ABSTRACT
There are no studies evaluating the glucose variability in different periods of Ramadan fasting in patients with type 2 diabetes using continuous glucose monitoring (CGM). This study examined the effect of Ramadan fasting on interstitial glucose (IG) variability in early,- late-, and post-Ramadan compared to pre-Ramadan days in non-insulin-treated type 2 diabetes patients. Participants had a CGM system connected 2 or 3 days before Ramadan start, which was removed on the third or fourth day of Ramadan. CGM performance continued for a total of 6 days. A second CGM performance started on the 27th or 28th day of Ramadan and ended on the 4th or 5th post-Ramadan day. First, CGM recordings were divided into pre-Ramadan and early-Ramadan CGM, and second recordings into late-Ramadan and post-Ramadan. At each visit, blood pressure, body weight, and waist circumference were measured, and fasting blood samples were collected for HbA1c and plasma glucose. All patients received recommended Ramadan education before Ramadan. Thirty-three patients (mean age 55.0 ± 9.8 years, 73% males) were prospectively included. IG variability, estimated as mean amplitude of glycaemic excursions (MAGE), increased significantly in early-Ramadan compared to pre-Ramadan (P = 0.006) but not in late-Ramadan and post-Ramadan recording days. Only patients on >2 anti-diabetic drugs (n = 16, P = 0.019) and those on sulphonylureas (n = 14, P = 0.003) showed significant increase in MAGE in early-Ramadan. No significant changes were seen in coefficient of variation, time in range, time in hyperglycaemia, or time in hypoglycaemia. Except for an initial increase in glucose variability, fasting Ramadan for patients with non-insulin-treated type 2 diabetes did not cause any significant changes in glucose variability or time in hypoglycaemia during CGM recording days compared to non-fasting pre-Ramadan period.
1. Introduction
Ramadan fasting for Muslims is refraining from food and fluid intake from dawn to sunset for a month every lunar calendar year. For patients with diabetes, this implies major changes in their daily routines, including mealtimes, medication frequency and doses, and daily activities. Consequently, there is an increased risk of affecting metabolic control for a whole month annually.
There is little information regarding how plasma glucose values and its fluctuation are affected during Ramadan fasting in adult patients with type 2 diabetes. Most studies done using continuous glucose monitoring (CGM) during Ramadan fasting included adolescent patients with type 1 diabetes [Citation1–Citation4]. In these studies, no significant difference in glucose fluctuation could be shown during Ramadan fasting compared to non-fasting periods. However, mean amplitude of glycaemic excursion (MAGE), a measure of glycaemic variability, was not used in these studies. In one study, that included patients with both type 1 and type 2 diabetes, a different pattern of excursions could be seen but no significant difference in glucose variability was measured. However, amplitude of excursions was greater among insulin- or sulphonylurea (SU)-treated patients [Citation5]. Hypoglycaemia during Ramadan fasting has been a matter of concern. In one study using CGM in patients with diabetes on insulin pump treatment, no difference in hypoglycaemic episodes could be seen during daytime and night-time [Citation4]. Kaplan et al. on the other hand reported significantly higher rates of hypoglycaemia during daytime compared to night-time [Citation2].
Until now, no studies on diabetes patients have assessed glucose variability in different periods of Ramadan fasting using CGM. The main objective of this study was to examine the effect of Ramadan fasting on interstitial glucose (IG) variability in early-, late-, and post-Ramadan compared to pre-Ramadan days in non-insulin-treated type 2 diabetes patients using CGM.
2. Material and methods
2.1. Study population and design
Thirty-three patients with non-insulin-treated type 2 diabetes mellitus followed at a tertiary diabetes centre (Rashid Centre for Diabetes and Research, Ajman, UAE) were included in this clinical observational study. Patients were included prospectively at the diabetic clinics according to inclusion criteria during planned control visits to their diabetes team starting 6 months before Ramadan start. All patients were Emirati national citizens. Inclusion criteria were type 2 diabetes mellitus, age ≥ 18 years, males and females, and having the intention to fast during the Ramadan month in 2017 (the first day of Ramadan was 26 May and the last day was 24 June). Overall exclusion criteria were insulin treatment, known liver insufficiency, defined as an elevation of serum alanine aminotransferase (ALAT) or aspartate aminotransferase (ASAT) levels twice higher than the normal superior limit or spontaneous international normalized ratio > 1.1, severe renal insufficiency, defined as estimated glomerular filtration rate < 30 mL/min/1.73 m2, cannot or do not intend to fast during Ramadan, untreated metabolic or endocrine diseases (e.g. pheochromocytoma, Cushing syndrome, or acromegaly), or on corticosteroid treatment. The study was approved by the regional ethics committee in Ajman Medical District, Ministry of Health and Prevention. Written consent was given by all subjects before beginning the study.
2.2. CGM performance and data collection
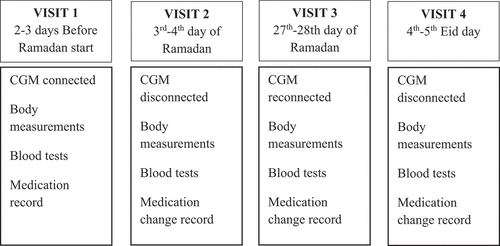
The Medtronic iPro®2 CGM system was used to perform CGM. IG was measured every 5 min. All subjects had the CGM system connected, after education about the system and calibration, for a first recording of IG values 2 or 3 days before Ramadan started (visit 1). The CGM device was removed on the third or fourth day of Ramadan (visit 2). The CGM performance continued for total of 6 days on each subject. During both visits, blood pressure, height, and body weight were measured, and fasting venous blood samples for HbA1c (measured in derived NGSP units, % to one decimal point), plasma glucose, and serum creatinine were taken after a 10-h fast. All samples consisted of whole blood drained into 6-mL serum separation tubes, plasma separation tubes, and BD Microtainer tubes with EDTA (BD Biosciences, Franklin Lakes, NJ, USA). Blood chemistries were analysed the same morning during the study. Blood pressure was measured in the supine position after a 5-min rest with the arm at the level of the heart. Height was measured to the nearest centimetre, weight to the nearest kilogram in subjects wearing light clothes, but not shoes, and BMI was calculated. Patients’ data including diabetes duration, medications, and reported episodes of hypoglycaemia were registered during study time. The second CGM performance started on the 27th or 28th day of Ramadan (visit 3) and ended on the 4th or 5th post-Ramadan day (visit 4). The first 6 days of recordings were considered. All body measurements, blood samples, and data collection done at visits 1 and 2 were repeated at visits 3 and 4. The first CGM recordings were divided into two periods: pre-Ramadan (days before Ramadan start) and early-Ramadan CGM, and the second recordings were divided into late-Ramadan (last days of Ramadan) and post-Ramadan (). At least a whole day reading was considered in analysis in each period.
Software KADIS®DCC (version 0.4.6.1, 2017, Institute of Diabetes, Karlsburg, Germany) was used to calculate sensor-derived mean and standard deviation (SD) of IG, and as MAGE for pre-, early-, late-, and post-Ramadan CGM recordings separately. Coefficient of variation was calculated from mean and SD of IG. Time in range (per cent of IG readings 70–180 mg/dl), time in hypoglycaemia (per cent of IG readings < 70 mg/dl), and time in hyperglycaemia (per cent of IG readings > 180 mg/dl) were calculated [Citation6]. Subjects were asked to report any symptomatic or serious hypoglycaemic events defined as hypoglycaemia associated with severe cognitive impairment requiring external assistance for recovery [Citation7] during CGM recording days or fast breaking during Ramadan month. Education and advice about adjustment of anti-diabetic treatment and doses for the Ramadan period were given to all subjects before Ramadan started according to clinical guidelines [Citation8].
2.3. Statistical analysis
Values are given as mean ± SD or median ± range. Normality assumption was tested using Shapiro–Wilk test. Comparisons between groups were made using the Mann–Whitney test. Statistical significance was defined as a P-value < .05. Statistical analysis was performed using SPSS statistics version 25 (IBM corporation, Armonk, NY, USA).
3. Results
3.1. General characteristics
Thirty-three patients were included in this study, of whom 73% were men. One patient dropped out in the second CGM recording, and thus only this patient’s pre-Ramadan and early-Ramadan readings were included. Average recording days of each period were 2.3 days. The mean age was 55 ± 9.8 years, and the mean diabetes duration 10.4 ± 6.8 years. At the first visit (), mean HbA1c was 6.7 ± 1.0%, and mean fasting plasma glucose was 133.5 ± 35.5 mg/dl. The average fasting time was 15 h and 8 min each day.
Table 1. Clinical patient characteristics
In accordance with the inclusion criteria, none of the patients had insulin treatment during the study time. Five patients were on anti-diabetic monotherapy (4 patients on metformin and 1 on SU), 12 patients had two anti-diabetic medications, and 16 patients had three or more anti-diabetic medications. At the start of the study, 32 patients had metformin as part of their treatment, 14 patients had SU, 21 patients had dipeptidyl peptidase 4 (DPP-4) inhibitor, 4 patients had glucagon-like peptide-1 receptor (GLP-1) agonist, 3 patients had pioglitazone, and 6 patients had sodium-glucose co-transporter-2 (SGLT2) inhibitor. During the follow-up, one patient was started on DPP-4 inhibitor instead of pioglitazone, which was stopped, and another patient had SGLT-2 inhibitor added to treatment. No other medication changes were made before the end of the study.
One patient had symptomatic hypoglycaemia during the early-Ramadan period. The patient was on metformin treatment only. No serious hypoglycaemic episodes were reported by the patients during Ramadan fasting, and none of the patients broke his/her Ramadan fasting.
3.2. Glucose variability
MAGE showed significant increase at early-Ramadan fasting days compared to pre-Ramadan days (70.0 [55.5–88.5] vs. 54.0 [42.5–89.5], P = 0.007), while no statistically significant changes were seen in late-Ramadan fasting days or post-Ramadan days compared to pre-Ramadan. Subgroup analyses showed significant increase in MAGE at early-Ramadan fasting days among male patients (P = 0.008), patients on >2 anti-diabetic medications (P = 0.027), and patients treated with SU (P = 0.009). However, no significant changes in MAGE at late-Ramadan or post-Ramadan periods were seen in these subgroups (). Male patients (26.0 [18.0–33.0] vs. 29.0 [21.0–38.0], P = 0.038), and patients on SU treatment (32.0 [20.0–40.5] vs. 34.0 [25.0–46.0], P = 0.034), showed increased SD at early-Ramadan fasting days compared to pre-Ramadan days. No significant changes in SD were seen at late-Ramadan or post-Ramadan periods in these subgroups.
Table 2. Interstitial glucose variability during CGM recording
Time in range (70–180 mg/dl), time in hyperglycaemia (>180 mg/dl), time in hypoglycaemia (<70mg/dl), CV, and mean IG showed no statistical significant changes during the four CGM study periods.
4. Discussion
In this study including non-insulin-treated patients with type 2 diabetes, fasting during Ramadan month resulted in an initial increased glucose variability measured by MAGE, which returned to pre-Ramadan levels in the late-and post-Ramadan period. Male patients and patients treated with SU showed an initial increase in both MAGE and SD. No significant differences were seen in the percentage of euglycemic, hypoglycaemic or hyperglycaemic values during early- and late-Ramadan compared to pre- and post-Ramadan. Glycaemic control measured by HbA1c showed no significant changes after Ramadan compared to pre-fasting period.
To our knowledge, there are no previous studies examining glucose variability using CGM during Ramadan fasting in patients with type 2 diabetes without insulin treatment. In one study including patients with both type 1 and type 2 diabetes as well as healthy subjects, no significant changes were seen in glucose variability measured as MAGE and mean IG, and no difference was seen in rate of hypoglycaemia [Citation5].
Studying glucose variability using CGM in adolescent patients with type 1 diabetes, Kaplan et al. could not show any significant changes in mean IG between Ramadan fasting and non-Ramadan periods [Citation3]. Afandi et al. also used CGM to compare well and poorly controlled adolescent patients with type 1 diabetes during Ramadan fasting. Both glucose variability, measured as mean IG and SD, percentage of hypoglycaemia and hyperglycaemia were higher in the poorly controlled group [Citation1]. However, unlike the present study, they included younger patients with type 1 diabetes and compared two different groups of patients rather than following glucose fluctuations for the same patient during different periods of Ramadan.
In our study, patients on SU and patients on more than two anti-diabetic drugs showed early significant increase in MAGE. SU has previously been associated with higher risk of hypoglycaemia during Ramadan fasting compared to other oral anti-diabetic drugs in patients with diabetes type 2 [Citation9–Citation15]. It is worth mentioning that no CGM was used in these studies to monitor glycaemic variation and hypoglycaemia. However, in contrast to these observational studies, SU (including gliclazide) has not been associated with higher rates of hypoglycaemia compared to DPP-4 inhibitors in other studies [Citation16,Citation17]. This is probably due to individualized patient education and advice about dose adjustments used in these studies.
In our study, no significant increase in the time in hypoglycaemia could be seen during CGM recording days of Ramadan fasting. There is scarce data on the rate of hypoglycaemia in non-insulin-treated patients with type 2 diabetes during Ramadan fasting. However, recent studies have shown that the newer anti-diabetic medications – DPP-4 inhibitors, GLP-1 agonists, and SGLT2 inhibitors – are safe during Ramadan fasting and have lower risk for hypoglycaemia compared to SU [Citation12,Citation14,Citation18,Citation19]. Pre-Ramadan individualized advice and education provided at our centre may have played a role in preventing significant increase in the rate of hypoglycaemia [Citation20,Citation21].
Some limitations should be considered in the present study. The patients who participated in our study had non-insulin-treated well-controlled diabetes and thus are not representative of all patients with type 2 diabetes. This may, at least partially, explain the lack of significant glucose variability changes during Ramadan fasting. Another consideration is the limited number of patients participating in the study. Because the early-Ramadan CGM recordings in our study were done in the first 3 days of Ramadan, the initial increased variability may be due to transition from non-fasting normal daily life routines to different daily routines with shift of mealtimes and altered physical activity in this group of patients with well-controlled diabetes. Another limitation to this study is the low number of CGM recording days that may reduce the possibility of exploring more glucose fluctuation including hypoglycaemias during Ramadan fasting period. More studies are needed in patients with type 2 diabetes, both less well-controlled and insulin-treated, to understand better the effect of Ramadan fasting on glucose fluctuation.
In conclusion, except for an initial increase in glucose variability, fasting during Ramadan for patients with non-insulin-treated type 2 diabetes did not cause any significant changes in metabolic control, glucose fluctuation, or time in hypoglycaemia during CGM recording days compared to the non-fasting pre-Ramadan period. Further pre- and early-Ramadan patient education and follow-up of high-risk patients may help avoid initial significant glucose variability in patients with non-insulin-treated diabetes.
Acknowledgments
The authors express their gratitude to Zainab Mahmoud and Sijomol Skaria for their help in patient recruitment and study conduction, to the nursing staff at Rashid Center for Diabetes and Research for their help in study conduction.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Afandi B, Kaplan W, Al Hassani N, et al. Correlation between pre-ramadan glycemic control and subsequent glucose fluctuation during fasting in adolescents with Type 1 diabetes. J Endocrinol Invest. 2017;40:1–5.
- Kaplan W, Afandi B. Blood glucose fluctuation during Ramadan fasting in adolescents with type 1 diabetes: findings of continuous glucose monitoring. Diabetes Care. 2015;38:e162–e163.
- Kaplan W, Afandi B, Al Hassani N, et al. Comparison of continuous glucose monitoring in adolescents with type 1 diabetes: Ramadan versus non-Ramadan. Diabetes Res Clin Pract. 2017;134:178–182.
- Khalil AB, Beshyah SA, Abu Awad SM, et al. Ramadan fasting in diabetes patients on insulin pump therapy augmented by continuous glucose monitoring: an observational real-life study. Diabetes Technol Ther. 2012;14:813–818.
- Lessan N, Hannoun Z, Hasan H, et al. Glucose excursions and glycaemic control during Ramadan fasting in diabetic patients: insights from continuous glucose monitoring (CGM). Diabetes Metab. 2015;41:28–36.
- Wright LA, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther. 2017;19:S16–S26.
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S55–S64.
- Ibrahim M, Abu Al Magd M, Annabi FA, et al. Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care. 2015;3:e000108.
- Al Sifri S, Basiounny A, Echtay A, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract. 2011;65:1132–1140.
- Anwar A, Azmi KN, Hamidon BB, et al. An open label comparative study of glimepiride versus repaglinide in type 2 diabetes mellitus Muslim subjects during the month of Ramadan. Med J Malaysia. 2006;61:28–35.
- Aravind SR, Ismail SB, Balamurugan R, et al. Hypoglycemia in patients with type 2 diabetes from India and Malaysia treated with sitagliptin or a sulfonylurea during Ramadan: a randomized, pragmatic study. Curr Med Res Opin. 2012;28:1289–1296.
- Azar ST, Echtay A, Wan Bebakar WM, et al. Efficacy and safety of liraglutide compared to sulphonylurea during Ramadan in patients with type 2 diabetes (LIRA-Ramadan): a randomized trial. Diabetes Obes Metab. 2016;18:1025–1033.
- Glimepiride in Ramadan Study G. The efficacy and safety of glimepiride in the management of type 2 diabetes in Muslim patients during Ramadan. Diabetes Care. 2005;28:421–422.
- Hassanein M, Echtay A, Hassoun A, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: results of the Canagliflozin in Ramadan Tolerance Observational Study (CRATOS). Int J Clin Pract. 2017;71:e12991.
- Khattab M, Mahmoud K, Shaltout I. Effect of vildagliptin versus sulfonylurea in muslim patients with type 2 diabetes fasting during Ramadan in Egypt: results from VIRTUE study. Diabetes Ther. 2016;7:551–560.
- Hassanein M, Abdallah K, Schweizer A. A double-blind, randomized trial, including frequent patient-physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manag. 2014;10:319–326.
- Mbanya JC, Al-Sifri S, Abdel-Rahim A, et al. Incidence of hypoglycemia in patients with type 2 diabetes treated with gliclazide versus DPP-4 inhibitors during Ramadan: A meta-analytical approach. Diabetes Res Clin Pract. 2015;109:226–232.
- Bashier A, Khalifa AA, Abdelgadir EI, et al. Safety of sodium-glucose cotransporter 2 inhibitors (SGLT2-I) during the month of Ramadan in muslim patients with type 2 diabetes. Oman Med J. 2018;33:104–110.
- Mudher Mikhael E. Effectiveness and safety of newer antidiabetic medications for Ramadan fasting diabetic patients. J Diabetes Res. 2016;2016:6962574.
- Ahmedani MY, Ahsan S, Haque MSU. Role of Ramadan specific diabetes education (RSDE): A prospective study. Pak J Med Sci. 2017;33:586–593.
- El Toony LF, Hamad DA, Omar OM. Outcome of focused pre-Ramadan education on metabolic and glycaemic parameters in patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2018 Apr 25;12: 761–767.