ABSTRACT
The validity of the GLI-2021 norms for SLVs in healthy Algerian adults has not been assessed. To ascertain how well do the GLI-2021 norms fit to contemporary SLVs data in Algerian adults. This was a cross-sectional study involving 481 (n = 242 females) healthy non-smoking adults recruited from the Algiers general population. All participants underwent a clinical examination and a plethysmography. Z-scores for slow vital capacity (SVC), functional residual capacity (FRC), residual volume (RV), total lung capacity (TLC), expiratory reserve volume (ERV), inspiratory capacity (IC), and RV/TLC were calculated. The mean difference between the determined and the predicted values (∆value) of SLVs were calculated. The GLI-2021 norms would be considered as reflective of contemporary Algerian SLVs if the total sample mean z-scores were in the normal range (ie; −0.5 to +0.5). The participants’ means ± SDs of age and height were 46.4 ± 16.4 years and 166 ± 10 cm, respectively. The determined SLVs were significantly different from those predicted (∆values means ± SDs were −170 ± 470 ml for IC, −100 ± 490 ml for SVC, 170 ± 400 ml for ERV, 240 ± 620 ml for TLC, 370 ± 340 ml for RV, 480 ± 480 ml for FRC, and 5.28 ± 4.38% for RV/TLC). The means ± SDs z-scores for IC, SVC, ERV, and TLC were in the normal range (−0.29 ± 0.88, −0.17 ± 0.94, 0.29 ± 0.77, and 0.35 ± 0.86, respectively), but those of RV, FRC, and RV/TLC were out of the normal range (0.74 ± 0.66, 0.75 ± 0.72, and 0.83 ± 0.75, respectively). In healthy Algerian adults, the GLI-2021 norms fit well to SVC, TLC, ERV, and IC, but they do not fit to FRC, RV, and RV/TLC.
1. Introduction
According to scholarly societies [Citation1], spirometric and static lung volumes (SLVs) norms should be derived from measurements carried out within a representative sample of the general population, i.e. an adapted population of ‘healthy/normal’ individuals possessing similar anthropometric, ethnic, and socio-economic conditions as the patients tested [Citation1–3]. Therefore, it is recommended to use norms that fit the population to be explored [Citation1]. Nowadays, lung function parameters (LFPs) are habitually reported as percentage predicted where predicted data are derived from a healthy non-smoking population [Citation1,Citation4]. Nevertheless, it appears that the use of percentage predicted leads to an age bias [Citation5], which can be avoided by the use of sex, age, height, and ethnicity-specific z-scores [Citation6,Citation7]. The z-score indicates how many standard deviations (SDs) a measurement is from its predicted value, with 90% of healthy individuals having a z-score between +1.645 and −1.645 [Citation6,Citation7]. Unlike percentage predicted, z-score is free of bias related to age, height, sex, and ethnic group, and it is accordingly suitable for defining lower- and upper limits of normal (LLN and ULN, respectively). They also simplify the uniform interpretation of LFPs [Citation6,Citation8–10].
In Algeria, spirometric and SLVs norms were developed for adults living in Constantine, an Eastern region of Algeria [Citation11]. For several reasons (eg; environmental factors relating to altitude, air pollution and humidity, in addition to anthropometric disparities), the aforementioned norms were judged inapplicable to Northern Algerian adult residents [Citation12]. In 2018, global lung function initiative multi-ethnic spirometric norms (GLI-2012) [Citation6] were found to be applicable to a representative sample of the Algiers region [Citation10]. However, the GLI-2012 norms only concern spirometric data [Citation10]. Currently, physicians do not have any norms for SLVs whose applicability is verified on the general Algerian population. In 2021, the GLI Task Force released SLVs norms (GLI-2021) including 7190 observations from healthy individuals between the ages of 5 and 80 years [Citation7]. The observations were collected from 17 centers in 11 countries, including one in Tunisia who contributed by SLVs values from 615 Tunisians (8.55% of the total data)) [Citation7]. The GLI-2021 sex-specific norms [Citation7], including height and age, were developed for total lung capacity (TLC), functional residual capacity (FRC), residual volume (RV), inspiratory capacity (IC), slow vital capacity (SVC), expiratory reserve volume (ERV), and RV/TLC. As done during the genesis of the GLI-2012 spirometric norms [Citation6], the LMS [lambda, mu, and sigma] method was used and the generalized additive models of location shape and scale were applied [Citation6,Citation13]. External validation of the GLI-2021 norms is recommended [Citation7], and further evaluations of their applicability to other parts of the world are required in order to verify their appropriateness in these areas [Citation7]. In Algeria, the GLI-2021 norms [Citation7] will shortly be implemented by manufacturers of plethysmography devices, and will therefore replace the applied local norms [Citation11]. Thus, verifying their applicability to the Algerian population seems to be crucial for care activities and research, and is urgently required. Hitherto, there is no publication evaluating the validity of the GLI-2021 norms [Citation7] in Algerian adults. Since the GLI-2021 norms [Citation7] may be unsuitable for use in Algerian adult population, it is essential that physicians be aware of the potential consequences of adopting these norms for clinical decision-making [Citation7].
Taking into account the above points, the aim of this study was to evaluate if the GLI-2021 norms [Citation7] are applicable to an adult Algerian population.
2. Population and methods
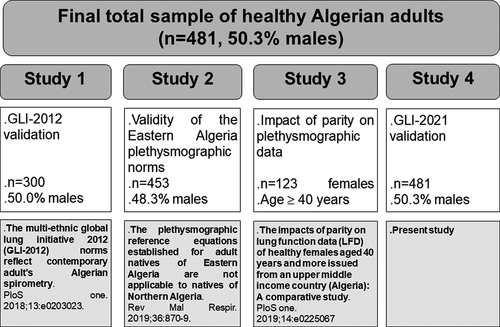
This study is part of a project involving four parts largely described in . The first part [Citation10] aimed at testing the validity of the GLI-2012 norms [Citation6]. The second part [Citation12] aimed at testing the validity of the Eastern Algeria plethysmographic norms [Citation11]. The third part [Citation14] aimed at evaluating the impact of parity on females’ plethysmographic data. The fourth part constitutes the topic of this study. The final total population included 481 healthy adults (50.3% males) living in Algiers (). exposes the present study flow-chart.
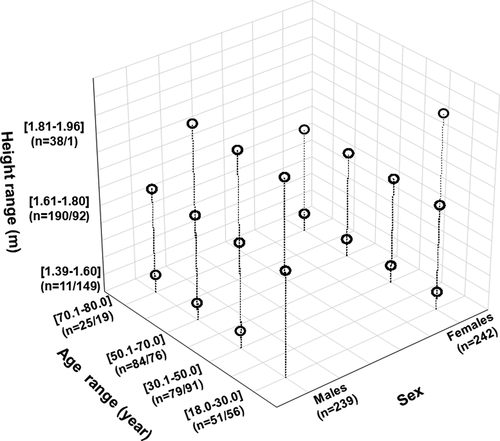
Figure 3. Distribution of the 481 participants according to sex, age and height ranges.
2.1. Study design
A cross-sectional study was performed at the Department of Pneumology, Phthisiology, and Allergology in Rouiba Hospital, Algiers, Algeria. The study was conducted in compliance with Helsinki ‘Ethical principles for medical research involving Human subjects’. Rouiba Hospital (Algiers) Medical Advice and Ethics Commission approved the study (approval number: 0601/2014). Written informed consent was obtained from all participants.
2.2. Study population
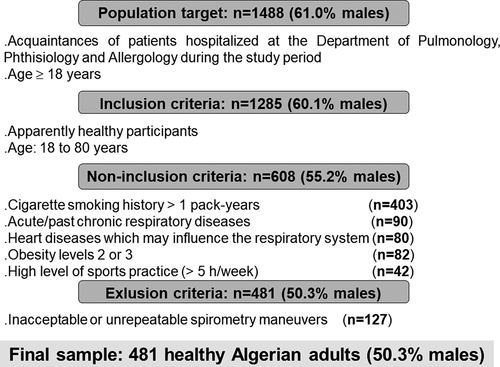
The target population consisted of a group of healthy Algerian adults. Participants were selected by convenience sampling from the acquaintances of patients hospitalized at the above department during, for example, the hospital visiting time. The inclusion, non-inclusion, and exclusion criteria, which were previously described [Citation10,Citation12,Citation14], are reported in . In this study, only healthy adults aged ≥18 years with technically acceptable and repeatable plethysmographic/spirometric maneuvers were included. In order to meet the GLI-2021 norms age limits, adults over 80 years of age were excluded from the final statistical analysis [Citation7].
2.3. Medical and anthropometric data
Medical data were collected using a simplified and modified medical questionnaire [Citation15]. Age (accuracy to 0.1 years) was calculated, and standing height and weight were measured. Depending on the calculated body mass index (BMI, kg/m2), participants were classified as: underweight (BMI < 18.5 kg/m2), normal weight (BMI: 18.5 to 24.9 kg/m2), overweight (BMI: 25.0 to 29.9 kg/m2), and obese (BMI ≥ 30.0 kg/m2) [Citation16]. Obesity was classified as stage-1 (BMI: 30.0 to 34.9.0 kg/m2), stage-2 (BMI: 35.0 to 39.9.0 kg/m2), and stage-3 (BMI > 40.0 kg/m2).
2.4. Plethysmographic and spirometric measurements
One qualified person (AK in the authors’ list) performed all the plethysmographic/spiromtric tests in the morning. All measurements were performed with a body plethysmograph (Body-box 5500, MediSoft, Belgium), following carefully the international guidelines [Citation8,Citation10–12,Citation14,Citation17,Citation18] in the following order: FRC maneuver and spirometry maneuver. The spirometer was calibrated daily with a 3-L syringe. The plethysmography was calibrated daily according to manufacturer’s instructions.
The plethysmography was performed, after a resting period of 10–15 minutes, in a seated position, back straight, with a nose clip. The maneuvers to be performed were explained and their demonstrations supported these explanations. Inspiratory and expiratory maneuvers were forced, maximal, performed without hesitation, and continued until the RV. The plethysmographic technique followed the succeeding steps [Citation18]: i) The procedure was explained in detail to participants; ii) The plethysmograph door was closed and time was allotted for thermal transients to stabilize and patients to relax; iii) Participants were instructed to attach the mouthpiece and breathe quietly until they achieved stable end-expiration; iv) When participants were at or near FRC, the shutter was closed at end-expiration for 2–3 s, and they were instructed to perform a series of gentle pants at a frequency of 0.5–1.0 Hz; v) After a series of 3–5 technically satisfactory panting maneuvers was recorded, the shutter opened and participants performed an ERV maneuver followed by an SVC maneuver.
The repeatability and acceptability criteria were respected for plethysmography and spirometry [Citation17,Citation18]. Regarding the FRC repeatability, at least three values were obtained and the difference between the highest and the lowest FRC values divided by the mean was ≤ 0.05 [Citation18]. The FRC average value was selected [Citation18]. For the forced vital capacity (FVC) manoeuvres, at least three repeatable FVC measurements were obtained [Citation17]. FVC and forced expiratory volume in 1 second (FEV1), the best two tests out of the three selected ones, did not differ by more than 0.150 L (if FVC ≥ 1 L), or 0.100 L (if FVC < 1 L). The highest FVC and FEV1 were computed even though the two data did not come from the same flow-volume curve [Citation17].
Three spirometric data were measured [FEV1, FVC, and forced expiratory flow at 25–75% of FVC (FEF25-75%], and FEV1/FVC ratio was calculated. The use of accompanying spirometry maneuvers allowed the measurement of some dynamic lung volumes (ie; SVC, ERV, and IC), and when combined with FRC, it allowed the calculation of additional SLVs [ie; RV (= FRC – ERV), TLC (= SVC + RV)], and the RV/TLC ratio (%) [Citation7]. More details related to the collected data during the spirometric/plethysmographic tests are recently reported in an Editorial aiming to review the current use of GLI-2012 [Citation6] and GLI-2021 [Citation7] in Great Arab Maghreb countries and steps required to improve their utilization [Citation19].
An online software for the GLI-2012 and the GLI-2021 [Citation6,Citation7] norms was used [Citation20]. An Excel file including the mandatory and optional input values for our calculator of interest (ie; age, height, sex, ethnicity, FEV1, FVC, FEF25-75%, FRC, TLC, RV, ERV, IC, and SVC) was uploaded on the calculator page. The software performed the calculation, and the results were returned automatically. Height-, age-, and sex-specific z-scores for spirometric and SLVs parameters were calculated using the GLI-2012 spirometric norms for Caucasians [Citation6], and GLI-2021 norms [Citation7], respectively. For each parameter, the software calculated several outcomes (ie; predicted value, LLN, ULN, and z-score). SLVs were divided into two categories: low (i.e. SLV z-score < −1.645) and high (SLV z-scores > + 1.645) volume [Citation1,Citation2,Citation7]. For each SLV, a delta volume (∆SLV = determined value minus predicted value) was calculated.
2.5. Statistical analysis
The distribution of quantitative variables was normal and the results were expressed by their means ± SDs and 95% confidence interval. The corpulence status results and sex were expressed as numbers (%). The two-sided chi-square test was used to compare percentages. The Student t-test was used to compare the anthropometric data and LFPs of males and females. The Wilcoxon test was used to compare the determined LFPs with those predicted from the GLI norms for spirometry and SLVs [Citation6,Citation7]. As proposed by Bland and Altman [Citation21], limits of agreement (ie; mean difference between measured and predicted value±1.96 SD) were used for comparison of measured SLVs (i.e. SVC, FRC, RV, TLC, ERV, and IC) with predicted values calculated from the GLI-2021 norms [Citation7], with individual difference (measured value minus predicted value) plotted against the corresponding mean value. The correlations between mean differences and mean values were evaluated by Pearson’s product-moment correlation ‘r’. The main judgment criterion related to the applicability or not of the GLI-2021 norms [Citation7] is the mean value of the total sample z-score of each SLV. The expected z-scores of the tested population (ie; determined SLV value) would have a mean of ‘> 0’ and a SD of ‘> 1’, and would therefore be considered ‘statistically’ significant [Citation6]. As previously done [Citation6,Citation8–10], and according to a consensus established by the GLI Task Force, a z-score out of the normal range (ie; < −0.5 or > + 0.5) was arbitrarily considered ‘clinically’ significant [Citation22]. Two additional secondary criteria are in favour of the validity of the GLI-2021 norms [Citation7] in the total sample: i) the absence of statistically (i.e. p < 0.05) [Citation12] and clinically (i.e. ∆SLV > 0.200 L for SVC [Citation1,Citation23,Citation24], or > 0.300 L for FRC, TLC, and RV [Citation25,Citation26]) significant differences between the determined and the predicted SLVs; and ii) less than 5% of participants have abnormal SLVs (i.e. low SVC or IC, or high FRC, TLC, RV, or RV/TLC) [Citation6,Citation12]. The associations between z-scores, and both sex and anthropometric data were evaluated, respectively, by Student t-test and Pearson’s product-moment correlation ‘r’. The latter was considered as ‘high’, ‘good’, ‘fair’, or ‘weak’ when it was, respectively, ‘> 0.70’, between ‘0.50 and 0.70’, between ‘0.30 and 0.50’, or ‘≤ 0.30’ [Citation27]. If the GLI-2021 norms [Citation7] are valid, no ‘good’ or ‘high’ relationships should exist [Citation28]. All mathematical computations and statistical procedures were performed using the statistical software (Statistica, version 12). Significance level was set at 0.05.
3. Results
Among the 1488 acquaintances of patients hospitalized at the Department of Pulmonology, Phthisiology and Allergology, 1285 (86.3%) were included in the initial stage. Non-inclusion criteria were found in 677 participants (52.7%). Among the remaining 608 participants, 127 (21.8%) failed to meet the acceptability and repeatability criteria of plethysmography/spirometry. Therefore, the final sample included 481 adults (239 males) aged between 18 and 80 years ().
exposes the participants’ distribution according to sex, age, and height ranges. Age distribution according to sex was similar. However, fewer adults (9.15%) were included in the age range 70.1–80.0 years. Only one female was included in the height range 1.81–1.96 m, and fewer males (2.29%) were included in the height range 1.39–1.60 m.
exposes the participants’ anthropometric data. Females and males had similar age and BMI. Compared to females, males were significantly taller and heavier, and the percentage of adults with an obesity stage-1 was significantly lower.
Table 1. Anthropometric data of the healthy Algerian adults aged 18–80 years
exposes the participants’ spirometric data. In the total sample: i) Only FEF25-75% z-score was out of the normal range; and ii) Compared to the predicted values, the measured FVC and FEF25-75% values were significantly higher. However, FEV1/FVC and FEV1 values were significantly lower.
Table 2. Spirometric data of the healthy Algerian adults aged 18–80 years
exposes the participants’ SLVs. In the total sample: i) The mean z-scores of FRC, RV, and RV/TLC were out of the normal range; ii) All the determined SLVs were significantly different from those predicted from the GLI-2021 norms. They were lower than the predicted values for SVC and IC, and higher than the predicted values for FRC, RV, TLC, ERV, and RV/TLC; and iii) The means (in mL) of ∆SVC, ∆IC, ∆ERV, and ∆TLC were not ‘clinically’ significant (=- 100; −170, 170, and 240, respectively), but ∆RV and ∆FRC were ‘clinically’ significant (=370, and 480, respectively).
Table 3. Static lung volumes of healthy Algerian adults aged 18–80 years
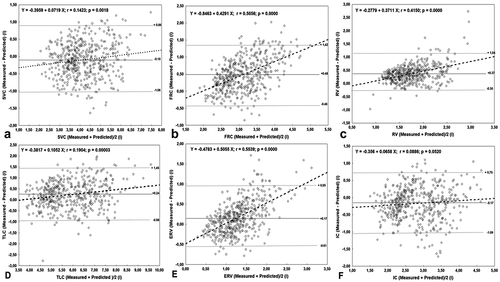
illustrates the Bland and Altman comparisons between measured and predicted SLVs from the GLI-2021 norms. There was a systematic bias between the measured and predicted values for all measured SLVs ( and ). The correlations between mean differences and mean values were significant for all the SLVs, and were ‘good’ for FRC () and ERV (), ‘fair’ for RV (), and ‘weak’ for SVC (), TLC (), and IC ().
Figure 4. Bland and Altman representation of measured and predicted static lung volumes determined from the 2021-GLI (n = 481).
exposes the percentages of participants having abnormal SLVs. In the total sample, more than 5% of adults had ‘clinically’ lower SVC (6.3%), and IC (5.82%), and ‘clinically’ higher FRC (10.60%), RV/TLC (9.36%), and RV (6.24%).
Table 4. Percentages of healthy Algerian adults having abnormal static lung volumes (SLVs)
The z-scores of SLVs were not related to sex (). exposes the ‘r’ between SLVs z-scores and anthropometric data. In the total sample: i) ‘Weak or no correlations’ were noted between SLVs, and both age and height; and ii) ‘Fair’ correlations were noted between weight or BMI, and FRC or ERV.
Table 5. Pearson correlation coefficient (r) between the static lung volumes z-scores and the anthropometric data
4. Discussion
The results of this study, performed in an Algerian population of 481 healthy non-smoking adults, are mixed. On the one hand, the GLI-2021 norms fit well to SVC, TLC, ERV and IC. On the other hand, they do not fit to FRC, RV, and RV/TLC. To the best of the authors’ knowledge, no previous study has aimed at evaluating the validity of the GLI-2021 norms in healthy adult populations.
Accurate norms of SLVs have several factors of primary care significance. First, TLC allows more meaningful interpretations of FEV1 and FVC [Citation1]. Second, the determination of TLC LLN facilitates accurate diagnosis of the restrictive ventilatory defect (i.e. TLC < LLN) [Citation1]. Third, a reduced TLC (e.g. TLC < LLN) correlates with both morbidity and mortality, quality of life, and physical activity [Citation29,Citation30]. Fourth, the determination of RV ULN or IC LLN eases the correct diagnosis of lung-hyperinflation (i.e. RV > ULN or IC < LLN) [Citation26]. Fifth, SLVs provide a physiological pattern, which can be used in combination with spirometry and transfer factor of the lung for carbon monoxide tests to refine a differential diagnosis [Citation1,Citation7]. By developing sex-specific norms that summarize the height- and age-related changes in SLV data, the GLI-2021 norms should improve the interpretation of SLVs values [Citation7].
4.1. Discussion of the methodology
One of the main strong points of this study is its prospective design. However, it would have been better if more than one centre was included. According to the GLI Task Force [Citation31], at least 300 participants (150 males) are required to validate norms and to avoid spurious variances due to sampling mistakes. This requirement was largely considered in this study (239 males and 242 females). The population from which the norms are derived should be representative of the general population [Citation28]. Age scattering and other anthropometric, ethnic, socioeconomic, and environmental factors should be equivalent since such factors can mark lung function [Citation28]. Furthermore, the methodology for performing plethysmographic tests (eg; protocol and equipment) must be stringent [Citation1]. In order to avoid biased assessment of outcomes [Citation32] and sex-related effect on lung function [Citation33], similar percentages of males and females were included in this study (). The present study included adults with large age range (18–80 years) and height range (149–196 cm for males, and 139–181 cm for females), and with obesity stage-1 in 23.49% of them. The present study height range was closer to the one reported by Hall et al. [Citation7], where the height ranges were 145–203 cm, and 134–186 cm, respectively, in males and females. The percentage of Algerian adults with overweight or obesity (60.9%) was closer to that reported in the GLI-2021 study (i.e. 54.8%) [Citation7]. Moreover, since 30% of Algerian adults have obesity [Citation34], the present study group composition reflected this ‘healthy’ population as it exists in real life. It would have been better if overweight and obese individuals were excluded in order to represent ‘ideal’ health and to allow assessment of the impact of weight on SLVs [Citation7]. However, the aforementioned strategy dismisses a significant fraction of participants with no documented evidence of lung condition [Citation7]. The above point was largely discussed by Hall et al. [Citation7].
Similar to the study generating the GLI-2021 SLVs norms [Citation7], where healthy participants were never-smokers with no history of self-reported or physician-diagnosed respiratory condition, in the present study, only healthy never-smokers were included. The external validity of this study was therefore increased. Unlike the study generating the GLI-2021 SLVs norms [Citation7], where different body plethysmography and gas dilution techniques were used, in this study, only one plethysmograph was used, which ensures more intern validity for the reported data. It is important to highlight that the SLVs collected for the two gas dilution techniques (eg; nitrogen washout and helium dilution) and body plethysmography demonstrate remarkable overlap [Citation7]. As recommended, and as done in the studies retained to generate SLVs norms [Citation7], the American thoracic society and the European respiratory society guidelines for spirometry and plethysmography [Citation17,Citation18] were applied.
We applied the same statistical type of analysis used in previous similar North African studies [Citation8,Citation10], aiming to ascertain how well do the GLI-2012 norms [Citation6] fit contemporary North African spirometric data. However, the suggested ‘fairly’ high cut-point of ‘0.5’ for a significant mean difference to the GLI-2021 norms [approximately equating to a change of 10% for FRC, 8% for IC, 8% for TLC (in older adults), 5% for TLC (in adults aged 30– 50 years), and 30% for ERV and RV (older adults) [Citation7]] requires to be further assessed for its relevance in clinical medicine, as well as in epidemiological studies. Moreover, the relationships between z-scores and age, height, weight, BMI, and sex were examined. The presence of any such relationship was in favour of the non-validity of the GLI-2021 norms.
4.2. How well did the GLI-2012 norms fit contemporary Algerian spirometric data?
Our results related to spirometric data confirm our previous conclusion [Citation10] that GLI-2012 norms [Citation6] are valid to interpret FEV1, FVC, and FEV1/FVC but not FEF25-75%. This point was largely discussed [Citation10].
4.3. How well did the GLI-2021 norms fit contemporary Algerian SLVs data?
According to the GLI Task Force, data from ‘non-European’ ancestries, eg the Arab World, are urgently required to allow the expansion of the GLI-2021 norms [Citation7] to the global population. Our results demonstrate that the application of GLI-2021 norms [Citation7] to a contemporary Algerian population provides mixed results. First, it ‘appears’ that the GLI-2021 norms [Citation7] fit well to SVC, IC, ERV, and TLC in Algerian adults. On the one hand, the above data mean z-scores were in the normal range (), and their observed variability (SD of the z-score) was close to one (), indicating a good overall fit of the GLI-2021 norms [Citation7]. On the other hand, the means of ∆SVC, ∆IC, ∆ERV, and ∆TLC were not ‘clinically’ significant (), and more than 5% of the total sample had ‘clinically’ lower SVC and IC (). Secondly, it is clear that the GLI-2021 norms [Citation7] do not fit to FRC, RV, and RV/TLC in Algerian adults. The above data mean z-scores were out of the normal range (), the means of ∆FRC and RV were ‘clinically’ significant (), and more than 5% of the total sample had ‘clinically’ higher FRC, RV, and RV/TLC ().
In this study, no SLV z-score was related to sex (), and there were ‘fair’ significant correlations only between weight or BMI, and both ERV and FRC (). These results support the use of the GLI-2021 norms [Citation7] to interpret SVC, IC, ERV, and TLC data in the Algerian adult population.
4.4. Why did the GLI-2021 norms fit perfectly well contemporary Algerian SVC, TLC, ERV, and IC data?
The GLI-2021 datasets [Citation7] were obtained from 17 centers in 33 countries, including Tunisia. Although representing only 8.55% of the total sample data, the inclusion of Tunisians could partially explain why the GLI-2021 norms [Citation7] fit contemporary Algerian SVC, TLC, ERV, and IC data.
4.5. Why didn’t the GLI-2021 norms fit contemporary Algerian FRC, RV, and RV/TLC data?
Four explanations could be advanced. The first is related to the impact of ethnicity on LFPs [Citation35]. In fact, the great majority (>90%) of the observations included in the GLI-2021 norms [Citation7] came from individuals having a ‘European’ ancestry. This point was clearly foreseen by the GLI Task Force noting that the derived GLI-2021 norms may not be appropriate for individuals of ‘non-European’ ancestry [Citation7]. The second explanation is related to the effects of parity on SLVs [Citation4,Citation14]. A previous Algerian study [Citation14] reported that compared to females with a parity ≤ 6, those with a parity > 6 had higher FRC and RV. Moreover, compared to females with a parity ≤ 4, those with a parity > 9 had higher RV. The third explanation is linked to the effects of obesity on SLVs [Citation4,Citation36,Citation37]. In our study, 60.9% of adults were overweight or obese, and BMI was negatively associated with some SLVs (e.g. the ‘r’ between BMI and FRC was significant at −0.33). BMI gain causes an accelerated decline in LFPs [Citation38], and induces a decrease in FRC [Citation4,Citation36,Citation37]. Moreover, reduced FRC or RV has been described in overweight and obese individuals [Citation39]. In the GLI-2021 study [Citation7], overweight and obese adults had lower FRC z-scores. However, it is necessary to relativize our findings. In fact, in the GLI-2021 study [Citation7], the FRC coefficient of variation was 20%, which is comparable to a range of normal of 60–140%, much wider than the commonly used threshold of 80–120%. Therefore, if the 140% threshold is applied as an ULN, the percentage of Algerian with higher FRC diminishes from 10.60% (FRC z-score > + 1.645, ) to 8.52%. The fourth hypothesis is related to the technique(s) applied for FRC measurements (ie; plethysmography (this study) vs. plethysmography and gas dilution (GLI-2021) [Citation7]). On the one hand, it is recognized that individual SLVs, measured by gas dilution and the plethysmography technique are not exchangeable [Citation18]. On the other hand, GLI-2021 primary analyses of the FRC values reported significant overlap between plethysmography and gas dilution techniques across all ages [Citation7]. In the GLI-2021 study [Citation7], the relative differences in FRC between plethysmography and gas dilution techniques were < 120 mL, which were within the limits of technical precision [Citation18].
4.6. Study limitations
This study presented three limitations. The first concerns the rational of the study itself. According to the GLI Task Force [Citation7], SLVs norms are limited to people of ‘European’ ancestry. As these different ethnic/racial groups do not appear to have LFPs that fit the same prediction formulae [Citation35], it is ‘irrational’ to compare the Algerian SLVs data with those of individuals of a ‘European’ ancestry. However, taking into consideration, that Tunisia participated in the GLI-2021 study [Citation7], and that both Tunisian and Algerian populations have similar ethnic backgrounds, the study becomes ‘rational’. Like Tunisians, Algerians are mainly genetically descendants of native Berber groups, with some Punic and Middle Eastern input [Citation40,Citation41]. To a lesser degree, Algerians are descendants of other North-African and/or European people [Citation40,Citation41]. Nowadays, the approach to compute the normal values using cross-sectional reference equations that include terms for ethnicity/race is of uncertain clinical benefit and may highlight inequalities [Citation42]. It appears that there is no evidence that ethnic/race-based spirometry norms improved the prediction of clinical events compared to ethnic/race-neutral norms [Citation42]. Therefore, the inclusion of ethnicity/race in spirometry norms should be reconsidered [Citation42]. The second limitation is related to the convenience sampling whose primary weaknesses are the hazard that the sample might not characterize the population as a whole, and the results might propose a bias in the answers from volunteers [Citation43]. On the one hand, due to our atypical method of inclusion, our results cannot be generalized to the target population because of the likely bias of the sampling procedure due to under-representation of subgroups in the sample in comparison to the population of interest. On the other hand, our convenience sampling is characterized with unsatisfactory power to recognize differences of population subcategories [Citation44]. However, the convenience sampling is the most often used due to the numerous advantages it provides: method extremely speedy, easy, readily available, and cost-effective [Citation43]. The third limitation concerns the non-determination of the individuals’ socioeconomic levels and/or occupational status. However, this omission might not have influenced the results, since there were no significant differences in some SLVs (eg; SVC, FRC, TLC, and RV) depending on the general socioeconomic status [Citation4].
4.7. Recommendations
First, since the GLI data repository accepts additional datasets from individuals of ‘non-European’ ancestry, Algerian SLVs data will be submitted to that repository. Second, in order to simplify comparative studies between countries, and to avoid mistakes due to age-related gaps in norms [Citation45], the authors acclaim the implementation of the GLI-2021 norms [Citation7] in healthcare in Algeria. However, as recommended by the GLI Task Force, if GLI-2021 norms [Citation7] are applied to Algerians, this should be clearly stated to ensure that results are not misinterpreted.
To conclude, our results, related to the validity of the GLI-2021 norms [Citation7] in healthy Algerian adults, are mixed. They fit well to SVC, TLC, ERV, and IC, but they do not fit to FRC, RV, and RV/TLC. However, although imperfect, the authors support the use of the GLI-2021 norms [Citation7] to interpret clinical and research results in contemporary Algerian adults.
Abbreviations
BMI: body mass index
ERV: expiratory reserve volume
FEF25-75%: forced expiratory flow at 25–75% of FVC
FEV1: forced expiratory volume in 1 second
FRC: functional residual capacity
FVC: forced vital capacity
GLI: global lung function initiative
IC: inspiratory capacity
LFP: lung function parameter
LLN: lower limit of normal
r: Pearson correlation-coefficient
RV: residual volume
SD: standard deviation
SLV: static lung volume
SVC: slow vital capacity
TLC: total lung capacity
ULN: upper limit of normal
∆: delta
Authors’ contributions
Abdelbassat Ketfi conceived the study, participated in its design, performed the spirometry tests and the statistical analysis, and helped to draft the manuscript.
Helmi Ben Saad conceived the study, participated in its design, performed the statistical analysis, helped to draft the manuscript and coordinated the study.
Both authors read and approved the final version of the manuscript.
Acknowledgments
Authors wish to thank Professor Samir BOUKATTAYA for his invaluable contribution in the improvement of the quality of the writing in the present article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pellegrino R. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–12.
- Ben Saad H. Interpretation of respiratory functional explorations of deficiency and incapacity in adult. Tunis Med. 2020;98:797–815.
- Solberg HE, Grasbeck R. Reference values. Adv Clin Chem. 1989;27:1–79.
- Ben Saad H, Tfifha M, Harrabi I, et al. Factors influencing pulmonary function in Tunisian women aged 45 years and more. Rev Mal Respir. 2006;23:324–338.
- Miller MR, Quanjer PH, Swanney MP, et al. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest. 2011;139:52–59.
- Quanjer PH, Stanojevic S, Cole TJ, et al.; Initiative ERSGLF. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343.
- Hall GL, Filipow N, Ruppel G, et al., contributing GLINm. Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J. 2021;57(3):2000289.
- Ben Saad H, El Attar MN, Hadj Mabrouk K, et al. The recent multi-ethnic global lung initiative 2012 (GLI2012) reference values don’t reflect contemporary adult’s North African spirometry. Respir Med. 2013;107:2000–2008.
- Hall GL, Thompson BR, Stanojevic S, et al. The global lung initiative 2012 reference values reflect contemporary Australasian spirometry. Respirology. 2012;17:1150–1151.
- Ketfi A, Gharnaout M, Bougrida M, et al. The multi-ethnic global lung initiative 2012 (GLI-2012) norms reflect contemporary adult’s Algerian spirometry. PloS One. 2018;13:e0203023.
- Bougrida M, Ben Saad H, Kheireddinne Bourahli M, et al. Spirometric reference equations for Algerians aged 19 to 73 years. Rev Mal Respir. 2008;25:577–590.
- Ketfi A, Gharnaout M, Ben Saad H. The plethysmographic reference equations established for adult natives of Eastern Algeria are not applicable to natives of Northern Algeria. Rev Mal Respir. 2019;36:870–879.
- Rigby RA, Stasinopoulos DM. Smooth centile curves for skew and kurtotic data modelled using the Box-Cox power exponential distribution. Stat Med. 2004;23:3053–3076.
- Ketfi A, Triki L, Gharnaout M, et al. The impacts of parity on lung function data (LFD) of healthy females aged 40 years and more issued from an upper middle income country (Algeria): a comparative study. PloS One. 2019;14:e0225067.
- Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis. 1978;118:1–120.
- Tsai AG, Wadden TA. In the clinic: obesity. Ann Intern Med. 2013;159:ITC3-1-ITC3-15;quiz ITC13-16.
- MR M, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338.
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522.
- Ben Saad H. Review of the current use of global lung function initiative norms for spirometry (GLI-2012) and static lung volumes (GLI-2021) in Great Arab Maghreb (GAM) countries and steps required to improve their utilization. Libyan J Med. 2022;17(1):2031596.
- Global lung function initiative calculators for spirometry, TLCO and lung volume. Availabe from this URL: http://gli-calculator.ersnet.org/index.html. Date last accessed: December 14th 2021.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310.
- Global lung function initiative. Available from this URL: http://www.lungfunction.org. Date last accessed: March 12th 2022.
- Ben Saad H. Promoting the inclusion of lung volumes in the reversibility evaluation. Respir care. 2017;62:255–256.
- Ben Saad H, Prefaut C, Tabka Z, et al. The forgotten message from gold: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulm Pharmacol Ther. 2008;21:767–773.
- O’Donnell DE, Laveneziana P. Lung hyperinflation in COPD: the impact of pharmacotherapy. Eur Respir Rev. 2006;15:85–89.
- Ben Saad H, Ben Amor L, Ben Mdalla S, et al. The importance of lung volumes in the investigation of heavy smokers. Rev Mal Respir. 2014;31:29–40.
- Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. In: Boston: Houghton Mifflin. 2003. p. 750–756. https://www.worldcat.org/title/applied-statistics-for-the-behavioral-sciences/oclc/50716608?page=citation
- Backman H, Lindberg A, Sovijarvi A, et al. Evaluation of the global lung function initiative 2012 reference values for spirometry in a Swedish population sample. BMC Pulm Med. 2015;15:26.
- Hassel E, Stensvold D, Halvorsen T, et al. Association between pulmonary function and peak oxygen uptake in elderly: the Generation 100 study. Respir Res. 2015;16:156.
- Waschki B, Kirsten AM, Holz O, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:295–306.
- Quanjer PH, Stocks J, Cole TJ, et al. Influence of secular trends and sample size on reference equations for lung function tests. Eur Respir J. 2011;37:658–664.
- Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4:8–11.
- Liptzin DR, Landau LI, Taussig LM. Sex and the lung: observations, hypotheses, and future directions. Pediatr Pulmonol. 2015;50:1159–1169.
- Atek M, Traissac P, El Ati J, et al. Obesity and association with area of residence, gender and socio-economic factors in Algerian and Tunisian adults. PloS One. 2013;8:e75640.
- Bibi H, Goldsmith JR, Vardi H. Racial or ethnic variation in spirometric lung function norms. Recommendations based on study of Ethiopian Jews. Chest. 1988;93:1026–1030.
- Littleton SW, Tulaimat A. The effects of obesity on lung volumes and oxygenation. Respir Med. 2017;124:15–20.
- Mandal S, Hart N. Respiratory complications of obesity. Clin Med (Lond). 2012;12:75–78.
- Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993;48:375–380.
- Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833.
- Holt P, Lambton A, Lewis B. The Cambridge History of Islam. CU:Press. editor 1977.
- Ben Saad H. Do Tunisians have a European ancestry? Eur Respir J. 2021;58:210076.
- Elmaleh-Sachs A, Balte P, Oelsner EC, et al. Race/ethnicity, spirometry reference equations and prediction of incident clinical events: the multi-ethnic study of atherosclerosis (Mesa) lung study. Am J Respir Crit Care Med. 2021;in press. doi:10.1164/rccm.202107-1612OC.
- Teddlie C, Yu F. Mixed methods sampling: a typology with examples. J Mixed Methods Res. 2007;1(1):77–100.
- Bornstein MH, Jager J, Putnick DL. Sampling in developmental science: situations, shortcomings, solutions, and standards. Developmental review. 2013;33(4):357–370.
- Kammoun R, Ghannouchi I, Rouatbi S, et al. Defining and grading an obstructive ventilatory defect (OVD): ‘FEV1/FVC lower limit of normal (LLN) vs. Z-score’ and ‘FEV1 percentage predicted (%pred) vs. Z-score’. Libyan J Med. 2018;13:1487751.