ABSTRACT
The widespread use of cardiovascular implantable electronic devices has increased concerns regarding using electronic apex locators in patients with these devices. This systematic review investigated the effects and safety of using electronic apex locators in patients with cardiovascular implantable electronic devices.
Methods: An electronic search in the Cochrane Library, PubMed (MEDLINE), ScienceDirect, and Scientific Electronic Library Online (Scielo) databases for relevant articles published between December 2000 and December 2018 was performed. The search strategy centered on terms related to electronic apex locators use during root canal treatment in patients with cardiovascular implantable electronic devices.
Results: Seven studies (five in vitro and two in vivo) fulfilled the inclusion criteria for this review. It was found that electronic apex locators can be used safely in patients with cardiovascular implantable electronic devices, when general precautions are followed.
Conclusions: Although the present review suggests that electronic apex locators can be used safely in patients with implantable cardioverter defibrillators, consultation with patients’ cardiologists remains advisable.
1. Introduction
Cardiovascular implantable electronic devices are small lithium battery-operated electronic devices that are inserted surgically beneath the skin, generally near the left clavicle [Citation1,Citation2]. They have flexible insulated wires (leads) that run through the veins to the heart and monitor heart rate continuously to detect heart rhythm disorders (i.e. arrhythmias) [Citation3,Citation4]. There are two basic types of arrhythmia: heart rates that are too slow [bradycardia]; and those that are too fast [tachycardia] [Citation5]. More than 66,000 implantable cardioverter defibrillators are implanted annually in the USA [Citation6], where a permanent pacemaker was supplied to 2.9 million individuals between 1993 and 2009. In the USA, this number is still increasing each year [Citation7,Citation8]. Similarly, in Europe, the number of implanted implantable cardioverter defibrillators has increased annually [Citation9].
This increased use of cardiovascular implantable electronic devices means that the number of patients with such an implanted device visiting dental offices will also increase. Electromagnetic waves released from electronic devices can disrupt the operation of these devices―an effect known as electromagnetic interference [Citation10]. Accurate determination of working length is clinically very important when performing root canal treatment [Citation11]. The electronic apex locator and periapical radiography are convenient tools to determine root canal working length in routine clinical practice [Citation11,Citation12]. The electronic apex locators are useful adjuncts in determining working length during endodontic therapy, with a reported accuracy of up to 93% [Citation13], which is higher than radiography [Citation14]. The widespread use of implanted cardiovascular implantable electronic devices has increased the concern regarding the use of electronic apex locators in patients with cardiovascular implantable electronic devices [Citation15]. Modern cardiovascular implantable electronic devices are usually well protected. Their covers are hermetically sealed against electromagnetic interference, and they are equipped with filters, rejection circuits, and bipolar modes [Citation16]. Despite these properties, magnetic resonance imaging and ionizing radiation devices should be avoided in medical settings [Citation17]. In dentistry, there is a conventional recommendation to avoid using electronic apex locators in patients with implanted cardiac pacemakers [Citation15,Citation18]. Over the past few decades, there has been debate on whether dental equipment could interfere with the correct functioning of pacemakers and implantable cardioverter defibrillators. Some authors have reported that electronic dental equipment can interfere with correct functioning [Citation18], whereas others have concluded that dental equipment has no significant effect on cardiovascular implantable electronic devices [Citation19].
The present systematic review investigated the effects of electronic apex locators on cardiovascular implantable electronic devices, and the safety of effects of electronic apex locators use in patients with cardiovascular implantable electronic devices.
2. Methods
An electronic search of the Cochrane Library, PubMed (MEDLINE), ScienceDirect, and Scientific Electronic Library Online (Scielo), databases for articles published between December 2000 and December 2018 was performed. The search strategy centered on terms related to using electronic apex locators use during root canal treatment in patients with cardiovascular implantable electronic devices. A combinations of the following terms were used: ‘apex locator,’ ‘cardiovascular,’ ‘implantable,’ ‘electronic devices,’ ‘pacemakers,’ ‘defibrillators,’ ‘cardiac,’ ‘pulp testers,’ ‘equipment safety,’ ‘endodontic,’ and ‘root canal working length.’ In addition, a manual search for relevant articles was performed in the following journals: Journal of Endodontics, International Endodontic Journal, and the Australian Endodontic Journal.
The titles and abstracts of the identified articles were reviewed separately by two researchers to evaluate eligibility. Subsequently, the selected articles were assessed thoroughly for final decision for inclusion in the systematic review. In case of any conflict, a third researcher was consulted for resolution. The inclusion criteria were English-language publication, and in vivo and in vitro investigations related to the topic. Review articles, case series, case reports, and studies based on surveys or expert opinion were excluded. In addition, in vitro studies designed without a model that simulated electrical resistance in the human body were excluded.
3. Results
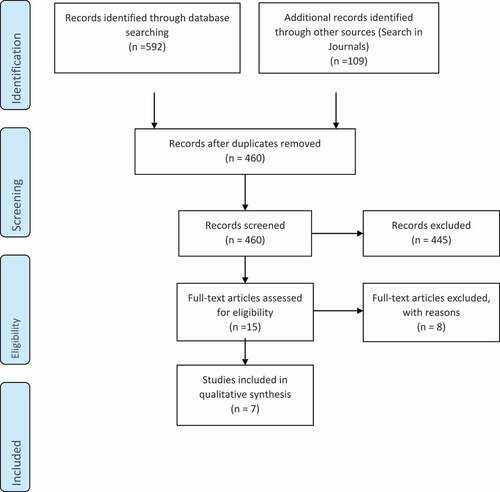
The initial database search identified 701 articles. After removal of duplicates, the search strategy yielded 460 publications. The initial screening of the retrieved studies focused on titles and abstracts. A total of 445 studies were excluded, and the full-text of 15 studies were assessed for eligibility. Among these 15 articles, seven fulfilled the inclusion criteria: five in vitro [Citation1,Citation3,Citation10,Citation15,Citation20]; and two in vivo [Citation21,Citation22] studies. summarizes the details and results of the search strategy. The main characteristics of these studies are summarized in . The quality of each study included in this review was given a grad out of 9. Four in vitro studies [Citation1,Citation3,Citation15,Citation20] reported that electronic apex locators do not interfere with cardiovascular implantable electronic devices and can be used safely. One in vitro study [Citation10], however, reported that electronic apex locators caused electromagnetic interference without altering cardiovascular implantable electronic devices function and suggested that electronic devices should be kept to use at minimum distances. One in vivo study [Citation21] reported that electronic apex locators did not interfere with the functioning of cardiac devices. In contrast, the results of the other in vivo study [Citation22] revealed that electronic apex locators are able to produce background noise interference or pauses in cardiovascular implantable electronic devices, and recommended caution when using electronic apex locators.
Table 1. The main characteristics of the included studies
4. Discussion
Cardiovascular implantable electronic devices are used in the treatment of patients with arrhythmias (i.e. tachycardia, bradycardia, or irregular) and poor cardiac function [Citation5]. They monitor heart rhythm continuously and, when necessary, impulses are delivered to restore normal heart function [Citation23]. In this systematic review, we attempted to assess the effect of using an electronic apex locator during root canal treatment on these devices.
We reviewed seven studies (four in vitro studies and two in vivo studies) that investigated the influence of electronic apex locators on cardiovascular implantable electronic devices function. Owing to clinical heterogeneity in experimental designs, it was not possible to conduct a meta-analysis.
The results of four in vitro studies demonstrated that electronic apex locators did not interfere with cardiovascular implantable electronic devices function and can be used safely [Citation1,Citation3,Citation15,Citation20].
One in vitro study by Dadalti et al. [Citation10] reported that electronic apex locators caused electromagnetic interference without altering cardiovascular implantable electronic devices function. The authors used four electronic apex locators from different manufacturers with two pacemakers and one defibrillator. The tests were performed at distances of 2 cm from the generator, electrode, and sensing arc and, in the case of electromagnetic interference, tests were performed at distances of 5, 10 and 15 cm. However, in real-world dental practice, electronic apex locators are not used at this distance.
There was also controversy in the results of the in vivo studies, in which one investigation [Citation21] revealed that electronic apex locators did not interfere with the functioning of cardiac devices, while the other in vivo study conducted by Moraes et al. [Citation22] demonstrated that electronic apex locators can produce background noise interference or pauses in cardiovascular implantable electronic devices and recommended caution when using electronic apex locators. No endodontic device produced permanent changes in implanted cardiac pacemakers or implantable cardioverter defibrillators. Moraes et al. [Citation22] used two electronic apex locators [Romiapex A-15 and Novapex] from the same manufacturer (Romidan, Kiryat, Israel). This study, however, included only 12 patients (i.e. a small sample size), and the implantable electronic devices were from two different manufacturers: St. Jude Medical (Fullerton, CA, USA) and Medtronic (Doral, FL, USA). Interpretation of the results may have been incorrect due to the possibility of telemetry interference.
Cardiovascular implantable electronic devices vary according to electrode polarity (unipolar or bipolar) [Citation24,Citation25], where the poles (anode and cathode) in the bipolar type were closer together than in the unipolar type [Citation24]. This will lead to reduction in the probability of interpreting extremal signals as a cardiac event(s) [Citation26]. Evidence in the literature suggests that unipolar devices are more prone to electromagnetic interference [Citation27–Citation29]. Interference signals with a frequency between 10 and 300 Hz can bypass the input circuits, as well as those calculated by the algorithm of the implanted cardiac pacemakers. Therefore, these devices may be unable to identify whether the signal source is the heart or an external source [Citation30].
Technological advances in cardiovascular implantable electronic devices have reduced their susceptibility to electromagnetic interference [Citation26,Citation28,Citation31]. Consequently, Crossley and Poole [Citation32] suggested that data interpretation may be impacted more by telemetry interference than interference with pacemaker function.
The new-generation-implanted cardiac pacemakers are considered to be impervious to most sources of interference because of their construction, which encapsulates components in a stainless steel or titanium cover, and are equipped with an interference mode [Citation7,Citation15,Citation33]. In addition, these new devices have bipolar leads and capacitors that effectively filter out electromagnetic interference and, thus, reduce external interference [Citation34,Citation35]. Direct contact between the electronic apex locator and pacemaker is not possible in practice [Citation15,Citation36], where electronic apex locators produce fields around the head with distance ranging from 25.5 to 30.5 cm from the heart [Citation36]. In addition to built-in precautions, electric and magnetic fields decrease inversely with the square of the distance from the source [Citation3,Citation37]. The patients’ body bulk (i.e. skin, fat, muscle, bone, and teeth) may resist the conduction of electromagnetic currents and act as a ‘second capsule’ for implantable electronic devices [Citation34,Citation38–Citation40].
The increased pacing observed during the use of dental devices in some patients is a normal, proper response of implantable electronic devices to a slowing of the patient’s natural underlying heart rate, and not to dysfunction of or interference with cardiac devices [Citation21].
5. Conclusion
The present review suggests that electronic apex locators can be used safely in patients with cardiovascular implantable electronic devices, especially when general precautions are followed to keep electrical appliances at least 10–20 cm away from an implantable cardioverter defibrillator and its leads. However, consultation with the patient’s cardiologist remains advisable.
Acknowledgments
We would like to thank Editage [www.editage.com] for English-language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Idzahi K, De Cock CC, Shemesh H, et al. Interference of electronic apex locators with implantable cardioverter defibrillators. J Endod. 2014;40:1–7.
- Cingolani E, Goldhaber JI, Marbán E. Next-generation pacemakers: from small devices to biological pacemakers. Nat Rev Cardiol. 2017.
- Maheshwari KR, Nikdel K, Guillaume G, et al. Evaluating the effects of different dental devices on implantable cardioverter defibrillators. J Endod. 2015;41:692–695.
- Boyer SL, Silka MJ, Bar-Cohen Y. Current practices in the monitoring of cardiac rhythm devices in pediatrics and congenital heart disease. Pediatr Cardiol. 2015;36:821–826.
- Fu D-G. Cardiac arrhythmias: diagnosis, symptoms, and treatments. Cell Biochem Biophys. 2015;73:291–296.
- Zhan C, Baine WB, Sedrakyan A, et al. Cardiac device implantation in the USA from 1997 through 2004: a population-based analysis. J Gen Intern Med. 2008;23:13–19.
- Lister T, Grant L, Lee S-M, et al. Electromagnetic interference from lasers and intense light sources in the treatment of patients with artificial pacemakers and other implantable cardiac devices. Lasers Med Sci. 2015;30:1619–1622.
- Gauter-Fleckenstein B, Israel CW, Dorenkamp M, et al. DEGRO/DGK guideline for radiotherapy in patients with cardiac implantable electronic devices. Strahlenther Onkol. 2015;191:393–404.
- Ector H, Vardas P. Current use of pacemakers, implantable cardioverter defibrillators, and resynchronization devices: data from the registry of the European Heart Rhythm Association. Eur Heart J Suppl. 2007;9(suppl_I):I44–I9.
- Dadalti M, Da Cunha AJLA, de Araújo MCP, et al. Electromagnetic interference of endodontic equipments with cardiovascular implantable electronic device. J Dent. 2016;46:68–72.
- Yılmaz F, Kamburoğlu K, Şenel B. Endodontic working length measurement using cone-beam computed tomographic images obtained at different voxel sizes and field of views, periapical radiography, and apex locator: a comparative ex vivo study. J Endod. 2017;43:152–156.
- AlRahabi MK. Evaluation of complications of root canal treatment performed by undergraduate dental students. Libyan J Med. 2017;12:1345582.
- de Vasconcelos BC, Chaves RDV, Vivacqua-Gomes N, et al. Ex vivo evaluation of the accuracy of electronic foramen locators in root canals with an obstructed apical foramen. J Endod. 2015;41:1551–1554.
- Khandewal D, Ballal NV, Saraswathi MV. Comparative evaluation of accuracy of 2 electronic apex locators with conventional radiography: an ex vivo study. J Endod. 2015;41:201–204.
- Gomez G, Duran‐Sindreu F, Jara Clemente F, et al. The effects of six electronic apex locators on pacemaker function: an in vitro study. Int Endod J. 2013;46:399–405.
- Sabzevari K, Oldman J, Herrey AS, et al. Provision of magnetic resonance imaging for patients with ‘MR-conditional’cardiac implantable electronic devices: an unmet clinical need. EP Europace. 2017;19:425–431.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the medical environment (part II). Clin Cardiol. 2012;35:321–328.
- Roedig JJ, Shah J, Elayi CS, et al. Interference of cardiac pacemaker and implantable cardioverter-defibrillator activity during electronic dental device use. J Am Dent Assoc. 2010;141:521–526.
- Brand H, Entjes M, Amerongen AN, et al. Interference of electrical dental equipment with implantable cardioverter-defibrillators. Br Dent J. 2007;203:577–579.
- Lahor‐Soler E, Miranda‐Rius J, Brunet‐Llobet L, et al. Capacity of dental equipment to interfere with cardiac implantable electrical devices. Eur J Oral Sci. 2015;123(3):194–201.
- Wilson BL, Broberg C, Baumgartner JC, et al. Safety of electronic apex locators and pulp testers in patients with implanted cardiac pacemakers or cardioverter/defibrillators. J Endod. 2006;32:847–852.
- Moraes A, Silva E, Lamas C, et al. Influence of electronic apex locators and a gutta‐percha heating device on implanted cardiac devices: an in vivo study. Int Endod J. 2016;49:526–532.
- Samad S, Khan SA, Haq A, et al. Classification of arrhythmia. Int J Electr Energy. 2014;2:57–61.
- Napp A, Stunder D, Maytin M, et al. Are patients with cardiac implants protected against electromagnetic interference in daily life and occupational environment? Eur Heart J. 2015;36:1798–1804.
- Schulman PM, Rozner MA, Sera V, et al. Patients with pacemaker or implantable cardioverter-defibrillator. Med Clinics. 2013;97:1051–1075.
- Stone KR, McPherson CA. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32:S155–S165.
- Ubee SS, Kasi VS, Bello D, et al. Implications of pacemakers and implantable cardioverter defibrillators in urological practice. J Urol. 2011;186:1198–1205.
- Yerra L, Reddy PC. Effects of electromagnetic interference on implanted cardiac devices and their management. Cardiol Rev. 2007;15:304–309.
- Sticherling C, Menafoglio A, Burri H, et al. Recommendations for the perioperative management of patients with cardiac implantable electronic devices. Cardiovasc Med. 2016;19:13–18.
- Della Chiara G, Primiani VM, Moglie F. Experimental and numeric investigation about electromagnetic interference between implantable cardiac pacemaker and magnetic fields at power line frequency. Ann Ist Super Sanita. 2007;43:e53.
- Allen M. Pacemakers and implantable cardioverter defibrillators. Anaesthesia. 2006;61:883–890.
- Crossley GH, Poole JE. More about pacemakers. J Am Dent Assoc. 2010;141:1053.
- Beinart R, Nazarian S. Effects of external electrical and magnetic fields on pacemakers and defibrillators: from engineering principles to clinical practice. Circulation. 2013;128:2799–2809.
- Glikson M, Hayes DL. Cardiac Pacing. Med Clinics. 2001;85:369–421.
- Yonemura T, Koyama J, Sakai Y, et al. Electromagnetic Interference with cardiac implantable devices by household and industrial appliances. J Arrhythm. 2011;27:49–56.
- Garofalo RR, Ede EN, Dorn SO, et al. Effect of electronic apex locators on cardiac pacemaker function. J Endod. 2002;28:831–833.
- Lakshmanadoss U, Chinnachamy P, Daubert JP. Electromagnetic interference of the pacemakers. In: Modern pacemakers-present and future. InTech. 2011.
- Kushner RF, De Vries P, Gudivaka R. Use of bioelectrical impedance analysis measurements in the clinical management of patients undergoing dialysis. Am J Clin Nutr. 1996;64:503S–509S.
- Shah PM, Ellenbogen KA. Life after pacemaker implantation: management of common problems and environmental interactions. Cardiol Rev. 2001;9:193–201.
- Tondato F, Bazzell J, Schwartz L, et al. Safety and interaction of patients with implantable cardiac defibrillators driving a hybrid vehicle. Int J Cardiol. 2017;227:318–324.