ABSTRACT
Hepatitis C virus is one of the leading causes of liver cirrhosis and hepatocellular carcinoma. The tumor-associated calcium signal transducer 2 (Tacstd-2) molecule is thought to be involved in the expression of a number of molecules that facilitate transport of hepatitis C into the cell. The aim of this study was to investigate Tacstd-2 concentrations in hepatitis C patients, with and without cirrhosis, and compare with uninfected controls. Sixty-one hepatitis C patients and twenty-nine control (hepatitis C antibody negative patients with dyspeptic complaints) cases were recruited between 2014 and 2016. Tacstd-2 concentrations in all hepatitis C and control patients were measured and compared. In addition, cirrhotic and non-cirrhotic hepatitis C patients were compared in terms of Tacstd-2 concentration, and comparison was made between patients with high and low concentrations of Tacstd-2. The mean Tacstd-2 concentration of patients with Hepatitis C was 691.2 ± 473.3 ng/U was significantly higher (p = 0.043) than in the healthy control group (524 ± 290.1 ng/U). Although the Tacstd-2 value was higher in cirrhotic than the non-cirrhotic patient group, the difference was not statistically significant (p = 0.78). Liver transferase concentrations were higher in hepatitis C patients with a Tacstd-2 concentration <500 ng/U compared to those with a Tacstd-2 concentration >500 ng/U. In patients with hepatitis C, Tacstd-2 level was detected at higher serum concentrations than healthy individuals. The introduction of hepatitis C virus into the cell can be relatively easy in people with a higher serum concentration of Tacstd-2.
KEYWORDS:
1. Introduction
Hepatitis C (Hep C) virus is one of the leading causes of chronic liver disease and affects more than 170 million people worldwide [Citation1]. Hep C virus is a positive-stranded, RNA virus of the Flaviviridae group [Citation2]. Although the detailed mechanism is uncertain, Hep C virus entry into hepatocytes includes a multi-stage process involving various host entry factors such as glycosaminoglycans (GAGs), tight junction binding (TJ) proteins, claudin-1 (CLDN1), and occludin (OCLN) [Citation3–Citation5].
Tumor-associated calcium signal transducer 2 (Tacstd 2), also known as trophoblast antigen 2 (Trop2), is a type-1 transmembrane glycoprotein [Citation6–Citation9] and an adhesion molecule that is expressed in a multistradiated epithelium, several stem/progenitor cell types, and carcinoma cells. Current evidence suggsts that Tacstd-2 plays a functional role in stem cells and also in cancer progression [Citation10,Citation11]. Modified expression and/or activity of Tacstd-2 has been implicated in growth, proliferation, migration, invasion, and survival of cancer cells [Citation12–Citation17].
Tacstd-2 binds directly to claudin 1 and 7 and is required for these proteins to act as carriers to the cell membrane or to localize to the plasma membrane without degradation by the ubiquitin proteasome system [Citation18]. Tacstd-2 has an effect on the expression of Occludin and Claudin, which play a role in the entry of Hep C virus into the cell. Thus, increased Tacstd-2 molecule expression may play a role in the virulence of hepatitis C infection. We hypothesized that Tacstd-2 expresson may play a role in Hep C and Hep C-related cirrhosis patients. The aim of this study was to investigate Tacstd-2 concentrations in Hep C patients, with and without cirrhosis, and compare with uninfected controls.
2. Methods
Sixty-one Hep C patients and 29 control (people who presented to the gastroenterology clinic with dyspeptic complaints and who did not have any additional liver disease were accepted as the control group. Individuals with HBs Antigen positivity were excluded from the study) cases, who were admitted to our gastroenterology clinic between 2014 and 2016, were included in the study. Criteria for inclusion in the patient group were those patients with positive Hep C RNA values who gave their informed consent to participate. Exclusion Criteria included any Hep C positive patient who had advanced heart failure or renal failure or any patient declining consent.
Hep C patients were further divided into groups based on those who had cirrhosis (patients with splenomegaly, platelet levels below 150,000 and parenchymal liver disease) and those who did not. Patients were also divided into two groups according to Tacstd-2 levels, and differences between them were examined according to statistical methods (those who had Tacstd-2 concentrations under 500 ng/U (Group A) and those with Tacstd-2 concentration >500 ng/U (group B)). The rounded harmonic mean (harmonic mean = 500 ng/U) calculated up for Tacstd-2 was used as a discriminator value between two groups. Comparisons in terms of laboratory results were made between these groups and between the whole cohort of Hep C positive patients and the control group. The study was approved by the Ethics Committee of Mersin University (number.2016/29, date:28/01/2016).
Blood samples were collected from all study participants at the first clinic visit (at 09.00 pm during fasting period) and separated immediately following collection. Serum samples collected for the study were stored at −80°C. In this study, the levels of the Tumor-associated Calcium Signal Transducer-2 protein (Manufacturer company: Cusabio Technology LLC, Houston/USD) (Tacstd-2) were measured in the DSX System [Dynex (14340 Sullyfield CircleChantilly, USA)]. Monoclonal antibodies against different epitopes of Tacstd-2 were used in this measurement. The standards and samples used were allowed to react with the capture monoclonal antibody (MAb1) and the peroxidase (HRP) labeled monoclonal antibody (MAb2) in the wells in which they were placed. After an incubation period to allow the formation of the sandwich (Mab1- (tacdstd-2) -MbA2-HRP), the wells were washed to remove unbound enzyme-labeled antibodies.
The bound enzyme-labeled antibody was measured by a chromogenic reaction to add chromogenic solution (TMB) and incubated. The reaction was stopped by addition of stop solution, and the wells were determined by colorimetric measurement of absorbance. The amount of substrate turnover determined by colorimetric measurement of absorbance was calculated in proportion to the concentration of Tacstd-2. In our study, a calibration curve was drawn and the Tacstd-2 concentrations in the samples were determined by interpolation from the calibration curve.
2.1. Statistical analysis
All analyses were performed using SPSS version 16.0 for Windows (IBM Inc, Chicago, Ilınois, USA). While the parameters of kurtosis and skewness values between +2 and −2 are considered as homogeneous, the parameters outside these limits are considered as non-homogeneous. Differential analysis between study groups and controls was performed by paired sample T-test on parametric variables and Mann Whitney-U for non-parametric variables. Spearmann correlation analysis was performed between Tacstd-2 and hematologic and biochemical variables. Factors affecting Tacstd-2 levels in Hep C patients were evaluated by a linear regression analysis method. P-value of<0.05 was considered statistically significant.
3. Results
Subjects consisted of 61 (67.8%) Hep C positive patients and controls (Hepatitis C antibody negative patients with dyspeptic complaints) were 29 (32.2%) healthy patients. Forty one (67.2%) of 61 Hep C patients were male and 20 patients (32.8%) were female. Forty-one (67.2%) of the Hep C patients were non-cirrhotic and 20 subjects (32.8%) were from the cirrhotic group. In the Hep C group, 44 patients (72.1%) patients had the Hep C 1b genotype 1b. Genotype and mean HCV RNA levels of Hep C patients in the study are shown in . Three of the cirrhotic patients had hepatocellular carcinoma. The mean Tacstd-2 concentration of patients with Hep C was significantly higher (p= 0.043) at 691.2 ± 473.3 ng/U than in the healthy control group (524 ± 290.1 ng/U) (see ). When the Hep C patient group was stratified by the presence of cirrhosis, cirrhotic patients had a mean Tacstd-2 concentration of 715.5 ± 333.9 ng/U while non-cirrhotic patients had a mean concentration of 679.3 ± 447.4 ng/U although this was not statistically significant (p= 0.78) ().
Table 1. Comparison of viral load and Tacstd-2 concentration in Hepatitis C positive patients by viral genotype
Table 2. Comparison of laboratory parameters of Hepatitis C patients with controls
Table 3. Comparison of Hepatitis C patients with and without cirrhosis
Patients were stratified according to low (<500 ng/U; Group A) or high (>500 ng/U; Group B) Tacstd-2 concentrations. Liver function tests showed that aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations were significantly higher in group A patients compared with group B patients. There was no difference between the groups in terms of alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT) or bilirubin concentrations. Conversely, the hemoglobin value was found to be statistically lower in group B than in group A ().
Table 4. The correlations between tumor-associated calcium-binding transducer-2 and liver function tests, hematologic parameters, and age
In our study, a correlation between Tacstd-2 and AST (r: −0.31, p: 0.02) and ALT (r: −0.29, p: 0.03) concentrations was found in Hep C patients ( and ). There was no correlation between Tacstd-2 and hematological parameters or Hep C RNA concentration (). In the linear regression model, only hemoglobin concentration was found to be dependent on Tacstd-2 concentration in Hep C patients (p= 0.007) as shown in .
Figure 1. Correlation relationship between Tacstd-2 and Alanine Aminotransferase in Hepatitis C group. Alt: Alanine aminotransferase, Tacstd-2: tumor-associated calcium signal transducer 2.
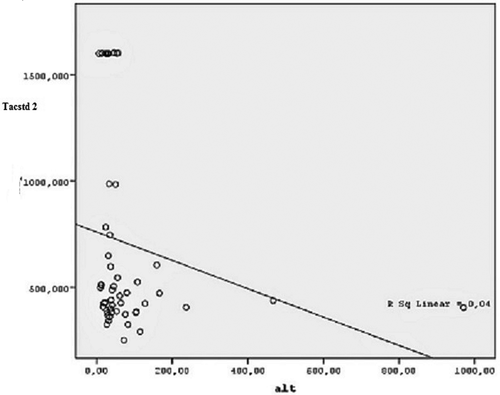
Figure 2. Correlation relationship between Tacstd-2 and Aspartate Aminotransferase in Hepatitis C group. Ast: Aspartate aminotransferase, Tacstd-2: tumor-associated calcium signal transducer 2.
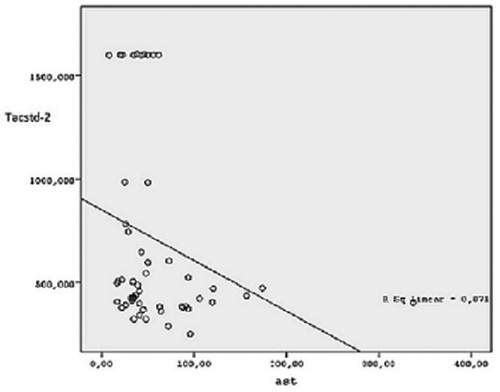
Table 5. Comparison of Hepatitis C patients with Group A (Tumor-associated calcium signal transducer-2 < 500 ng/U) and Group B (Tumor-associated calcium signal transducer-2 > 500 ng/U) according to laboratory parameters
Table 6. Hematological and biochemical parameters affecting Tacstd-2 according to linear regression analysis (Backward method)
4. Discussion
This study analyzed and compared Tacstd-2 molecule concentrations in Hep C positive patients with a healthy control group. It was shown that the Hep C patient group as a whole exhibited higher concentrations of Tacstd-2 than Hep C negative controls. However, no difference in Tacstd-2 concentration was found between cirrhotic and non-cirrhotic Hep C patients. It was also shown that aminotransferase concentrations were negatively correlated with Tacstd-2 concentrations, with higher enzyme concentrations being found in patients with lower Tacstd-2 concentrations. Regression analysis showed that Tacstd-2 concentrations may affect patients hemoglobin levels.
Sekhar et al. [Citation18] investigated the relationship between the tacstd-2 molecule and hepatocellular carcinoma due to Hep C. They reported that both Hep C virus cellular infection and viral replication rates were decreased in cells with increased tacstd-2 expression in patients with hepatocellular carcinoma. In our study, there were only three patients with hepatocellular carcinoma thus we did not attempt separate statistical analysis for these patients.
Shimizu et al. [Citation19] analyzed the relationship between the occludin molecule, a tight junction protein and the expression of which is dependent on Tacstd-2, and Hep C virus cell entry. Using a mouse model, it was shown that Hep C viral entry was prevented with anti-occludin antibodies. It was also demonstrated that anti-occludin antibodies may be a treatment for Hep C patients. This suggests that there may be a role for anti-Tacstd-2 antibodies in the treatment of Hep C patients too.
Fofana et al. [Citation20] reported a study in which they investigated the effect of antibodies against claudin-1, another tight junction protein, also regulated by Tacstd-2. In this study, it was shown that Hep C virus can be reduced by anti-claudin antibody treatment. This study also supports the role of antibodies directed against molecules known to facilitate Hep C viral cell entry into hepatocytes in the treatment of Hep C.
Tacstd-2 is a highly expressed molecule in many cancer types, such as colorectal, esophagus, pancreas, lung, and ovarian cancer [Citation21,Citation22]. In addition to tumor growth, Tacstd-2 is also thought to be involved in metastasis formation [Citation21]. High levels of tacstd-2 may be an indicator of the oncogenic potential of the virus in Hep C patients and measurement of tacstd-2 may have some prognostic value. However, we found that Tacstd-2 concentrations were not different between cirrhotic and non-cirrhotic patients which may indicate that Tacstd-2 does not play a role in the development of cirrhosis. Longer term studies in newly diagnosed cases of Hep C infection are needed to clarify this hypothesis. However, the difference between control group and Hep C patients may reveal the relationship between Tacstd-2 and Hep C infection. The fact that it is easy for hepatitis C virus to enter the cell with the height of Tacstd-2 level can explain our study results.
Correlation analysis demonstrated a negative correlation between Tacstd-2 and aminotransferase levels in people with Hep C infection. In patients with lower Tacstd-2 concentrations (<500 ng/U) aminotransferase concentrations were higher. Tacstd-2 concentrations were lower in Hep C patients with high AST and ALT values. Cell damage caused by the introduction of hepatitis virus into the cell may be related to increased levels of transaminases and a decrease in the Tacstd-2 concentration. There was also a negative association between Tacstd-2 concentration and hemoglobin concentration. In this study, no relation was found between Hep C RNA concentration, indicative of viral burden, and tacstd-2 concentrations.
In the literature, aminotransferase values are found to be lower in cirrhotic patients than non-cirrhotic patients [Citation23]. Although it was not statistically significant in our study, aminotransferase values were higher in the non-cirrhotic group compared to cirrhotic patients. Tacstd-2 values were measured at higher concentrations in cirrhotic patients, but these results were not significant. Although Tacstd-2 and aminotransferase levels were inversely correlated, this condition could not be observed between the evaluation of cirrhotic and non-cirrhotic patient groups. Since liver cirrhosis is a precursor condition to hepatocellular carcinoma [Citation24], it can be expected that Tacstd-2 values which are elevated in most malignant diseases may be higher in patients with cirrhosis. However, since our results are statistically insignificant, it is necessary to investigate the relationship between aminotransferase and Tacstd-2 levels in cirrhotic and non-cirrhotic groups with further studies. Also Tacstd-2 levels should be evaluated in Hepatitis C positive hepatocellular carcinoma patients.
One of the limitations of our study was the small size of the group of patients with hepatocellular carcinoma patients making statistical analysis unreliable. In addition, additional investigation of occludin and claudin-1 concentration may have provided additional information on the relationship between Tacstd-2 and these molecules.
In conclusion, our study is the first to evaluate Tacstd-2 levels in patients with Hep C and to compare this with a control group. Higher concentrations of Tacstd-2 in patients infected with Hep C virus suggest that the Tacstd-2 molecule may play a role in Hep C virulence with higher concentrations indicating easier cell entry for the virus. There does not appear to be a relationship between Tacstd-2 concentration and the likelihood of developing cirrhosis although there is a direct relationship between aminotransferase concentrations and Ttacstd-2 concentration. There is a need for further, larger, long-term studies of the relationship between Tacstd-2 and Hepatitis C infection which should include more patients with hepatocellular carcinoma in order to elucidate the role of Tacstd-2 in Hepatitis C infection and disease progression.
Acknowledgments
The authors thank Onur Bobusoglu for laboratory tests, Jeremy Jones for proofreading and editing the manuscript and also thanks to all personel working in the Department of Gastroenterology for their assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the USA, 1999 through 2002. Ann InternMed. 2006;144:1–6.
- Lohmann V, Körner F, Koch J-O, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatomacellline. Science. 1999;2851:10–13.
- Barth H, Schafer C, Adah MI, et al. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem. 2003;278:41003–41012.
- Evans MJ, vonHahn T, Tscherne DM, et al. Claudin-1 is a hepatitis C virusco-receptor required for a late step in entry. Nature. 2007;2007(446):801–805.
- Ploss A, Evans MJ, Gaysinskaya VA, et al. Humanoccludin is a hepatitis C virus entry factor required for infection of mousecells. Nature. 2009;12:882–886.
- Fong D, Moser P, Krammel C, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295.
- Mühlmann G, Spizzo G, Gostner J, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–158.
- Fong D, Spizzo G, Gostner JM, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191.
- Ohmachi T, Tanaka F, Mimori K, et al. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063.
- Goldstein SA, Lawson DA, Cheng D, et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci U S A. 2008;105:20882–20887.
- Okabe M, Tsukahara Y, Tanaka M, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960.
- Cubas R, Zhang S, Li M, et al. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPKpathway. Mol Cancer. 2010;9:253.
- Pak MG, Shin DH, Lee CH, et al. Significance of EpCAM and TROP2 expression in non-small cell lung cancer. World J Surg Oncol. 2012;10:53.
- Li Z, Jiang X, Zhang W. TROP2 overexpression promotes proliferation and invasion of lung adenocarcinoma cells. Biochem Biophys Res Commun. 2016;470:197–204.
- Chen R, Lu M, Wang J, et al. Increased expression of Trop2 correlates with poor survival in extranodal NK/T cell lymphoma, nasal type. Virchows Arch. 2013;463:713–719.
- Trerotola M, Jernigan DL, Liu Q, et al. Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res. 2013;73:3155–3167.
- Ning S, Liang N, Liu B, et al. TROP2 expression and its correlation with tumor proliferation and angiogenesis in human gliomas. Neurol Sci. 2013;34:1745–1750.
- Sekhar V, Pollicino T, Diaz G, et al. Infection with hepatitis C virus depends on TACSTD2, a regulator of claudin-1 and occludin highly down regulated in hepatocellular carcinoma. PLoSPathog. 2018;14:e1006916.
- Shimizu Y, Shirasago Y, Kondoh M, et al. Monoclonal antibodies against occludin completely prevented hepatitis C virus infection in a mouse model. J Virol. 2018; Apr:92(8)7;pii: JVI.02258–17.
- Fofana I, Krieger SE, Grunert F, et al. Monoclonal anti-claudin 1 antibodies prevent hepatitis C virus infection of primary human hepatocytes. Gastroenterology. 2010;139:953–964.
- Nakashima K, Shimada H, Ochiai T, et al. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer. 2004;20:1029–1035.
- Wang J, Day R, Dong Y, et al. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7:280–285.
- Hyder MA, Hasan M, Mohieldein AH. Comparative Levels of ALT, AST, ALP and GGT in Liver associated diseases. Euro J Exp Bio. 2013;3:280–284.
- Okuda H. Hepatocellular carcinoma development in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):161–173.
