ABSTRACT
Introduction: Paediatric cardiomyopathies are rare but serious and often life-threatening conditions. In the absence of cardiac transplant and ventricular assist device as treatment options in our region, it is very important to identify patients at higher risk. The aim of this study was to determine the outcome of patients diagnosed with cardiomyopathies and their prognostic indicators.
Patients and methods: This study included 92 cases representing all patients diagnosed with cardiomyopathy who were admitted into the pediatric cardiac intensive care unit during the period from January 2012 to September 2018. The patients were classified into two groups according to the outcome: the first group comprised 69 patients who survived, and the second group comprised 23 patients who died. All medical records were reviewed, and data were recorded and analysed.
Results: Patients with cardiomyopathies represented 8.6% (92/1071) of all patients with cardiac diseases who were admitted in the study period and in the target age group (0.5–12 years). Dilated cardiomyopathy (DCM) was the most frequent type of cardiomyopathy among the admitted patients (80 patients), while 6 patients were diagnosed with hypertrophic cardiomyopathy (HCM), 4 were diagnosed with restrictive cardiomyopathy (RCM), and only 2 were diagnosed with mixed DCM-RCM. Seventy patients required inotropic support (76.1%). Assisted mechanical ventilation was used on 15 patients (16.3%). Twenty-three patients (25.0%) died during the 7-year study period.
ConclusionsConclusions The occurrence of hypotension, abnormally high liver enzymes, the need for mechanical ventilation and the need for multiple inotropic drugs were found to be statistically significant predictors of mortality, while age, sex, fractional shortening, ejection fraction, presence of mitral regurgitation, mural thrombus, electrolyte disturbance and arrhythmias did not predict or affect patients’ outcomes.
1. Introduction
Paediatric cardiomyopathies are considered rare, with an international incidence of 1 in every 100,000 individuals under 18 years of age [Citation1]. Cardiomyopathy is a chronic illness and usually obeys a progressive natural history with a high mortality rate [Citation2]. The worldwide incidence is increasing [Citation3–5].
The national incidence of paediatric cardiomyopathies in Egypt remains underestimated because of the lack of registration outside the main tertiary centres, it is estimated to be around 6.6% of children with cardiovascular disease at one report [Citation6]. However, since the establishment of a paediatric cardiology subdivision dedicated to cardiomyopathies, the number of children diagnosed and followed up at Cairo University Children’s Hospital, which is the largest tertiary centre in Egypt and the Middle East, has been growing significantly, particularly with increased awareness and referral of cases. The paediatric cardiomyopathy clinic follows more than 1400 patients with various phenotypes, with the majority being dilated cardiomyopathy (DCM) (72%), followed by hypertrophic cardiomyopathy (HCM) (22%), hypertrophic obstructive cardiomyopathy (7%), restrictive cardiomyopathy (RCM) (3.2%), left ventricular non-compaction (2.7%), and only 1 case of endocardial elastofibrosis. The aetologic diagnosis in most cases is limited due to the lack of kits necessary to perform endomyocardial biopsy (EMB), however, while writing this paper the authors started the EMB project for cardiomyopathy cases. Around 29% of our patients have passed away, and another 13% have dropped follow-up.
A major difference exists in our region, as the options of left ventricular assist techniques and cardiac transplantation are not available. A significant impact is therefore posed on the medical system because of repeated and prolonged hospital admissions, and a high mortality rate is ensued. Prognostic indicators have been pursued by many researchers to identify cases that require a higher level of care in the context of limited resources. In a systematic review, most articles indicated that younger age at diagnosis, higher left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF), and the presence of myocarditis indicated a better prognosis. Other factors, such as severe mitral regurgitation, arrhythmias, and a family history of cardiomyopathy, conflicted across studies, and some studies identified no prognostic factors [Citation7].
Our work aimed to identify the factors that correlate with mortality in patients with cardiomyopathy who were admitted to the paediatric cardiac intensive care unit (PCICU).
2. Patients and methods
This is a retrospective analytical cohort single-centre study that included all consecutive patients admitted with the diagnosis of idiopathic cardiomyopathy to the PCICU of Cairo University Specialized Children’s Hospital during the period from January 2012 to September 2018, excluding patients with congenital structural heart disease, chronic arrhythmias, rheumatic heart disease or any systemic disease known to cause cardiomyopathies.
A total of 92 patients were enrolled after obtaining informed consent from the parent or guardian, and these patients were divided into 2 groups according to outcome: 69 survivors (75%) and 23 non-survivors (25%). The files were examined for the following:
Demographic data: gender; weight and height (from which body surface area ‘BSA’ was calculated); and age at presentation;
Presenting manifestations: heart failure, arrhythmias, syncope and shock;
History of previous hospitalizations or intravenous immunoglobulin treatment;
Vital signs on admission and on follow up. These were referenced to BSA-specific percentiles. Normal values were defined between the 5th and 95th percentiles. Hypertension was defined as persistent systolic blood pressure above the 99th percentile + 5 mmHg.
Laboratory work-up including complete blood picture, electrolytes, alanine transaminase (ALT) and aspartate transaminase (AST), blood urea nitrogen (BUN) and creatinine. Anaemia was defined as haemoglobin 9 g/dL or less [Citation8]; ALT and AST were considered abnormal if more than double-fold the reference value; BUN and creatinine were considered abnormal in values exceeding the reference ranges or rising more than 50% of baseline values.
Chest radiograms;
Electrocardiogram and Holter results, if present;
Echocardiographic studies on admission including left ventricular dimensions: left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD), from which left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) were derived; inter-ventricular septal (IVS) and left ventricular posterior wall thickness (LVPW); mitral valve regurgitation (MR); left atrial dimension; and intramural thrombi. We analysed LVFS as a raw value but converted LV dimensions and wall thicknesses to BSA-appropriate z-scores for analysis;
Medication history, response to treatment and duration of ICU stay.
Cardiomyopathy phenotype was based on echocardiographic picture, cardiac MRI confirmed the diagnosis in some cases.
Myocarditis was diagnosed in patients presenting with manifestations of low cardiac output with echocardiographic evidence of myocardial affection [Citation9] plus an elevation of serum troponin I with or without the elevation of serum CK-MB.
2.1. Statistical analysis
Data were analysed using SPSS© Statistics version 23 (IBM© Corp., Armonk, NY, USA). Normality of numerical data distribution was examined using the Shapiro-Wilk test. Normally distributed numerical data are presented as the mean and standard deviation (SD), and intergroup differences were compared using the unpaired t-test. Categorical data are presented as a number and percentage, and differences were compared using Fisher’s exact test (for nominal data) or the chi-squared test for trend (for ordinal data). Survival analysis was performed using the Kaplan-Meier method. The log-rank test was used to compare Kaplan-Meier curves. Multivariable binary logistic regression analysis was used to identify independent predictors of mortality. P-values <0.05 were considered statistically significant.
3. Results
A total of 92 patients were included in the study, representing 8.6% of the total admissions (n = 1071) to the PCICU. Males represented 55.4% of the patients and females were 44.6% (n = 51 and 41, respectively). The mean age at admission was 20.5 months (range 6–144 months; median 72 months), and the mean weight was 20.5 kg (range 4.5–55 kg). The types of cardiomyopathy included DCM (n = 80, 87%), HCM (n = 6, 6.5%), RCM (n = 4, 4.3%) and mixed DCM-RCM (n = 2, 2.2%). There were no cases of LVNC or arrhythmogenic right ventricular dysplasia (ARVD) encountered during the period of study. Twelve per cent of the patients were readmitted once, and 5.4% were readmitted more than once. The survival rate was 75% among our patients (n = 69). The most common cause of mortality was intractable heart failure (in about 63%); followed by malignant arrhythmias in 21%; and infections in 10%. A comparison of survivors with non-survivors showed no statistically significant correlation with the type of cardiomyopathy, gender, age, weight, height, or body/surface area (BSA).
Congestive heart failure and/or low cardiac output, which was present in 72.8% of patients (n = 67), was the most common presentation. Pneumonia was the presenting manifestation in 17.4% of patients (n = 16), and 6.5% had pleural effusions (n = 6).
Two-thirds of our patients were normotensive (68.5%, n = 63); hypotension was observed in 21.7% of the patients (n = 20); and hypertension was observed in 9.8% (n = 9). Hypotension was found to be a risk factor for mortality (odds ratio = 9.6, 95%CI = 1.71–54.24, p-value = 0.024).
Arrhythmias was present in 25% of our study group (n = 23): 19 patients (82.6%) survived, while 4 patients (17.4%) did not. The most common arrhythmia was supraventricular tachycardia (SVT), and amiodarone was the standard therapy in the treatment of arrhythmias associated with cardiomyopathy in most of our patients (n = 22/23). In our study, arrhythmia was not found to be a statistically significant risk factor for mortality (p > 0.05).
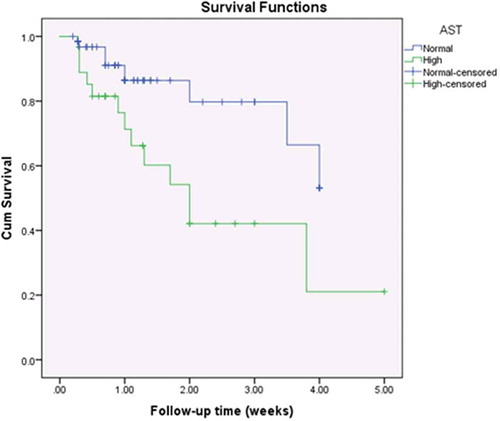
Cardiomegaly on chest X-ray (CXR) was observed in 69.6% (n = 64) of patients. Electrolyte disturbances upon admission were frequently observed among our patients: hyponatraemia was present in 32.6% (n = 30); hypokalaemia was present in 15.2% (n = 14); and hyperkalaemia was observed in only 2.1% (n = 2). Anaemia was present in 37% of patients (n = 34); leucocytosis in 17.4% (n = 16); impaired coagulation profile in 40.2% (n = 37); increased BUN in 12% (n = 11); elevated serum creatinine in 6.5% (n = 6); increased ALT in 27.2% (n = 25); and increased AST in 29.3% (n = 27). On correlating laboratory abnormalities on admission with survival (), only elevated ALT and AST were correlated with unfavourable outcomes (p-values of 0.001 and 0.003, respectively).
Table 1. Comparison of survivors and non-survivors: Laboratory findings.
Echocardiographic measurements showed no statistical correlation with survival (): all studied echocardiographic parameters had poor predictive value, with areas under the ROC curves ranging from 0.52 to 0.60 and p-values >0.05. The degree of mitral regurgitation (MR) did not correlate with outcome: mild, moderate and severe MR was present in 59.4% (n = 41), 26.1% (n = 18), and 14.5% (n = 10) of survivors, respectively, and in 56.6% (n = 13), 21.7% (n = 5), and 21.7% (n = 5) of non-survivors, respectively. Even severe MR was not a statistically significant risk factor for mortality (odds ratio = 1.64, 95%CI = 0.52–5.21, p-value = 0.418). Moreover, an LV thrombus was documented in both survivors (7.2%, n = 5) and non-survivors (4.3%, n = 1) but had no statistically significant association with mortality (p > 0.05).
Table 2. Comparison of survivors and non-survivors: Echocardiographic parameters.
Intravenous immunoglobulin (IVIG) therapy was administered in 16 patients (17.4%) presenting with clinical features of acute myocarditis and elevated cardiac enzymes (troponin I and CK-MB). A total of 68.7% (n = 11) of these patients survived, while 31.3% (n = 5) did not, with no significant correlation with outcome (p > 0.05).
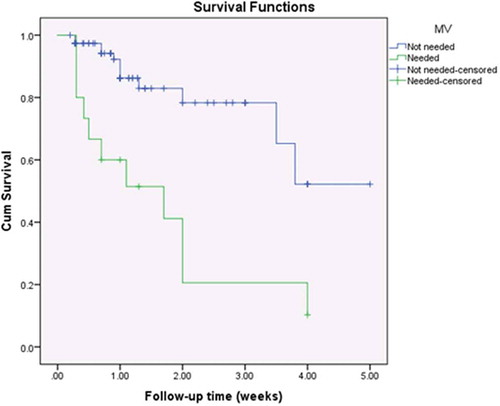
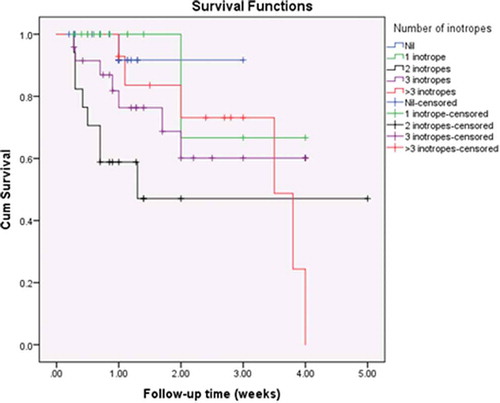
Inotropic support was administered to 76.1% of patients (n = 70). Digoxin was used in 51 patients (55.4%), dobutamine infusion in 48 (52.2%), dopamine in 44 (47.8%), milrinone in 22 (23.9%), epinephrine in 12% (n = 11), and levosimendan in 5 (5.4%). More than one inotrope was used in 49.3% (n = 34) of survivors and in 91.3% of non-survivors (n = 21) (), which may be attributed to the more severe conditions of patients who did not survive. The number of inotropes used was found to be proportionately related to unfavourable outcomes (p = 0.004). The need for epinephrine was found to be a statistically significant risk factor for mortality (odds ratio = 4.52, 95%CI = 1.29–15.81, p-value = 0.023). Mechanical ventilation was required in 16.3% of patients (n = 15), of whom only 4 survived (26.7%). The need for mechanical ventilation was also found to be a statistically significant risk factor for mortality (odds ratio = 14.9, 95%CI = 4.28–51.81, p-value <0.0001).
Table 3. Comparison of survivors and non-survivors: Inotropic support.
Kaplan-Meier survival curves showed statistically significant differences among the study population with regards to ALT (p = 0.016, ); AST (p = 0.007, ); the need for mechanical ventilation (p < 0.001, ); and the number of inotropes needed (p = 0.044, ).
Figure 1. Kaplan-Meier survival curves for the study population by the level of alanine transaminase.
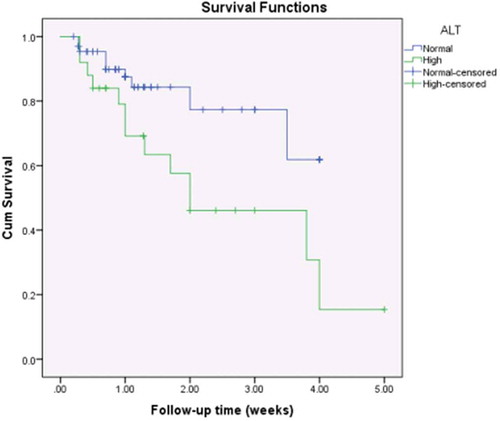
Figure 2. Kaplan-Meier survival curves for the study population by the level of aspartate transaminase.
4. Discussion
We aimed to evaluate the outcome of cardiomyopathies in patients admitted to the PCICU of the largest tertiary centre in Egypt, and the various clinical, radiological and laboratory indictors of mortality. The overall survival rate was 75%, which is similar to national rates at other centres in Egypt [Citation10] and comparable to international rates [Citation11].
Notably, in our region, advanced mechanical support by a left ventricular assist device (LVAD) and extra-corporeal oxygenation (ECMO), as well as heart transplantation, are not available as treatment options [Citation12]. This factor highlights the importance of medical management, the evaluation of its efficacy and the prediction of patients with a poor outcome to justify more intensive management [Citation13].
Cardiomyopathy is a chronic illness, and hospital readmission for non-transplanted survivors is common, which demonstrates the economic burden of such cases and the burden on the medical system. The majority of patients in our study had DCM, followed by HCM, RCM and then the mixed DCM-RCM types, with an equal male/female ratio, which is similar to the international distribution of cases [Citation14,Citation15]. Hollander and colleagues reported in 2012 a slight predisposition in the male gender [Citation13], supported by Towbin and colleagues, who hypothesized that this difference could be related to X-linked genetic causes and neuromuscular disorders [Citation16]. Gender, in our study, was found to have no prognostic significance towards outcome which is in agreement with most of the other reports [Citation17]; however, Tsirka and colleagues reported significantly higher risk of mortality and transplantation in the female gender (hazard ratio, HR = 3.0) [Citation12] with uncertainty, and postulated potential immunologic or hormonal factors.
Age of presentation did not correlate with survival. In DCM, Weng and colleagues found that age at initial diagnosis did not predict poor outcome [Citation17]. However, two other studies reported that age above 5 years old at presentation was a risk factor for early death or transplantation in children [Citation18,Citation19]. On the other hand, another study identified that age groups beneath 1 year and above 12 years were at a higher risk of death or transplantation (OR = 7.1 and 4.5, respectively) [Citation12]. Miranda et al. reported that the younger the patient, the worse the prognosis with highest rates of mortality below 1 year of age [Citation20]. In addition, Colan et al. stated that patients who presented with HCM before 1 year of age had the poorest outcome [Citation21].
The presence of hypotension was found to be a significant predictor of mortality in our study (OR = 9.63). This might reflect a state of circulatory failure in these cases and/or impaired myocardial perfusion. Other researchers have demonstrated a correlation between resting hypotension and lower exercise capacity on cardiopulmonary testing which is an important prognostic marker in idiopathic DCM [Citation22]; and similarly, a hypotensive response to exercise in HCM [Citation23].
A history of myocarditis did not show an association with outcome in our study. Kumar and Sudha (2017) likewise reported a preceding viral illness in 25.5% of cases with no association with outcome (p = 0.650) [Citation11]. All of our myocarditis patients received IVIG, but no survival benefit was found. Both Herath et al. [Citation24] and El-Saiedi [Citation25] reported similar results. In contrast, a report from the Pediatric Cardiomyopathy Registry (PCMR) study demonstrated that patients with DCM secondary to biopsy-proven or clinically diagnosed myocarditis had better outcomes with lower rates of mortality and transplantation, and higher rates of normalization of ventricular function than DCM patients without myocarditis [Citation26].
Several studies have investigated the utility of radiographically determined cardiac measurements, ratios and patterns to predict outcome. However, use of cardiomegaly, which is commonly observed at presentation, obscures the ability to differentiate outcomes at baseline and explains the larger number of negative study results as in our study. Breaking from the trend, one study found that the presence of cardiomegaly and pulmonary congestion were associated with unfavourable short-term outcome on univariate analysis [Citation11].
Echocardiography plays an important role in both the diagnosis and evaluation of patients with cardiomyopathy. In our study, we found no association between any echocardiographic measurement and outcome. These findings are in disagreement with other reports, such as the Italian Trieste Heart Muscle Disease Registry that included 47 paediatric patients and found that a low LVEF was a powerful independent predictor of mortality or heart transplantation (p = 0.046) [Citation27]. Additionally, Kampel and colleagues reported that LVFS was significantly lower in non-survivors (mean 13.8% vs 17.2% in survivors, p = 0.014); for every per cent decrease in FS, there was a 1.22-fold higher risk of death [Citation28]. In multivariate survival analysis, age-adjusted LVFS z-scores were an independent predictor of death or transplant [Citation12,Citation16,Citation20], other researchers similarly reported similar associations in univariate analysis [Citation29–Citation31]. However, few earlier reports [Citation32–Citation34] found no association as in the current study. It is well established that patients with cardiomyopathy develop tolerance to low EF, therefore, maybe a follow-up functional assessment is more useful than a single measurement especially with the newer tissue Doppler indices [Citation35] that have the advantage of assessment of regional function but have the limited applicability in emergency settings and absence of specialized echocardiographers.
Whatever the degree of MR, no impact was found on outcome. Our results are in accordance with the findings of Kumar and Sudha [Citation11]. However, a large Brazilian pediatric study found that moderate to severe mitral, tricuspid or pulmonary regurgitation were associated with mortality: only severe MR was an independent predictor of mortality in a multivariate survival analysis [Citation29]. Similarly, another study reported a hazard ratio of 1.9 (p = 0.01) for death or transplant [Citation36]. Again, the follow-up assessment is more important, as the degree of MR was found to improve in survivors [Citation37].
A mural thrombus in the LV also was not a predictor of poor outcome in our study, which is different from other reports [Citation11]. Similarly, arrhythmias did not correlate with outcome in our study, which is in accordance with the studies by Tsirka et al. [Citation12] and Decker et al. [Citation23] on DCM and HCM patients, respectively. In contrast, another study reported that the presence of arrhythmia was a significant predictor of early mortality in idiopathic DCM [Citation17,Citation30,Citation33,Citation34,Citation38]. The QRS width is also an important predictor and should be considered in such cases [Citation30]. Arrhythmias could indicate a terminal myocardial status, and could be a complication to inotropic treatment, or a precipitating factor to cardiovascular events and should therefore be addressed thoroughly in such a cohort.
We found that the need for inotropic support was correlated with poor outcome, and a higher association with the use of multiple inotropic agents, which might reflect the severity of the condition at the time of presentation. This finding is in agreement with other reports [Citation32]: this questions the escalating manner that we follow according to the clinical status of the patient and whether we should start more aggressive from the start? The other side of the coin is whether inotropic agents themselves are a risk for mortality and therefore we should be more conservative when using them? Similarly, the need for ventilatory support was significantly higher in non-survivors in our study, a finding that is consistent with other studies [Citation28,Citation39], but also raises the question: are we too late to ventilate, and should we have a lower threshold to ventilate those patients?
The abnormality in levels of transaminases indicates cardiac hepatopathy and was associated with mortality in our study. This finding might indicate congestive hepatopathy and/or acute cardiogenic liver injury are associated with circulatory failure. In chronic heart failure, cell death is due to apoptosis whereas necrotic cell death is prominent in acute heart failure [Citation40]. AST, together with cholestasis markers including γ-glutamyl-transpeptidase, total bilirubin and alkaline phosphatase were correlated with poor outcome in congestive hepatopathy, while ALT and lactate dehydrogenase were important markers in acute cardiogenic liver injury [Citation41].
Similarly, many mechanisms contribute to the pathogenesis of renal dysfunction in patients with impaired systolic function, such as salt and water retention, venous congestion, anaemia and other systemic comorbidities. Assessment of kidney functions is conducted as part of the routine inpatient work-up of children with cardiomyopathy. However, elevated serum creatinine was not a significant predictor of outcome in our study. In contrast, in one study, elevated serum creatinine was found to be associated with increased mortality or the need for mechanical ventricular assistance [Citation42].
5. Conclusion
By multivariable binary logistic regression analysis, we found that the indicators associated with an unfavourable outcome in pediatric cardiomyopathies admitted to the intensive care unit were the occurrence of hypotension, the need for mechanical ventilation and abnormal transaminases; marking cases with these risk-factors as higher risk for mortality could therefore rationalize more intensive management. Lower threshold for mechanical ventilation could also be justified, and also more judicious use of inotropic agents. In the absence of heart transplantation and ventricular assist devices, our findings may influence an improved approach to medical management of these patients.
6. Limitations
The retrospective observational nature of the study limited the number of variables to be studied. Additionally, the study included a heterogeneous population of patients with cardiomyopathies. There were a low number of cardiac deaths, and therefore the study may not be powered to detect all risk factors.
Ethical considerations
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on care and have been approved by the local ethics committee in the Department of Paediatrics, Faculty of Medicine, Cairo University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wilkinson JD, Sleeper LA, Alvarez JA, et al. For the pediatric cardiomyopathy study group: the pediatric cardiomyopathy registry: 1995–2007. Prog Pediatr Cardiol. 2008;25(1):31–9. .
- Lipshultz SE, Law YM, Asante-Korang A, et al. Cardiomyopathy in children: classification and diagnosis: a scientific statement from the American Heart Association. Circ. 2019 Jul 2;140(1):e9–e68. .
- Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the USA. N Engl J Med. 2003;348:1647–1655.
- Nugent AW, Daubeney PE, Chondros P, et al. National Australian childhood cardiomyopathy study: the epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646.
- Nugent AW, Daubeney PE, Chondros P, et al. National Australian childhood cardiomyopathy study: clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–1338.
- Elmasry OA, Kamel TB, El-Feki NF. Pediatric cardiomyopathies over the last decade: a retrospective observational epidemiology study in a tertiary institute, Egypt. J Egypt Public Health Assoc. 2011;86(3and 4):63–67.
- Lee TM, Hsu DT, Kantor P, et al. Pediatric cardiomyopathies. Circ Res. 2017;121(7):855–873. .
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011; [cited 2020 Aug 22. (WHO/NMH/NHD/MNM/11.1) http://www.who.int/vmnis/indicators/haemoglobin pdf
- Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circ. 2014;129(1):115–128.
- Bakeet MA, Mohamed MM, Allam AA, et al. Childhood cardiomyopathies: a study in tertiary care hospital in upper Egypt. Electron Physician. 2016;8(11):3164–3169. .
- Kumar S, Sudha S. Profile and outcome of dilated cardiomyopathy in children: a short-term follow up. JMRP. 2017;6(1):9–16.
- Tsirka AE, Trinkaus K, Chen SC, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–397.
- Hollander SA, Bernstein D, Yeh J, et al. Outcomes of children following a first hospitalization for dilated cardiomyopathy. Circ Heart Fail. 2012;5(4):437–443. .
- Oh JH, Hong YM, Choi JY, et al. Idiopathic cardiomyopathies in Korean children. 9-year Korean multicenter study. Circ J. 2011;75(9):2228–2234. .
- Harmon WG, Sleeper LA, Cuniberti L, et al. Treating children with idiopathic dilated cardiomyopathy (from the pediatric cardiomyopathy registry). Am J Cardiol. 2009;104(2):281–286. .
- Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296(15):1867–1876. .
- Weng KP, Lin CC, Huang SH, et al. Idiopathic dilated cardiomyopathy in children: a single medical center’s experience. J Chin Med Assoc. 2005;68(8):368–372.
- Daubeney PE, Nugent AW, Chondros P, et al. On behalf of the national Australian childhood cardiomyopathy study: clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006;114:2671–2678.
- Alexander PM, Daubeney PE, Nugent AW, et al. National Australian childhood cardiomyopathy study: long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of childhood cardiomyopathy. Circulation. 2013;128:2039–2046.
- Miranda JO, Costa L, Rodrigues E, et al. Paediatric dilated cardiomyopathy: clinical profile and outcome. The experience of a tertiary centre for paediatric cardiology. Cardiol Young. 2015;25(2):333–337. .
- Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the pediatric cardiomyopathy registry. Circulation. 2007;115(6):773–781. .
- Suh YS, Youn JC, Park SH, et al. Hypotension rather than hypertension is more correlated with lower exercise capacity in idiopathic dilated cardiomyopathy. Europ Heart J. 2013;34(1):5735. .
- Decker JA, Rossano JW, Smith EO, et al. Risk factors and mode of death in isolated hypertrophic cardiomyopathy in children. J Am Coll Cardiol. 2009;54(3):250–254. .
- Herath VC, Gentles TL, Skinner JR. Dilated cardiomyopathy in children: review of all presentations to a children’s hospital over a 5-year period and the impact of family cardiac screening. J Paediatr Child Health. 2015;51(6):595–599.
- El-Saiedi SA. Randomized controlled trial on the use of intravenous immune globulin in acute pediatric myocarditis. J Clin Res Bioeth. 2013;5:170.
- Foerster SR, Canter CE, Cinar A, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the pediatric cardiomyopathy registry. Circ Heart Fail. 2010;3(6):689–697. .
- Puggia I, Merlo M, Barbati G, et al. Natural history of dilated cardiomyopathy in children. J Am Heart Assoc. 2016;5(7):e003450. Published online 2016 Jun 30. .
- Kampel L, Perles Z, Rein AJ, et al. The outcome of infants and children with heart muscle disease: prognosis in centers without heart transplantation option. Open J Pediatrics. 2014;4:34–41. .
- Azevedo VM, Albanesi Filho FM, et al. How can the echocardiogram be useful for predicting death in children with idiopathic dilated cardiomyopathy? Arq Bras Cardiol. 2004;82(6):505–514. .
- Nogueira G, Pinto FF, Paixao A, et al. Idiopathic dilated cardiomyopathy in children: clinical profile and prognostic determinants. Revista Portuguesa de Cardiologia. 2000;19:191–200.
- Venugopalan P, Houston AB, Agarwal AK. The outcome of idiopathic dilated cardiomyopathy and myocarditis in children from the west of Scotland. Int J Cardiol. 2001;78:135–141.
- Arola A, Tuominen J, Ruuskanen O, et al. Idiopathic dilated cardiomyopathy in children: prognostic indicators and outcome. Pediatrics. 1998;101:369–376.
- Lewis AB, Chabot M. Outcome of infants and children with dilated cardiomyopathy. Am J Cardiol. 1991;68:365–369.
- Wiles HB, McArthur PD, Taylor AB, et al. Prognostic features of children with idiopathic dilated cardiomyopathy. Am J Cardiol. 1991;68:1372–1376.
- McMahon CJ, Nagueh SF, Eapen RS, et al. Echocardiographic predictors of adverse clinical events in children with dilated cardiomyopathy: a prospective clinical study. Heart. 2004;90:908–915.
- Fernandes FP, Manlhiot C, McCrindle BW, et al. Usefulness of mitral regurgitation as a marker of increased risk for death or cardiac transplantation in idiopathic dilated cardiomyopathy in children. Am J Cardiol. 2011;107(10):1517–1521. .
- Patange A 1, Thomas R, Ross RD. Severity of mitral regurgitation predicts risk of death or cardiac transplantation in children with idiopathic dilated cardiomyopathy. Pediatr Cardiol. 2014;35(2):232–238.
- Griffin ML, Hernandez A, Martin TC, et al. Dilated cardiomyopathy in infants and children. J Am Coll Cardiol. 1988;11:139–144.
- Kirk R, Naftel D, Hoffman TM, et al. Pediatric heart transplant study investigators: outcome of pediatric patients with dilated cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28(12):1322–1328. .
- Herzer K, Kneiseler G, Bechmann LP, et al. Onset of heart failure determines the hepatic cell death pattern. Ann Hepatol. 2011;10:174–179.
- Çağlı K, Başar FN, Tok D, et al. How to interpret liver function tests in heart failure patients? Turk J Gastroenterol. 2015;26:197–203.
- Price JF, Mott AR, Dickerson HA, et al. Worsening renal function in children hospitalized with decompensated heart failure: evidence for a pediatric cardiorenal syndrome? Pediatr Crit Care Med. 2008;9:279–284.