ABSTRACT
Objectives: Our research investigated the relationship between childhood leukemia and breastfeeding in the P. R. of China.
Methods: We conducted a retrospective case-control study from March 2008 to April 2017 at the Children’s Hospital of Zhejiang University, Zhejiang province, P. R. of China, which reviewed 958 children who had been diagnosed with leukemia in case group and 785 healthy children in control group. Data were obtained from medical records, and if the medical records were incomplete, we called mothers of children by phone to complete the data.
Results: Breastfeeding reduces the risk of childhood leukemia; the effect is greater, if feeding continued for 7–9 months (p = 0.002). In addition, we suggest that some factors such as maternal age, smoking during pregnancy, abortion history, genetic factors, parents use of hair dye, and the history of using birth control pills before pregnancy can increase the risk of childhood leukemia.
Conclusions: This study indicates that promoting breastfeeding for 7–9 months may help lower the childhood leukemia incidence. Our study firstly demonstrates that breastfeeding has protective effects against childhood leukemia in the P. R. of China.
Abbreviations: ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia
1. Introduction
Childhood cancer is rare among the childhood diseases. The prevalence of childhood cancer is on the increase and seriously threatens the health of children [Citation1]. Leukemia accounts for over one-third of childhood cancer under 15 years old. There are two main types of childhood leukemia, acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML). AML is not very common, accounting for about 17% of childhood leukemia [Citation2]. Its etiology and pathogenesis remain unknown, which may be linked to genetic factors and environmental factors including infection, radiation, and living conditions [Citation3]. Impressive advancements in childhood leukemia treatment entail cure rates reaching 90% for ALL and 60–70% for AML [Citation4,Citation5]. As a result of improved cancer treatments, there has been a dramatic increase in the number of young survivors who are living well into adulthood, but many children have long-term complications because of radiotherapy and chemotherapy. The long-term complications include obesity, metabolic syndrome, impaired glucose tolerance, and insulin resistance, which are the risk factors for cardiovascular disease [Citation6,Citation7]. Long-term and late-appearing secondary effects include impaired neurocognitive development, mental health, endocrine system function, and general health [Citation8], which not only affects children’s health but also imposes a heavy financial burden on families and society. Thus, primary prevention plays an important role in childhood leukemia.
Breast milk also can be effective for preventing many diseases such as diabetes, cardiovascular diseases, obesity, and even cancer [Citation9]. Breastfeeding has been shown to decrease the risk of gastrointestinal infections. Any volume of breast milk is protective, associated with a 64% reduction in the incidence of nonspecific gastrointestinal infections [Citation10]. The duration of lactation is inversely related to the risk of overweight; each extra month of breastfeeding is associated with a 4% decrease in risk [Citation11]. Those not breastfed at discharge had a 33% higher risk of developing diabetes within the first 20 years of life [Citation12]. Breastfeeding is not only beneficial for children but also good for mothers [Citation13]. It can reduce the risk of breast cancer, ovarian cancer, and osteoporosis for mothers. However, the relationship between breastfeeding and childhood leukemia is elusive. Several studies reported no association between breastfeeding and ALL [Citation14,Citation15]. A meta-analysis indicated that promoting breastfeeding for 6 months or more might help lower the prevalence of childhood leukemia, in addition to its other health benefits for children and mothers [Citation16]. But these data were inadequate, and there is a lack of research among the 1.4 billon people in the P. R. of China. Given the conflicting findings within the existing literature, the exact impact of breastfeeding on childhood leukemia remains unclear. Therefore, the purpose of this study was to investigate whether breastfeeding had a protective effect against childhood leukemia in the P. R. of China, while looking for the best period of time for breastfeeding infants.
2. Subjects and methods
2.1. Patients
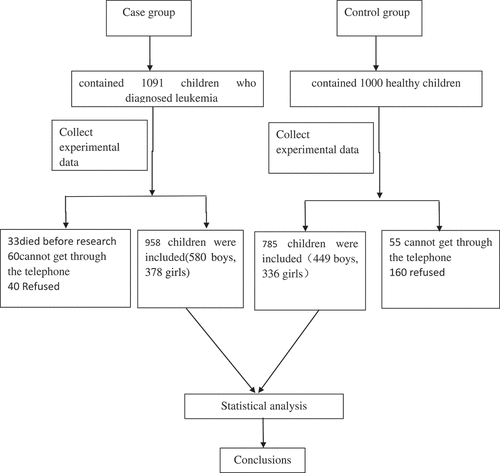
We conducted a case-control study from March 2008 to April 2017 at the Children’s Hospital of Zhejiang University, Zhejiang province, P. R. of China. The case group enrolled 958 children (580 boys, 378 girls) who had been diagnosed with childhood leukemia, and the control group contained 785 healthy children (449 boys, 336 girls) within the period of the study. All subjects volunteered to participate in the study, and ethical approval was given by the medical ethics committee of the Children’s Hospital of Zhejiang University. The screening process is shown in .
The following demographic characteristics and clinical factors were obtained from medical records: age, sex, family address, maternal age, delivery, mother’s education, maternal marital status, family history of malignant tumors, family history of neoplasm of the lymphatic/hematopoietic systems, history of housing decoration, smoking history during pregnancy, history of using birth control pills before pregnancy, abortion history, abortion times, Down’s syndrome, duration of breastfeeding, breastfeeding pattern, parents use of hair dye, and birth weight. If the medical records were incomplete, we called the mothers of children by phone to complete the data.
2.2. Inclusion and exclusion criteria
Inclusion criteria for cases contained (1) children aged 0–14 years old; (2) children who had been diagnosed childhood leukemia; (3) children who lived in Zhejiang province, P. R. China; and (4) the mother who remembered the time of breastfeeding exactly.
Exclusion criteria for cases included (1) a second primary tumor, (2) no answer or no connection, (3) death before the interview date, (4) biological mother unavailable for an interview (child adopted, mother deceased), and (5) children who did not agree to participate in the study.
Inclusion criteria for healthy group contained (1) children aged 0–14 years old; (2) children who lived in Zhejiang province, P. R. China; (3) children’s parents volunteered to participate in this study; (4) healthy children without prior history of cancer; and (5) the mother who remembered the time of breastfeeding exactly.
Exclusion criteria for healthy group included (1) no answer or no connection, (2) biological mother unavailable for an interview (child adopted, mother deceased), and (3) children who did not agree to participate in the study.
2.3. Statistical analysis
-All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) Version 17.0 (SPSS, Chicago, USA). Data were expressed as mean and standard deviation or frequency and percentage. The differences between two independent groups were compared by using Student’s t-test for continuous variables. A chi-square (χ2) test was used for categorical variables. Multivariable logistic regression analysis was used to evaluate risk factors for childhood leukemia. Odds ratios (ORs) and 95% confidence intervals (CIs) are presented. A two-sided p value <0.05 was considered statistically significant for all analyses.
3. Results
3.1. Demographic characteristics
The demographic characteristics of cases and controls were generally similar, with some exceptions (). The mean age was 7.72 ± 3.77 years in the case group, and the mean age of the control group was 7.96 ± 3.71 years. Case group and control group members had a similar mean age (t = −1.307, p = 0.191) and an identical distributions by gender (χ2 = 1.997, p = 0.158). Case group and control group members did not differ by the type of delivery (χ2 = 0.249, p = 0.618), maternal marital status (χ2 = 4.756, p = 0.190), and birth weight (t = 2.018, p = 0.063). However, in present study, the case group members were significantly different compared to the control group in the maternal age (χ2 = 22.716, p < 0.001). Mothers of case group members had a higher number of abortions (t = 2.001, p = 0.046) than in the control group. Control group mothers were more likely to have a college/university education (χ2 = 286.86, p < 0.001). Children with leukemia were mainly from Hangzhou city of Zhejiang province (29.4%).
Table 1. Sociodemographic characteristics
3.2. Environmental factors
In multivariable logistic regression analysis, smoking during pregnancy (OR: 3.551, 95% CI: 1.051–12.416, p = 0.034), a history of using birth control pills before pregnancy (OR: 1.795, 95% CI: 1.081–2.982, p = 0.022), and parents use of hair dye during breastfeeding (OR: 13.556, 95% CI: 1.112–165.206, p = 0.041) were significant risk factors associated with childhood leukemia ().
Table 2. Risk factors for childhood leukemia
3.3. Genetic factors
Compared with the control group, a family history of malignant tumors (OR: 4.143, 95% CI: 2.521–6.808, p < 0.0001), a family history of neoplasm of the lymphatic/hematopoietic systems (OR: 7.648, 95% CI: 2.334–25.062, p < 0.0001), and Down’s syndrome (OR: 8.604, 95% CI: 2.024–36.581, p < 0.0001) were significant risk factors associated with childhood leukemia ().
3.4. Breastfeeding
The results indicated that ever breastfed (OR: 7.431, 95% CI: 5.124–10.777, p < 0.0001) was significant difference compared to never breastfed. Breastfeeding period of 7–9 months (OR: 0.498, 95% CI: 0.318–0.780, p = 0.002) was significantly different in the case group and the control group. There were no differences in breastfeeding period of 1–3 months (OR: 1.037, 95% CI: 0.636–1.691, p = 0.884), breastfeeding period of 4–6 months (OR: 0.751, 95% CI: 0.479–1.178, p = 0.231), and 10–12 months (OR: 1.114, 95% CI: 0.718–1.727, p = 0.631) in the two groups. Pure milk powder might increase the incidence of leukemia, while exclusive breastfeeding could reduce the risk of childhood leukemia. The detail results were shown in .
4. Discussion
To our knowledge, this study was the first to demonstrate that breastfeeding had protective effect against childhood leukemia in the P. R. of China. In the present study, we have demonstrated that children who were ever breastfed (p < 0.0001) had a lower risk of childhood leukemia. In the case group, the breastfeeding period was shorter than in the control group. Furthermore, we confirmed that the 7–9-month duration of breastfeeding had a protective effect against leukemia. These results indicated that breastfeeding was found to be closely associated with the childhood leukemia. In the context of these findings, our results strongly suggested that the barrier protective effects of breastfeeding could be used to open a new strategy for preventing the development of childhood leukemia.
Today, the exact etiology of childhood leukemia and the protective effect of breastfeeding against cancer are still elusive. A population-based case-control study from Israel suggested that breastfeeding had a significant influence in reducing the risk of leukemia (OR: 0.36, 95% CI: 0.21–0.60, p < 0.001) [Citation17]. Another case-control study suggested that there was no significant difference between the patient and control groups (p = 0.641) [Citation18]. However, in our study, we indicated that 7–9 months duration of breastfeeding was better for infants. Breastfeeding too long did not reduce the risk of leukemia, which might be related to differences in nutrient components with lactation time. Protein, lactose, mineral content, and breast milk density decreased with the increase of lactation time. The fat content of mature milk is relatively high, which is not a benefit for infants. Chinese social and cultural background are different from other societies, and there is a different perception of breastfeeding, which could lead to differences in breastfeeding time. The result might also due to the rapid development of the Chinese economy. With the development of the economy and changes in society in the past few years in China, more and more women are joining the workforce and becoming the backbone of their work place. Another reason was that the differences of culture and understanding of breastfeeding might also affect the breastfeeding time. For all the above reasons, the best breastfeeding time for infants was determined to be 7–9 months in China.
In addition, this study found an association between mothers having a lower education level and an increased risk of leukemia in children, which was also reported by Ashok Kumar et al. [Citation19]. Our study indicated that there was no difference between advanced maternal age and risk of childhood leukemia. This could be partly explained by younger age of marriage and pregnancy especially in rural areas in the P. R. of China. A family history of cancer and a family history of neoplasm of the lymphatic/hematopoietic systems were significantly different in the two groups. Therefore, genetic susceptibility was important in terms of childhood leukemia etiology. This parameter can absolutely not be ignored. Children whose mothers had exposure to hair dye during the breastfeeding and smoking during pregnancy (OR: 3.551) were at greater risk of developing leukemia than controls (p = 0.041). Another study had reported that children whose mothers dyed their hair during breastfeeding had an increased risk of leukemia [Citation20]. This suggests that carcinogens enter the body through breast milk and that an embryo in the uterus might be particularly sensitive to carcinogenic effects. As was reported by Saldivar et al., children whose father had been in contact with carcinogenic materials also had a higher risk of ALL in childhood [Citation21].
Due to the developments in society and the economy, currently only 37% of infants under 6 months in low- and middle-income countries are exclusively breastfed, and this figure is even lower in high-income countries [Citation22]. The number of children who were given bottled milk powder in the case group (26.3%) was also greater than in the control group (3.7%), which might also affect the prevalence of leukemia. Because of the use of formula, the baby’s gut microorganisms are altered, which affects the immune system’s response to pathogens [Citation23–Citation26]. A great number of natural-killer cells, suggesting a more mature immune system, have been found in breastfed infants than in formula-fed infants [Citation27]. In addition, the pH level (hydrogen ion concentration, which can activate pepsin and promote protein digestion and absorption) in the stomach of breastfed children is better for the promotion of the protein-lipid α-lactalbumin, which induces apoptosis like death of tumor cells [Citation28,Citation29]. Infant formulas cannot mimic the array of protective properties of breast milk, which fits the infant owing to the dyadic connection between a mother and her baby.
In conclusion, the current study shows that the breastfeeding may have a great protective effect against childhood leukemia. Especially, breastfeeding for 7–9 months was found to be better. In the future, we should focus on the mechanism of breastfeeding that reduces childhood leukemia and provide stronger evidence for this conclusion.
The limitation of this research includes (1) this is just a single-center study, and we should focus on multicenter studies in the future; (2) regarding the relation between breastfeeding and childhood leukemia, the presence of confounding factors should be considered in future studies. In the future, more high-quality studies are needed to clarify the biological mechanisms underlying this association between breastfeeding and lower childhood leukemia morbidity.
Acknowledgments
We thank professor Hong-Mei Ma (People’s hospital of Wuhan University, China) for critical reading. The authors are grateful to all the mothers and children for warmly collaborating and for their support and participation in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barrington-Trimis JL, Cockburn M, Metayer C, et al. Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer. 2017;140(5):1–6.
- Metayer C, Milne E, Clavel J, et al. The childhood leukemia international consortium. Cancer Epidemiol. 2013;37(3):336–347.
- Wiemels J. Perspectives on the causes of childhood leukemia. Chem Biol Interact. 2012;196(3):59–67.
- Pui CH, Mullighan CG, Evans WE, et al. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120(6):1165–1174.
- Moore AS, Kearns PR, Knapper S, et al. Novel therapies for children with acute myeloid leukemia. Leukemia. 2013;27(7):1451–1460.
- Faienza MF, Delvecchio M, Giordano P, et al. Metabolic syndrome in childhood leukemia survivors: a meta-analysis. Endocrine. 2015;49(2):353–360.
- Lipshultz SE, Franco VI, Miller TL, et al. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–176.
- Iyer NS, Balsamo LM, Bracken MB, et al. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015;126(3):346–353.
- Brahm P, Valdes V. Benefits of breastfeeding and risks associated with not breastfeeding. Rev Chil Pediatr. 2017;88(1):15–21.
- Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial(PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420.
- Johnston M, Landers S, Noble L, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841.
- Jones ME, Swerdlow AJ, Gill LE, et al. Pre-natal and early life risk factors for childhood onset diabetes mellitus: a record linkage study. Int J Epidemiol. 1998;27(3):444–449.
- Turck D, Vidailhet M, Bocquet A, et al. Breastfeeding: health benefits for child and mother. Arch Pediatr. 2013;20(Suppl 2):S29–48.
- Kwan ML, Buffler PA, Wiemels JL, et al. Breastfeeding patterns and risk of childhood acute lymphoblastic leukemia. Br J Cancer. 2005;93(3):379–384.
- MacArthur AC, McBride ML, Spinelli JJ, et al. Risk of childhood leukemia associated with vaccination, infection, and medication use in childhood: the Cross-Canada childhood leukemia study. Am J Epidemiol. 2008;167(5):598–606.
- Amitay EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: A meta-analysis and systematic review. JAMA Pediatr. 2015;169(6):e151025.
- Amitay EL, Dubnov Raz G, Keinan-Boker L. Breastfeeding, other early life exposures and childhood leukemia and lymphoma. Nutr Cancer. 2016;68(6):968–977.
- Karimi M, Haghighat M, Dialameh Z, et al. Breastfeeding as a protective effect against childhood leukemia and lymphoma. Iran Red Crescent Med J. 2016;18(9):e29771.
- Kumar A, Vashist M, Rathee R. Maternal factors and risk of childhood leukemia. Asian Pac J Cancer Prev. 2014;15(2):781–784.
- Couto AC, Ferreira JD, Rosa AC, et al. Pregnancy, maternal exposure to hair dyes and hair straightening cosmetics, and early age leukemia. Chem Biol Interact. 2013;205(1):46–52.
- Perez-Saldivar ML, Ortega-Alvarez MC, Fajardo-Gutierrez A, et al. Father’s occupational exposure to carcinogenic agents and childhood acute leukemia: a new method to assess exposure (a case-control study). BMC Cancer. 2008;8:7.
- Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490.
- Bener A, Hoffmann GF, Afify Z, et al. Does prolonged breastfeeding reduce the risk for childhood leukemia and lymphomas? Minerva Pediatr. 2008;60(2):155–161.
- Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521.
- Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr. 2012;3(3):383S–91S.
- Coppa GV, Zampini L, Galeazzi T, et al. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(suppl 2):S291–S4.
- Hanson LA. Session1: feeding and infant development breast-feeding and immune function. Proc Nutr Soc. 2007;66(3):384–396.
- Hawkes JS, Neumann MA, Gibson RA. The effect of breastfeeding on lymphocyte subpopulations in healthy term infants at 6 months of age. Pediatr Res. 1999;45(5 Pt 1):648–651.
- Svanborg C, Agerstam H, Aronson A, et al. Hamlet kills tumor cells by an apoptosis-like mechanism: cellular, molecular, and therapeutic aspects. Adv Cancer Res. 2003;88:1–29.