ABSTRACT
This study was conducted to evaluate the characteristics, treatment outcome and risk factors associated with 223 drug-resistant tuberculosis (DR-TB) cases in the State of Qatar. A descriptive records-based retrospective study was conducted on patients registered at Communicable Disease Centre (CDC), Qatar to all consecutive microbiologically confirmed tuberculosis cases for the period January 2010 – March 2015. Demographic, clinical data, drug-resistance pattern of isolated mycobacteria and treatment outcome was assessed for the patient who completed their treatment in Qatar. Of 3301 patients with positive M. tuberculosis culture were analyzed; 223 (6.7%) were resistant to at least one drug. The overall prevalence of multi-d rug resistant TB (MDR-TB) was 1.2% (n = 38) of patients. A former resident of Indian sub contents was the most common demographic characteristic observed (64.1%). The outcome of treatment was assessed for 85 resistant cases with follow-up after completion of treatment. Cure and relapse rates were 97.6%, and 2.4%, respectively. Drug-resistant TB in Qatar is influenced by migration where the patients were probably infected. Rapid sputum sampling performed in the early stages of the disease, patient isolation, and drug-susceptibility testing should be the standard of care.
1. Introduction
Tuberculosis remains a major health problem through the word, it is a major cause for morbidity and mortality. According to WHO in 2017, TB killed 1.6 million people (0.3 million HIV-positive) [Citation1] and caused 240 000 deaths of MDR-TB in 2016 [Citation1]. The emergence of drug-resistant strains of TB (DR-TB) is a global threat to tuberculosis prevention and control efforts (WHO, 2004). The most important problem in drug resistance is MDR-TB and extensively drug resistance (XDR). MDR\XDR-TB is higher in countries with sub-standard national tuberculosis programs or lower socio-economic groups. It is a consequence of poor/late diagnosis, inadequate treatment, poor patient compliance and poor drug supplies [Citation2]. Moreover, several other factors such as homelessness, poverty, lack of infrastructure in public health, and inadequate access to health services have played an important role in worsening the situation [Citation3].
Drug resistance of Mycobacterium tuberculosis showed marked geographic variation from one country to the other. Among the Gulf Cooperation Council (GCC) member countries the overall MDR-TB prevalence rate was recorded as 4.0% with a maximum resistance was present in UAE [Citation4].
In Qatar, all expatriates applying for residency or a visiting visa for more than a month must undergo a full history, physical examination and chest radiography in the Department of Medical Commission upon arrival since 1985. The drug-resistant TB pattern in the period between January 1996 to December 1998 was found to be 15%, of which isoniazid-resistant was 12.4% followed by Streptomycin (5.2%), rifampicin (2%) and ethambutol (0.8%). Less than 1% of TB isolates was found to be MDR [Citation5]. Since that time, the number of populations increases in Qatar, with more people from other countries mainly south Asia, which change the trend of TB cases and the emergence of more cases of drug-resistant TB.
We aim in this study to assess these changes in TB profile in 6 years from 2010 to 2015, with an assessment of the treatment outcome.
The results of this study will provide important additional information on the epidemiology of DR-TB and preventive strategies against it in Qatar.
2. Materials and methods
A descriptive records-based retrospective study was conducted on patients registered at Communicable Disease Centre (CDC), Qatar, the only national referral TB Centre in the country. All consecutive microbiologically confirmed tuberculosis cases were eligible for the study. The study included a review of the records for the period of January 2010–March 2015. Demographic and clinical data extracted included: patient’s age, sex and country of origin; disease site (pulmonary or extra-pulmonary); HIV/AIDS status, sputum smear mycobacteriology studies status, previous chemoprophylaxis and/or previous treatment for TB and the resistance pattern of isolated mycobacteria.
The objective of this study was to: (1) determine the prevalence of DR-TB in Qatar from January 2010 to March 2015; (2) pattern of DR-TB in the country; (3) characterize the population of patients with DR-TB (i.e. Demographic and clinical data) and: (4) outcome of the treatment.
3. Patients (study population)
All consecutive microbiologically confirmed pulmonary and extra-pulmonary tuberculosis cases were eligible for the study; those which lacked confirmed MTB culture were excluded. All patients included are registered in the National TB registry located at the CDC in Qatar and TB national laboratory.
Definitions: Drug resistance in mycobacteria is defined as a decrease in sensitivity to a sufficient degree to be reasonably certain that the strain concerned is different from a sample of wild strains of human type that have never come into contact with the drugs [Citation2]. Mono-resistance is defined as a resistance to one first-line anti-TB drug only.
Poly-resistance is the resistance of M. Tuberculosis strain to more than one first-line anti-TB drug, other than both isoniazid and rifampicin. MDR-TB is a special subgroup of poly-resistance, in which there is resistance to at least rifampicin and isoniazid. Furthermore, extensively drug-resistant TB (XDR-TB) cases, defined as MDR-TB plus resistance to a Fluoroquinolone and at least one of the three injectable second-line drugs (Amikacin, Kanamycin or Capreomycin) [Citation3].
New patients defined as never been treated for TB or have taken anti-TB drugs for less than 1 month. Treatment outcome was assessed for patients who complete their course of therapy in Qatar with 1 year follow up in case of mono-resistant or 2-year follow up in case of MDR/XDR-TB infection. For mono-resistant cases, cured was defined as pulmonary TB patient with bacteriologically confirmed TB at the beginning of treatment who was smear- or culture-negative in the last month of treatment and on at least one previous occasion [Citation4]. Furthermore, treatment failure was defined as sputum smear-positive at 4 months or later during treatment [Citation4]. A patient whose most recent treatment outcome was ‘cured’ or ‘treatment completed’, and who is subsequently diagnosed with bacteriologically positive TB by sputum smear microscopy or culture was defined as a relapse [Citation6].
Cured for MDR/XDR-TB patient was defined as a completed treatment per programme protocol and has at least five consecutive negative cultures from samples collected at least 30 days apart in the final 12 months of treatment [Citation6]. While failure for MDR/XDR Treatment will be considered if two or more of the five cultures recorded in the final 12 months of therapy are positive, or if anyone of the final three cultures is positive.
4. Microbiology
Sputum and other samples from patients registered in CDC were sent to Qatar National TB Reference laboratory. All samples were examined using smear microscopy and the Ziehl-Neelsen acid-fast stain method then cultured on Mycobacterium Growth Indicator Tube MGIT liquid media (BACTEC™ MGIT™ 960) which can give a positive result in 7–14 days and final negative culture after 6 weeks.
All positive culture will be incubated in Löwenstein-Jensen (LJ media) for the preservation of the sample if need it for second line drug sensitivity and to differentiate NTM (non-tuberculous mycobacteria) from MTB.
Culture-positive specimens were tested for susceptibility to isoniazid (INH), rifampicin (RMP), streptomycin (SM), and ethambutol (EMB). Susceptibility testing was performed using BACTEC™ 460TB radiometric susceptibility method, generally takes from 4 to 12 days. It is based on the production of radioactive 14 C-labeled carbon dioxide by the growing mycobacteria, manifested by a Growth Index increase in the system [Citation3].
A specimen that is found to have MDR strains is sent to Mayo Clinic reference laboratory for confirmation and for the second-line drug-susceptibility testing [Citation4].
5. Disease response
Clinical response was defined as an improvement of signs and symptoms tuberculosis associated with tuberculosis as judged by the physician. Radiological response was defined as reduction or disappearance of the previously noted radiological with previously picture consistent with tuberculosis. Responders for anti-TB treatment defined as patients in whom consecutive AFB smear or mycobacterial culture had become consistently negative. Non-responders were defined as anti-TB medications who continue to be smear or culture positive.
6. Data collection
A pre-validated data collection form was used to collect patients’ data from the National TB registry database obtained from the Laboratory and subsequently from the HMC electronic medical record system (Cerner). Data extracted included: socio-demographic characteristics (e.g., age, gender, country of origin, type of residential accommodation), clinical characteristics (e.g., past medical history, pulmonary lesions on chest x-ray, results of the sputum smear, culture and drug-susceptibility testing), TB treatment regimens (current and past), and treatment outcomes.
7. Ethical statement
The study protocol was approved by The Institutional Review Board of Hamad Medical Corporation (15096/15).
8. Statistical analysis
Descriptive statistics were used to summarize demographic, clinical, laboratory and other characteristics of the participants. Associations between two or more qualitative variables were assessed using chi-square (χ2) test, Fisher Exact test or Yates corrected Chi-square as appropriate. Quantitative data means between the two independent groups were analyzed using unpaired student’s t-test or Mann Whitney U test as appropriate. All P values presented were two-tailed, and P values <0.05 was considered as statistically significant. All Statistical analyses were done using statistical packages SPSS 22.0 (SPSS Inc. Chicago, IL) and Epi-info (Centers for Disease Control and Prevention, Atlanta, GA) software.
9. Results
Of the 3301 cases of culture-positive TB identified between 2010 and 2015, 3078 (93.3%) were fully sensitive and 223 (6.7%) were resistant to one or more anti-tuberculous drugs. During the six-year period, the rate of isoniazid resistance, streptomycin resistance, rifampicin resistance, ethambutol resistance, MDR and XDR were 3.1% (n = 102), 1.2% (n = 41), 0.2% (n = 6), 0.15% (n = 5), 1.2% (n = 38) and XDR in 0.1% (n = 2) of patients. Levels of resistance to isoniazid and rifampicin and frequencies of any resistance are shown in . The majority of drug-resistant cases were from the Indian subcontinent [defined as India, Pakistan, Bangladesh and Nepal], which represents 64%, followed by 19.7% from South East Asia [defined as Philippines, Sri Lanka, Thailand and Indonesia].
Table 1. Pattern of drug-resistance tuberculosis in the State of Qatar from 2010 to 2015
Qatari Nationals represent only 1.8% and 4% from other Arab nationals as shown in . Male patients accounted for 79.8% (N = 78) and females for 20.2% (N = 45). The median age was 29 with a range of 6 months to 70 years. The percentage of drug-resistant TB was higher in patients who were new immigrants and referred from the Department of Medical Commission (61.4%) than indigenous cases (38.6%). The majority of resistant cases where pulmonary TB (80%) followed by pleural TB (9%) as shown in .
Table 2. Demographic data for drug-resistant tuberculosis in the State of Qatar
History of anti-TB medication was not a risk factor with drug resistance in our cohort[only 2 patients had a history of previous TB treatment] which may be due to denial of the previous history. Of the 223 patients infected with resistant TB, an HIV screening test was available for 112 (50.2%) patients and none of them were positive.
There was no significant correlation between comorbid diseases and drug-resistance. However, there is significant correlation between drug-resistance and chest radiographic finding, with 23.3% of patients had cavity with p value 0.019 ().
Table 3. Crosstable between risk factors and type of resistant TB
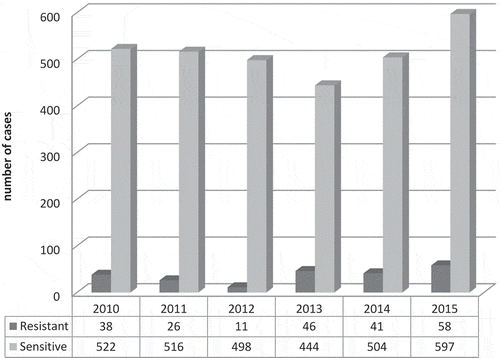
The trend of resistance was assessed during those years [2010–2015] showed an increasing number of resistant cases along with the increase in the total number of TB in the year of 2015 (Graph 1). Moreover, the outcome of treatment was assessed for 85 resistant cases with follow-up after completion of treatment, which showed a cure rate of 97.6% and relapse of 2.4%. However, 137 cases (61.4% from total) they left the country before completion of therapy. There were 2 XDR TB cases (2011 and 2013) both lost follow up as they travelled back to their home countries.
Sputum culture conversion rate was assessed in 101 resistant cases at 2-month therapy show culture conversion of 94%, whereas 122 cases (54.7%) lost to follow-up.
10. Discussion
The WHO reported that the global incidence of TB peaked around 2003 and appeared to stabilize or began to decline. However, the number of new cases increased between 2005 and 2006 includes drug-resistant tuberculosis [Citation3].
This study describes a 6-year national surveillance of drug-resistant TB in Qatar, a country that receives expatriates from high TB prevalence countries. Continuous surveillance is indeed the best approach to monitor time trends and is recommended by the WHO [Citation7].
The Global Project on Anti-tuberculous Drug Resistance Surveillance (GPADRS) investigated the prevalence of TB in 83 countries from 2002 to 2007 [Citation8] and found that the median prevalence of primary resistance to any drug was 11.1% and that resistance values ranged from 0% in Iceland to 56.3% in Azerbaijan. The data from Arab countries showed drug primary resistance rates of 9.8% in Yemen [Citation9] and 35% in Jordan. Furthermore, in a literature review of drug resistance in tuberculosis in GCC countries, the highest MDR TB prevalence rate was noted for UAE 9.2%, followed by Kuwait 5.9% and Saudi Arabia 4.3% [Citation4]. The proportion of MDR-TB in our study (1.2%) was higher than reported by a study performed in Qatar during the years 1996–1998, showing 0.8% MDRTB5. However, our rate was much lower than reported from other Gulf Cooperation Council (GCC) countries (overall 4.0%) [Citation4] and the global average (4.6%)12. Factors that may contribute to the low incidence of MDR-TB/RR-TB in Qatar include the low prevalence of HIV among patients diagnosed with TB, early diagnosis of TB, initiating TB treatment immediately upon proper diagnosis and bacteriologically checking for resistance to anti-TB medications for every patient diagnosed with TB.
The median prevalence of resistance to any drug in new cases of tuberculosis has been reported in 2011 to be as high as 11.1% [Citation8]. It should be borne in mind that this data is not entirely reflective of the local transmission dynamics in the country, but rather duplicate the high prevalence of resistant TB in endemic or high burden countries and this fact may explain an increasing number of MDR-TB in the year 2015. It is to be noted that the majority of the new cases of MDR in Qatar were detected in the newly arrived immigrants/expatriates. Local census data reflect the influx of male laborers from the Indian subcontinent which also explains the young male gender predominance in our study.
Drug-susceptibility testing coverage remained 100% in our study. Resistance to INH as a single drug occurred most frequently in other studies. This was also observed from other studies done from around the region [Citation10–Citation12]. Worldwide, there is considerable geographical heterogenicity in the percentage of TB cases with resistance to isoniazid. The higher resistance seen in our study population may be reflective of the situation in the Indian subcontinent [Citation13,Citation14].
A previous history of tuberculosis has been identified as one of the strongest independent predictors for MDR-TB previously [Citation15]. Rates of the previous history of TB in newly diagnosed cases of MDR tuberculosis have varied from 74% to 85% [Citation14,Citation16]. Our study aimed to address this issue. However, there are few limitations to this study include denial of the previous history of TB and treatment in expatriates due to the risk of deportation and this might explain the low proportion of the previous history of tuberculosis observed here.
Most patients in our study had pulmonary tuberculosis which is in agreement with the data already available. Pleural tissue was the major site of extra-pulmonary tuberculosis (EPTB) which has also been observed earlier. An association between the lower risk of MDR TB with extrapulmonary TB as compared to pulmonary TB has been proposed earlier [Citation10] and was observed in our study. Locally, the rate of EPTB does not compare with the global trend and it has been suggested that this may be due to early and advanced detection methods in Qatar.
The proportion of treatment success among MDR TB patients in our study [100%]. The high treatment success could be attributed to the practice of providing strict DOT for the entire duration of treatment. Latest treatment outcome data show treatment success rates of 52% for MDR/RR TB and 28% for XDR TB [2013 cohort] [Citation17].
Studies done earlier have identified that HIV-infected TB patients where at higher risk for MDR TB [Citation18], though there are older studies in which association could not be found [Citation19].
A relationship between MDR TB and HIV status could not be ascertained in our study due to the large proportion of unknown HIV status in our study population. However, it could be explained as the majority of them were referred from the medical commission centre, in which routine HIV screening is done for all new immigrants. Earlier study from the Gulf also had the same limitation [Citation15]. In 2015, only 55% of notified TB cases had a documented HIV test result (WHO Global tuberculosis report 2016). Jordan, Oman and UAE from the GCC region were enlisted among the 89 countries in which, more than 75% of TB patients had a documented HIV test result in 2015 [Citation17]. WHO, as a part of collaborative TB/HIV interventions recommends that routine HIV testing should be offered to all TB patients, to all those with TB signs and symptoms, and to partners of known HIV-positive TB patient [Citation20]. Qatar remains a low HIV prevalence country with a steady low rate of new infections diagnosed every year. Most recent available data from Qatar show that only 18 new cases of HIV were reported in 2013. [UNAIDS.ORG]
Diabetes mellitus is also a well-described comorbidity in MDR TB patients but does not appear to have worse outcomes [Citation14,Citation18].
11. Conclusion
Drug-resistant TB in Qatar is influenced by migration where the patients were probably infected. Rapid sputum sampling performed in the early stages of the disease, patient isolation and drug-susceptibility testing should be the standard of care.
Acknowledgments
The authors thank the study participants; the nursing and medical staff of the Communicable Disease Centre at Hamad Medical Corporation. Special thanks to Ms. Ameera Fabella (TB coordinator for providing registry data).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. Global tuberculosis report 2017 [Internet]. [cited 2019 Sep 6]. Available from: https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf
- Mitchison DA. Drug resistance in mycobacteria. Br Med Bull. 1984 Jan;40(1):84–6.
- WHO. Global tuberculosis report 2015 [Internet]. WHO. [cited 2016 Oct 2]. Available from: http://www.who.int/tb/publications/global_report/en/
- Areeshi MY, Bisht SC, Mandal RK, et al. Prevalence of drug resistance in clinical isolates of tuberculosis from GCC: a literature review from January 2002 to March 2013. J Infect Dev Ctries [Internet]. 2014 Sep 12 [cited 2016 Sep 20];8(9):1137–1147. Available from: http://www.jidc.org/index.php/journal/article/view/4053
- Al Marri M, Al Hail L, Al Otaibi S, et al. The time of reactivation of tuberculosis in expatriates in the state of Qatar. Qatar Med J. 2006;2006(2):12.
- Al-Marri MR. Pattern of mycobacterial resistance to four anti-tuberculosis drugs in pulmonary tuberculosis patients in the state of Qatar after the implementation of DOTS and a limited expatriate screening programme. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2001 Dec;5(12):1116–1121.
- Zignol M, van Gemert W, Falzon D, et al. Modernizing surveillance of antituberculosis drug resistance: from special surveys to routine testing. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011 Apr 1;52(7):901–906.
- Wright A, Zignol M, Van Deun A, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the global project on anti-tuberculosis drug resistance surveillance. Lancet. 2009 Jun 5;373(9678):1861–1873.
- Al-Akhali A, Ohkado A, Fujiki A. Nationwide survey on the prevalence of anti-tuberculosis drug resistance in the Republic of Yemen, 2004. Int J Tuberc Lung Dis. 2007 Dec;11(12):1328–1333.
- Al-Hajoj S, Varghese B, Shoukri MM, et al. Epidemiology of antituberculosis drug resistance in Saudi Arabia: findings of the first national survey. Antimicrob Agents Chemother. 2013 May;57(5):2161–2166. .
- Cakir E, Erdem E, Ozlu N, et al. Demographic and microbial characteristics and drug resistance of childhood tuberculosis in Istanbul: analysis of 1,541 cases. J Infect Dev Ctries. 2014 March;8(3):304–309.
- Pourakbari B, Mamishi S, Mohammadzadeh M, et al. First-line anti-tubercular drug resistance of mycobacterium tuberculosis in IRAN: a systematic review. Front Microbiol. 2016;7:1139.
- Gupta A, Kulkarni S, Rastogi N, et al. A study of mycobacterium tuberculosis genotypic diversity & drug resistance mutations in Varanasi, north India. Indian J Med Res. 2014 Jun;139(6):892–902.
- Subhash HS, Ashwin I, Jesudason MV, et al. Clinical characteristics and treatment response among patients with multidrug-resistant tuberculosis: a retrospective study. Indian J Chest Dis Allied Sci. 2003 Jun;45(2):97–103.
- Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006 Feb;61(2):158–163.
- Zhang C, Wang Y, Shi G, et al. Determinants of multidrug-resistant tuberculosis in Henan province in China: a case control study. BMC Public Health [Internet]. 2015 Dec [cited 2016 Nov 26];16(1). Available from: http://www.biomedcentral.com/1471-2458/16/42
- World Health Organization. Global tuberculosis report 2016 [Internet]. 2016 [cited 2016 Dec 14]. Available from: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf
- Mor Z, Goldblatt D, Kaidar-Shwartz H, et al. Drug-resistant tuberculosis in Israel: risk factors and treatment outcomes. Int J Tuberc Lung Dis. 2014 Oct 1;18(10):1195–1201.
- Robert J, Trystram D, Truffot-Pernot C, et al. Surveillance of mycobacterium tuberculosis drug resistance in France, 1995–1997. AZAY mycobacteria study group. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2000 Jul;4(7):665–672.
- Sculier D, Gétāhun H, World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders [Internet]. 2012 [cited 2016 Dec 14]. Available from: http://whqlibdoc.who.int/publications/2012/9789241503006_eng_Annexes.pdf