?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We aimed to compare the efficiency of the first dose of Hepatitis B (HB) vaccine: at Birth versus at 3 months and to evaluate the efficacy of HB vaccine. We conducted a cohort study in the governorate of Monastir. Vaccinated Cohort (VC) included populations receiving the first dose at 3 months (Protocol 1), and at birth (HepB-BD) (Protocol 2). First dose was followed by at least two doses. We collected, from January 2000 to December 2017, cases diagnosed by serological markers (hepatitis B surface antigen (HBsAg) and anti-HBc). We calculated Absolute Risk (AR) per 100,000 PY and the Relative risk reduction (RRR). Twenty-five cases were notified among VC and 1501 cases among not vaccinated cohort (NVC). Twenty-three cases were notified among the cohort receiving the first dose at 3 months and two cases in Protocol 2. The AR per 100,000 PY was 5.67 (CI95%: 3.36–7.99) in Protocol 1 and 0.11 (CI95%: 0.001–0.26) in Protocol 2. The RRR was 77% (95% CI: 66; 85) in Protocol 1 and 99.4% (95% CI: 97.8; 99.9) in Protocol 2. We identified 4 HB cases for children aged between 5 and 11 who benefited from protocol 1 (born between 2000 and 2006) and zero cases for children of the same age group benefiting from protocol 2 (born between 2011 and 2017). The annual number of HB has decreased from 112 in 2000 to 48 in 2017. We predicted 40 new cases of HB in 2030. HepB-BD was 99.4% effective at preventing HB. The continuity of HepB-BD worldwide would achieve WHO‘s goal of eliminating HB as a threat to health by 2050.
Abbreviations
AR: Absolute Risk; ARR: Absolute Risk Reduction; G1: Group1; G2: Group2; HB: Hepatitis B; HepB-BD: Hepatitis B Birth Dose; MENA: Middle East and North Africa; NNV: Number Needed to Vaccine; HIV: Human Immunodeficiency Virus; NVC: Not Vaccinated Cohort; PY: Person Year; RRR: Relative Risk Reduction; RR: Relative Risk; VC: Vaccinated Cohort; WHO: World Health Organization
1. Background
Hepatitis B (HB) infection is the seventh leading cause of mortality worldwide, responsible for 1.34 million deaths in 2015 with an estimated prevalence of 1.3% in the age under 5 [Citation1,Citation2]. Tunisian prevalence rate of Hepatitis B surface antigen (Ag-HBS) was 1.7% (95% CI [1.6%–1.9%]) [Citation3]. WHO has implemented child immunizations and prevention of mother-to-child transmission to reduce the number of new cases and deaths from chronic hepatitis B.
In 2016, the first global health sector strategy on viral hepatitis was endorsed with the goal of eliminating viral hepatitis as a public health threat by 2030 [Citation4,Citation5]. Thus, WHO has revised the age of administration of the first dose of HB vaccine to be dispensed at birth within 24 hours (HepB-BD) instead at the age of 3 months recommended previously [Citation6]. HB immunization was included to the Tunisian Expanded Program of Immunization (EPI) since April 1995 as Protocol 1 (G1) (first dose at 3 months). Protocol 2 (G2) (HepB-BD) started in April 2006 for babies born to positive mothers as a post-exposure prophylaxis. In 2014 protocol 2 was expanded to all newborns [Citation7,Citation8]. In Tunisia, a high vaccination coverage has been reported (94%) [Citation9], particularly in Monastir governorate (98%) [Citation10]. Similarly, the prenatal care was adequate for 82.5% in the study area (including screening of HB serological markers) [Citation11]. In Tunisia, HB’s screening is done by all physicians to achieve WHO’s goal by 2030 and declaration is mandatory [Citation12].
2. Objective
We aimed to compare the efficiency of the first dose of HB vaccine: at Birth versus at 3 months and to evaluate the efficacy of HB vaccine.
3. Methods
3.1. Study design
We performed a cohort study in the governorate of Monastir from January 2000 to December 2017.
3.2. Study Setting
Monastir governorate is an industrial-oriented and touristic region, including 13 delegations with 580,760 inhabitants [Citation13]. During the study period, there was no change in medical practices.
Periods of recruitment: we started data collection of HB cases in January 2000. In December 2017 we started to analyze according to vaccination status and according to both protocols. Exposure duration at endpoint varied from 11 to 22 years for G1 and from one to ten years for G2.
3.3. Study population
ł
3.3.1. Exposed/not exposed population
Not exposed population (NVC) were born before 1995 and exposed population to vaccination (VC) were born after this date. VC born from 1995 to 2006 benefited from Protocol 1 (G1: first dose at 3 months) and those from 2006 benefited from Protocol 2 (G2: first dose at birth). The two protocols were followed by at least two more doses. Patients living in other governorate were not included. Each vaccination is recorded on the personal immunization card.
3.3.2. Event
We studied all HB diagnosed from 1 January 2000 to 31 December 2017. Diagnosis criteria of HB is based on serological markers. Since two decades a mass screening program gets started in our country including serological markers of HB infection (hepatitis B surface antigen (HBsAg) and anti-HBc). Screening has been done in the prenuptial visit, at pregnancy, in clinically suspected cases, in blood donation, in the relatives of HB positive cases, in people living with HIV and in people living with other diagnosed sexually transmitted infections. Positive HB screening tests were associated with a spouse and family investigation. Screening program is done by all physicians to achieve WHO’s goal by 2030. In Tunisia, HB’s declaration is mandatory [Citation12].
3.4. Variables
Data included demographic variables (age, sex), vaccination status, and year at diagnosis. The vaccination status was verified by an anamnestic survey and by the immunization card control. We considered incident cases the new diagnosed cases (hepatitis B surface antigen (HBsAg) and anti-HBc) during the study period.
3.5. Data collection method and measurement
HB cases were registered in a database (‘epi info’ software) in the Regional Directorate of Primary Health of Monastir.
3.6. Bias
Several training days on HB screening and case management were carried out for the majority of physicians and laboratory to decrease the risk of underestimation.
3.7. Statistical analysis
Data were verified and analyzed using IBM SPSS Statistics version 22.0 software (IBM Corp., Armonk, NY, USA). Chi square tests were used to compare distribution of cases according to age groups and sex. Trends and prediction were calculated using Log-linear Poisson model. A p-value of <0.05 was considered statistically significant. The Absolute Risk (AR) per 100,000 person year (PY) was calculated based on Tunisian National Institute of Statistics data [Citation13].
AR, Absolute risk reduction (ARR); Relative risk (RR), Relative risk reduction (RRR), and Number Needed to Vaccine (NNV) were calculated using Microsoft Excel 2013.
3.8. Ethical considerations
The protocol was approved by the ethics committee of Faculty of medicine of Monastir. The study was conducted under Good Clinical Practice conditions and according to ethical standard collections. Each patient was assigned a unique identifying code and all documents were labeled accordingly to maintain anonymity. Investigations around all diagnosed cases were conducted and managed according to ethical standards.
4. Results
4.1. Effectiveness of birth-dose versus 3-month-dose (G1 vs G2)
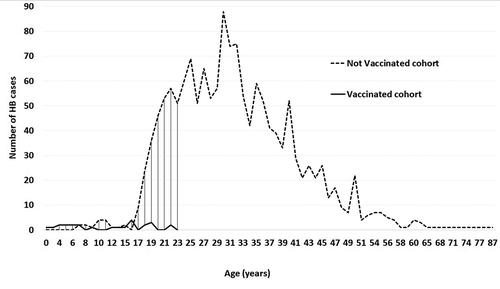
Twenty-three incident cases were notified in G1 and two cases in G2. The RRR of HB vaccination was 77% (95%CI: 66; 85) with protocol 1 and 99.4% (95%CI: 97.8; 99.9) with protocol 2. The RR of developing HB vaccine (G2 vs G1) was 0.019 (p < 0.001) (). We identified 4 HB cases for children aged between 5 and 11 who benefited from protocol 1 (born between 2000 and 2006) and zero cases for children of the same age group benefiting from protocol 2 (born between 2011 and 2017).
Table 1. Effectiveness of hepatitis B vaccine according to the two protocols, Monastir, Tunisia 2000–2017.
4.2. Effectiveness of HB vaccine (VC vs NVC)
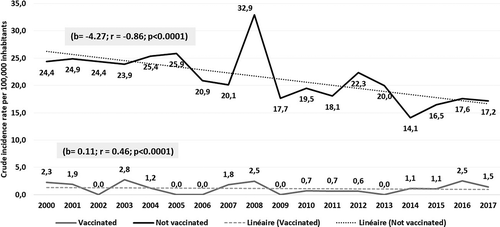
During study period 1,526 new cases of HB were diagnosed being 1,501 in NVC and 25 in VC. The AR/100,000 PY, was 22.06 (95% CI: 20.94; 23.17) in NVC and 1.11 (95% CI: 0.68; 1.55) in VC (P < 0.0001). The RRR of was 95% (95% CI: 92.5, 96.6). NNV was 4,775 (95%CI: 4,367; 5,268) (). In age group 0–22 years HB vaccine prevented 95% of HB cases (n = 217 cases) ().
Table 2. Effectiveness of HB vaccine (Monastir (2000–2017)).
4.3. Other results
For all groups, the AR/100,000 PY was 16.85 with a significant regional disparity passing from 23.08 in urban region (Monastir city) to 7.04 in rural one (Bekalta) (). Sex ratio was 3.95 in NVC (p < 0.001) and 1.5 in VC (p = 0.317).
Table 3. Absolute risk of developing HB, by delegations (Monastir; Tunisia, 2000–2017).
According to age, AR was the highest among 20–39 years age group (36.40/100,000 PY) and the lowest in 0–19 years age group.
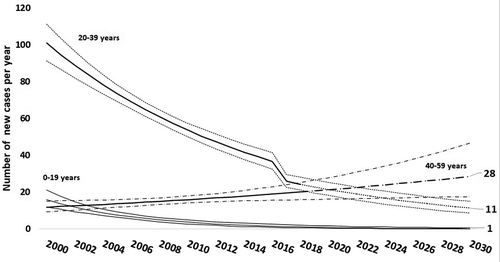
A negative and significant trend was noted in both sex, in NVC (b = −0.052; CI95% (−0.042; −0.062); p < 0.0001)) () and in 20–39 years age group (b = −0.063; CI95% (−0.05; –0.07); p < 0.0001)) (). We estimated 40 the number of new cases in 2030 (CI95%: 28–63)().
Table 4. Absolute risk and trends of HB, by age groups and gender (Monastir, Tunisia, 2000–2017).
5. Discussion
Key results: HepB-BD protocol had more effectiveness than protocol 2. AR was concordant to national rates. HB trends were significantly decreasing. A herd immunity effect was established in NVC.
5.1. Interpretations
According to WHO; HB vaccination is the most effective way to prevent HB virus infection. If given within 24 hours after birth, followed by at least two more doses, is effective at preventing perinatal HB virus infection and inducing immunity to HB virus [Citation14].
Tunisia located in the Eastern Mediterranean Region had an intermediate prevalence of HB infection in 2009 [Citation15] and low prevalence in 2015 [Citation3]. It was one of the 95 countries which had included HepB-BD as a part of national immunization schedule for all children in 2014. In our study, we determinate the efficacy of HepB-BD protocol proving the prevention of mother-to-child HB virus transmission. No cases of HB aged less than 5 years were reported since 2014, confirming the reduction of perinatal HB virus transmission by positive mothers [Citation16]. Our results were concordant with studies conducted in South-East Asia, America, and Western Pacific [Citation17-Citation19]. According to WHO, 90% of perinatal infections and horizontal transmission of household contacts can be prevented by HepB-BD followed by at least 2 additional doses in early childhood [Citation20]. Our results determining that 99% of cases can be preventable by HepB-BD.
The negative trend of notified new cases of HB and the low AR in younger than 20 years approved the high HB vaccine effectiveness. Our results were concordant with literature [Citation3].
The high AR notified in the 20–39 age group may be related to prenuptial screening and to the high-risk sexual activity in this age group. The decrease of HB new cases in this age group can be explained by herd immunity effect and by couple immunization during prenuptial visit. Indeed, it is advisable for HB-negative couples to be vaccinated, while vaccination is mandatory for discordant couples [Citation21].
The AR difference according to sex was consistent with a local study conducted at Monastir University Hospital (2002–2013, Citation22), in northern India [Citation23] and in France [Citation24]. This may be related to that men are more exposed to transfusions (road and work accident), sexual behavior, and imprisonment [Citation25]. In addition, females have a better immune response due to the lower plasma disappearance rate for Ag-HBs in males [Citation26–Citation28]. In VC we determine an equivalent number of new cases according to sex.
In our study, the general trend of HB infection was decreasing, concordant with the conclusion in the eastern Mediterranean regions [Citation29]. Iran (MENA) described increasing trends from 2008 to 2013 [Citation30]. In 2030 forty new cases were predicted to be diagnosed in Monastir governorate. These results demonstrate difficulties to achieve WHO’s goal by 2030, we suppose that the year 2050 is more reasonable to eliminate hepatitis in our region.
6. Limitations
This study confirmed the effectiveness of WHO strategies but some limitations related to data collection which can underestimate the number of new cases. In addition, NVC could include some people who could be immunized in vaccination campaigns launched before 1995.
7. Conclusion
HepB-BD followed by at least two doses was 99.4% effective at preventing HB. The continuity of HepB-BD worldwide would achieve WHO’s goal of eliminating HB as a threat to health by 2050.
Ethics approval and consent to participate
The data were obtained from a passive surveillance system focused on reportable diseases and conducted under the approval of the Regional Directorate of Primary Health according to ethical standards with the maintenance of anonymity of each patient. Thus, according to the Tunisian National Committee on Medical Ethics (http://www.comiteethique.rns.tn/index.php), it was not necessary to obtain the personal consent of the study participants.
Authors’ contributions
WD: Conceptualization, Formal analysis, Methodology, Writing - original draft.
ASB: design of the work Formal analysis, Methodology, , and substantively revised the draft.
MK, CBN, MBF, HA, IZ, and SC: Formal analysis, interpretation of data, and draft revision. IM and SG: data collection and verification.
All authors agreed to be personally accountable for the author’s own contributions, have read and approved the manuscript.
Acknowledgments
The authors thank the team of the regional direction of primary health, department of Epidemiology and Preventive Medicine at the university hospital of Monastir. Authors offer this paper to our eminent Professor Soltani Mohamed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Competing interests No Competing interests for all authors.
Additional information
Funding
References
- WHO. Global hepatitis report; 2017. Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report 2017/en/
- Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388(10049):1081–7.
- Ben Hadj M, Bouguerra H, Saffar F, et al. Observational study of vaccine effectiveness 20 years after the introduction of universal hepatitis B vaccination in Tunisia. Vaccine. 2018 18;36(39):5858–5864.
- Organization WH. Mediterranean RO for the E. Regional action plan for the implementation of the global health sector strategy on viral hepatitis 2017–2021; 2017. Available from: http://apps.who.int/iris/handle/10665/258729
- Chen D-S. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50(4):805–816.
- WHO. Hepatitis B position paper. Available from: http://www.who.int/immunization/policy/position_papers/hepatitis_b/en/
- Tunisian. Expanded program of immunization. Available from: http://www.santetunisie.rns.tn/fr/34-infos-pratiques/126-programme-de-vaccination
- Kacem M, Dhouib W, Bennasrallah C, et al. Programme élargie de vaccination aux pays du Maghreb. Etude de cas de la Tunisie. Revue systématique de la littérature. Tunis Med. 2018;96 (10/11):696–705.
- Ben Farhat, E, et al. Bulletin of the national vaccination programme. Tunisian Ministry of Health; 2015 Apr.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107.
- El Mhamdi S, Soltani MS, Haddad A, et al. New criteria and quality of health care services in the governorate of Monastir, Tunisia. East Mediterr Health J. 2010;16(1):107–12.
- Institut de santé et de securité au travail - ISST Tunisie. Available from: http://www.isst.nat.tn/fr/article/la-liste-des-maladies-transmissibles-a-declaration-obligatoire
- National institute of statistics. Available from: http://census.ins.tn/fr/recensement
- WHO. Preventing perinatal hepatitis B virus transmission. Available from: https://apps.who.int/iris/bitstream/handle/10665/208278/9789241509831_eng
- Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14(1):1–vii.
- Committee on infectious diseases, committee on fetus and newborn. Elimination of perinatal hepatitis B: providing the first vaccine dose within 24 hours of birth. Pediatrics. 2017 Sep;140:3.
- Wiesen E, Diorditsa S, Li X. Progress towards hepatitis B prevention through vaccination in the western Pacific, 1990–2014. Vaccine. 2016;34(25):2855–2862.
- Álvarez AM, Pérez-Vilar S, Pacis-Tirso C, et al. Progress in vaccination towards hepatitis B control and elimination in the region of the Americas. BMC Public Health. 2017 Apr 17;17(1):325.
- Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the south-east Asia region, 1992–2015. Vaccine. 2018;36(1):6–14.
- Hepatitis B vaccines: WHO position paper. World Health Organization; 2017 July [cited 2018 Jan]. Available from: http://www.who.int/immunization/policy/position_papers/hepatitis_b/en/
- Lahariya C. Vaccine epidemiology: A review. J Fam Med Prim Care. 2016;5(1):7–15.
- Ben Fredj M, SrihaBelguith A, Abroug H, et al. Hospitalizations for communicable diseases in a developing country: prevalence and trends-Monastir, Tunisia, 2002–2013. Int J Infect Dis IJID Off PublIntSoc Infect Dis. 2017;55:102–108.
- Behal R, Jain R, Behal KK, et al. Seroprevalence and risk factors for hepatitis B virus infection among general population in Northern India. Arq Gastroenterol. 2008;45(2):137–140.
- Antona D, Letort M-J L-BD. Estimation du nombre annuel de nouvelles infections par le virus de l’hépatite B en France, 2004–2007. Bull Epidemiol Hebd. 2009;20(21):196–199.
- Mikhail J. Massive transfusion in trauma: process and outcomes. J Trauma Nurs Off J Soc Trauma Nurses. 2004;11(2):55–60.
- Baghianimoghadam MH, Shadkam MN, Hadinedoushan H. Immunity to hepatitis B vaccine among health care workers. Vaccine. 2011;29(15):2727–2729.
- Thursz MR. Host genetic factors influencing the outcome of hepatitis. J Viral Hepat. 2003;4(4):215–220.
- Morris CA, Oliver PR, Reynolds F, et al. Intradermal hepatitis B immunization with yeast-derived vaccine: serological response by sex and age. Epidemiol Infect. 1989;103(2):387–394.
- Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555.
- Khazaei S, Karami M, Ayubi E. et al. Trends in epidemiology of hepatitis B and C infections in ilam province: national notifiable diseases surveillance system data. Casp J Intern Med. 2018;9(1):16–21.