a lipophilic-hydrophilic barrier to both SLN-NAC and free NAC. The SLN-NAC crosses the cell membrane to embed deep in the cell construct whereas free NAC is more concentrated on the membrane with less amount being able to permeate in.
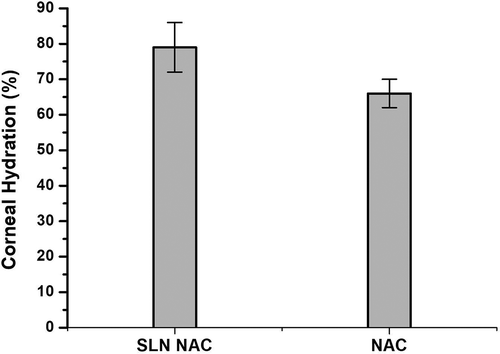
3.8. Corneal hydration studies
Corneal hydration was calculated and depicted in . The corneal hydration for SLN-NAC treated cornea was 79% whereas it is 72% in case of free NAC treated one. Normal corneal hydration is between 75% and 80% [Citation37]. Hence the corneal hydrations studies depict that was no corneal damage on treatment with SLN-NAC.
3.9. Cytotoxicity studies
Cytotoxicity of the NAC and SLN-NAC is clearly demonstrated in . We know from the control study the cells were completely viable at the beginning of the experiment. But it is interesting to know that the cell toxicity of the NAC and SLN-NAC is not significantly different from the control.
4. Discussion
We know from the previous investigations that concurrent culturing of each corneal cell type with extracellular matrix, collagen type I, within which epithelial, stromal, and endothelial cells may intermingle in-vitro and in-vivo identically. Additionally, culturing at air–liquid interface has a profound effect on the growth of a resilient, beehive styled epithelium that is multilayered [Citation25]. Keeping these points in mind, we had designed our study process so that the corneal constructs may prove a great equivalent replica of human cornea. This may help a long way in studying the SLN-NAC in comparison with only NAC for effective cataract reversal and treatment. Only factors which marks the difference is the absence of lachrymal fluid and eye blinking, apart from which the layers of cell are very similar. Along with corneal constructs, HENC line was cultured along to investigate the drug permeation and impedance studies. Although the drug permeation studies could have been done in freshly excised cornea as in corneal hydration studies, intraocular immortalised HENC line was preferred since cornea may be a bit less permeable to the drugs because of its state [Citation16]. Moreover for impedance studies we needed a cell line where cataract could be induced to compare the effectiveness of SLN-NAC and free NAC for treatment and reversal of the condition.
The novel bead mill method of preparation of SLN-NAC was preferred over the standard microemulsion and ultrasonication methods. The reason here lies was to elucidate any corneal toxicity of the SLN-NAC and allow better penetration of nanoparticulate [Citation17] as can be seen from the in-vitro release studies of SLN-NAC and drug permeation studies. The novel method also ensures sizes small enough to avoid aggregation over the shelf life of drug. Another very important reason is the cost-effectiveness of the preparation method. Mill method ensures preparation of SLN within 2 h and the cost would be effectively only the expenses incurred upon buying of reagents.
The shapes of the SLN-NAC were spherical and obtuse which further elucidated that the SLN would not cause irritation to the eye cornea as explained by Khare et al [Citation18]. Such nanoparticles also result in better tolerance into the eye because of concentration of small amount of drug in nanoparticles than eye drop. Moreover due to the general toxicity of free drug, there is potential danger to other tissues surrounding the lens which is not in case of nanoparticles as pointed out by. Moreover the TEM micrographs also point out that the there is no drug leakage due to membrane breakage of the nanoparticles despite the drug loading. The increased zeta potential indicates that neither the SLN-NAC would sediment nor will it aggregate to form larger particles. Outright large negative value of the zeta potential also indicates electrostatic repulsion between the nanoparticles which will avoid nanoparticle aggregation. Encapsulation efficiency and drug loading efficiency are found to be around 86% and 8% respectively. These are extremely important indices since with the in-vitro release profile of SLN-NAC; these provide important information for therapeutic purposes. Higher entrapment efficiency is as a result of the very small sized nanoparticles (65–80 nm) formed as a consequence of using bead mill method. Additionally, due to the hydrophobicity and the small size of the SLN-NAC, these penetrate the ocular barrier easily and remain long in the circulation. The in-vitro release studies agree with the solid lipid nanoparticles characteristic feature. The SLN are associated with burst release of drugs [Citation38] which is a disadvantage turned into advantage in this case because of the tear and blinking mechanism. The burst release is an advantage in this case because of the major drug release over a short period of is because of the drug adsorption over the surface of nanoparticle and not in the core which will be easily available for the treatment of cataract.
The FTIR studies made an interesting observation. It was seen that the peaks in the region of 1000–1339 cm−1 were not visible. These may be reasoned that the amount of NAC loaded in SLN-NAC is lesser than pure drug. The more visible peaks claimed successful drug loading in the SLN-NAC. The Bode plot of the impediametric studies indicates an array of impedance pattern which yields typical pattern of least cell death (highest impedance values) in normal HENC cell line which is very similar to the SLN-NAC treated cells, followed by NAC treated cells. This typically indicates that the NAC is known to reduce the oxidative stress [Citation8] and the synergistic effects of nanoparticles and NAC causes a membrane stabilizing effect and an antioxidant effect which tends to treat as well as reverse cataract. Corneal hydration is an integral part of study. The percentage of corneal hydration indicates that there is normal deturgescence in the corneal stroma. This also specifies that there is no loss or damage of the endothelial cells on exposure to SLN-NAC and free NAC. There is no fluid retained causing excessive corneal hydration which may coincide with the clarity of lens. Briefly to say, there is no corneal damage on contact with SLN-NAC and free NAC.
We know that not only for ocular therapeutic drug delivery, any application of nanoparticles for human health has to be looked seriously into. The nanoparticulate system should not interfere with the living tissues by causing toxicity or adverse reactions. This is being exhibited by the cytotoxicity studies. Although cytotoxicity studies indicate that there is not a significant difference between the control NAC and SLN-NAC. This may be attributed to the fact that NAC has been previously regularly used as eye drops for treatment of cataract in a lot of studies [Citation6–Citation8]. Our work proves that SLN-NAC has even lower cell toxicity than NAC. This study by us reinforces the physicochemical characterization of SLN and pronounces them compatible for human ocular use. The key characterization protocol like shape, size, entrapment efficiency, drug loading, in-vitro studies and permeation studies have specified that SLN-NAC may be more useful than free NAC in eye drops for reversal and treatment of cataract.
5. Conclusion
As evident from the study, we have invested much effort in preparation and introduction of SLN in ocular drug delivery. It was very logical to choose NAC because of its established effects on cataractuous lenses. Although the human corneal constructs need to be worked upon, this seemed the only feasible way of testing the efficacy of SLN-NAC. From a therapeutic point of view, we have achieved the way to reduce the number of injection to eye for treatment and reversal of cataract by using SLN-NAC. The desired effect was obtained with lowering of drug concentration in eye formulation and without causing any damage to surrounding ocular tissues. This may point out a significant extension of NAC for treatment of cataract. Nevertheless, in spite of opening up a new perspective, this studies mentioned above needs to be replicated and thoroughly investigated for any lacunae for further clinical implementation.
Authors Contribution
Ling Wang and Weixian Lui performed the experiments jointly and wrote the manuscript partially. Xionggao Huang analyzed the results, and was a major contributor in writing the results & discussion. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Pillay V, Choonara YE, Du Toit LC. Intraocular drug delivery technologies: advancing treatment of posterior segment disorders of the eye. In: Pathak Y, Sutariya V, Hirani AA, Edited by. Nano-biomaterials for ophthalmic drug delivery. Cham: Springer International Publishing; 2016. p. 1–10.
- Lindfield R, Vishwanath K, Ngounou F, et al. The challenges in improving outcome of cataract surgery in low and middle income countries. Indian J Ophthalmol. 2012;60(5):464–469.
- Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010;1(3):435–456.
- Cetinel S, Montemagno C. Nanotechnology for the prevention and treatment of cataract. Asia Pac J Ophthalmol (Phila). 2015;4(6):381–387.
- Azharuddin M, Dasgupta AK, Datta H. Gold nanoparticle conjugated with curcumin and curcumin nanoparticles as a possible nano-therapeutic drug in cataract. Curr Indian Eye Res. 2015;1:71.
- Babizhayev MA, Deyev AI, Yermakova VN, et al. Efficacy of N-acetylcarnosine in the treatment of cataracts. Drugs R D. 2002;3(2):87–103.
- Babizhayev MA, Deyev AI, Yermakova VN, et al. N-Acetylcarnosine, a natural histidine-containing dipeptide, as a potent ophthalmic drug in treatment of human cataracts. Peptides. 2001;22:979–994.
- Dubois VDP, Bastawrous A. N‐acetylcarnosine (NAC) drops for age‐related cataract. Cochrane Database Syst Rev. 2017;2.
- Somasundaram S, Blickenstaff G. A non-invasive approach for cataracts: efficacy of carnosine in the treatment and prevention of crystallin aggregation in vitro. JESS. 2013;5: 5–7.
- Mark A, Yermakova VN. Erratum to “Nα-Acetylcarnoslne is a prodrug of L-carnosine in ophthalmic application as antioxidant”[Clin. Chim. Acta 254 (1996) 1-21]. Clin Chim Acta. 1997;259(199):201.
- Li X, Zhang Z, Li J, et al. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale. 2012;4(15):4667–4673.
- Gupta H, Aqil M, Khar R, et al. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J Drug Target. 2011;19(6):409–417.
- Misra R, Upadhyay M, Mohanty S. Design considerations for chemotherapeutic drug nanocarriers. Pharm Anal Acta. 2014;5:279.
- Zhou HY, Hao JL, Wang S, et al. Nanoparticles in the ocular drug delivery. Int J Ophthalmol. 2013;6(3):390.
- Rafie F, Javadzadeh Y, Javadzadeh AR, et al. In vivo evaluation of novel nanoparticles containing dexamethasone for ocular drug delivery on rabbit eye. Curr Eye Res. 2010;35(12):1081–1089.
- Diebold Y, Calonge M. Applications of nanoparticles in ophthalmology. Prog Retin Eye Res. 2010;29(6):596–609.
- Nagai N, Ito Y. A new preparation method for ophthalmic drug nanoparticles. Pharm Anal Acta. 2014;5(6):1000305.
- Khare A, Singh I, Pawar P, et al. Design and evaluation of voriconazole loaded solid lipid nanoparticles for ophthalmic application. J Drug Deliv. 2016;11.
- Sunkireddy P, Jha SN, Kanwar JR, et al. Natural antioxidant biomolecules promises future nanomedicine based therapy for cataract. Colloids Surf B. 2013;112:554–562.
- Baeyens V, Gurny R. Chemical and physical parameters of tears relevant for the design of ocular drug delivery formulations. Pharm Acta Helv. 1997;72(4):191–202.
- Mudgil M, Pawar PK. Preparation and in vitro/ex vivo evaluation of moxifloxacin-loaded PLGA nanosuspensions for ophthalmic application. Sci Pharm. 2013;81(2):591–606.
- Kalam MA, Sultana Y, Ali A, et al. Preparation, characterization, and evaluation of gatifloxacin loaded solid lipid nanoparticles as colloidal ocular drug delivery system. J Drug Target. 2010;18(3):191–204.
- Khalil RM, Abd-Elbary A, Kassem MA, et al. Nanostructured lipid carriers (NLCs) versus solid lipid nanoparticles (SLNs) for topical delivery of meloxicam. Pharm Dev Technol. 2014;19(3):304–314.
- Budama-Kilinc Y, Cakir-Koc R, Kecel-Gunduz S, et al. Novel NAC-loaded poly(lactide-co-glycolide acid) nanoparticles for cataract treatment: preparation, characterization, evaluation of structure, cytotoxicity, and molecular docking studies. Peer J. 2018;6:4270.
- Reichl S, Bednarz J, Müller-Goymann C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol. 2004;88(4):560–565.
- Kahn C, Young E, Lee IH, et al. Human corneal epithelial primary cultures and cell lines with extended life span: in vitro model for ocular studies. Invest Ophthalmol Vis Sci. 1993;34(12):3429–3441.
- Araki-Sasaki K, Ohashi Y, Sasabe T, et al. SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci1995. 36(3):614–621.
- Toropainen E, Ranta V-P, Talvitie A, et al. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci. 2001;42(12):2942–2948.
- Kawazu K, Shiono H, Tanioka H, et al. Beta adrenergic antagonist permeation across cultured rabbit corneal epithelial cells grown on permeable supports. Curr Eye Res. 1998;17(2):125–131.
- Huang Y, Ning J, Zhou T, et al. Multidrug resistance reversal study of Psoralen-loaded solid lipid nanoparticles. J Biomater Tissue Eng. 2015;5(10):780–787.
- Katara R, Majumdar DK, Eudragit RL. 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf B. 2013;103:455–462.
- Gupta SK, Kalaiselvan V, Srivastava S, et al. Evaluation of anticataract potential of Triphala in selenite-induced cataract: in vitro and in vivo studies. J Ayurveda Integr Med. 2010;1(4):280.
- Benson K, Cramer S, Galla HJ. Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS. 2013;10:5.
- Dou QL, Wei YY, Gu YN, et al. Investigating the therapeutic effects of N-acetylcysteine decorated poly(L-lactic acid) nanoparticles on transfusion induced acute lung injury. J Biomater Tissue Eng. 2017;7(1):69–76.
- Gong T, Su XT, Xia Q, et al. Biodegradable combinatorial drug loaded pH-sensitive liposomes for enhanced osteosarcoma therapeutics. J Biomater Tissue Eng. 2017;7(10):952–961.
- Banerjee S, Sen K, Pal TK, et al. Poly (styrene-co-maleic acid)-based pH-sensitive liposomes mediate cytosolic delivery of drugs for enhanced cancer chemotherapy. Int J Pharm. 2012;436(1–2):786–797.
- Hulse WL, Forbes RT, Bonner MC, et al. The characterization and comparison of spray-dried mannitol samples. Drug Dev Ind Pharm. 2009;35(6):712–718.
- Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17(7):467–489.