ABSTRACT
In nature, bacteria predominantly reside in structured, surface-attached communities embedded in a self-produced, extracellular matrix. These so-called biofilms play an important role in the development and pathogenesis of many infections, as they are difficult to eradicate due to their resistance to antimicrobials and host defense mechanisms. This review focusses on the biofilm-forming periodontal bacterium Porphyromonas gingivalis. Current knowledge on the virulence mechanisms underlying P. gingivalis biofilm formation is presented. In addition, oral infectious diseases in which P. gingivalis plays a key role are described, and an overview of conventional and new therapies for combating P. gingivalis biofilms is given. More insight into this intriguing pathogen might direct the development of better strategies to combat oral infections.
Introduction
Biofilms are aggregates of microorganisms adherent to each other and/or to a surface and encapsulated within a self-produced matrix [Citation1]. These organized communities represent a significant health risk due to their resistance to host defense mechanisms and their decreased susceptibility to conventional antimicrobials [Citation1,Citation2]. Biofilm-mediated resistance has been attributed to impaired penetration of antimicrobials through the matrix, increased expression of drug-resistance genes, and reduced metabolic activity of cells residing in the biofilm [Citation3]. Because of their involvement in >80% of all bacterial infections in humans, biofilms have been the subject of intensive research for many years [Citation4].
The oral cavity provides a habitat for approximately 700 microbial species forming complex and dynamic multispecies biofilms, also referred to as ‘dental plaque’ [Citation5,Citation6]. The oral Gram-negative anaerobic bacteria P. gingivalis is typically a late colonizer of subgingival biofilms and has been correlated with several destructive periodontal diseases, including periodontitis and peri-implantitis [Citation7–Citation9]. P. gingivalis’ pathogenicity is reflected in an arsenal of virulence factors involved in tissue colonization and destruction, and interference with host defense systems [Citation10,Citation11].
In this review, an overview of the current knowledge on P. gingivalis biofilm formation is first presented. Next, biofilm infections in which P. gingivalis plays a key role are described, and finally conventional treatment strategies and new approaches to combat P. gingivalis biofilms are discussed.
Biofilm formation by P. gingivalis
Biofilm development is a complex, dynamic, multistage process [Citation1]. Initially, bacteria adhere to abiotic or biotic surfaces by production of surface appendages (initial adherence). Next, biofilms mature by the development of a three-dimensional structure containing microcolonies in which different species can interact with each other (biofilm maturation). In the last phase, cells disperse from the biofilm, allowing the formation of new biofilms (biofilm dispersal) [Citation1,Citation12]. Novel strategies to treat P. gingivalis infections benefit from thorough insight into the virulence mechanisms underlying biofilm formation. However, to date, knowledge on the molecular basis of biofilm formation by P. gingivalis is largely fragmentary. Approximately 18% of the P. gingivalis genome is differentially expressed when the bacteria is grown as a biofilm, demonstrating the complexity of biofilm development [Citation13]. Below, we describe the involvement of surface structures, quorum sensing, heme uptake, and nutritional interactions in in vitro biofilm formation by this pathogen ().
Figure 1. Determinants involved in biofilm formation by Porphyromonas gingivalis. A schematic representation of the involvement of surface structures (fimbriae, lipopolysaccharides, internalines, and capsules), quorum sensing (LuxS/AI-2), and heme uptake (gingipains, hemagglutinins and HmuY/HmuR) in in vitro biofilm formation.
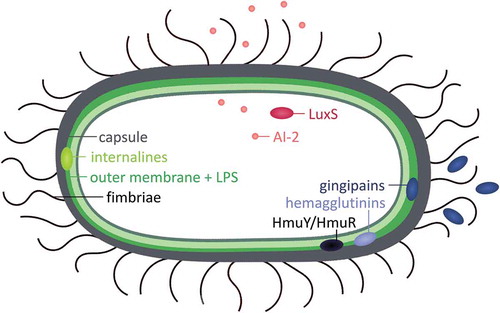
Role of surface structures in biofilm formation
Given the wide variety of substrates to which P. gingivalis can attach in the oral cavity (e.g. oral soft tissues, implant materials, and other bacteria), many extracellular structures play a role in mediating specific and stable substrate attachment. Examples include fimbriae, lipopolysaccharides (LPS), internalines, and capsules.
Fimbriae are proteinaceous appendages that are anchored to the outer membrane and play a role in biofilm formation (). P. gingivalis is known to express two types of fimbriae: long fimbriae, which are composed of FimA subunits, and short fimbriae, which are composed of Mfa1 subunits [Citation14]. Loss of FimA results in reduced adherence to human gingival fibroblasts and epithelial cells, demonstrating that FimA fimbriae play a role in the initial attachment of bacteria to host cells [Citation15,Citation16]. Furthermore, long fimbriae are involved in P. gingivalis auto-aggregation [Citation17,Citation18] and P. gingivalis co-aggregation with Actinomyces viscosus [Citation19], Treponema denticola [Citation20], Streptococcus gordonii [Citation21], and Streptococcus oralis [Citation22]. Of note, mutants deficient in gtfA, a putative glycosyltransferase gene (PG0750), are affected in biofilm development. These mutants lack mature FimA fimbriae but have an unchanged fimA expression, indicating that sugar transfer is involved in fimbriae development [Citation23].
Figure 2. Overview of interactions of P. gingivalis fimbriae FimA and Mfa1 with epithelial cells and other bacteria. A question mark indicates a hitherto unclear effect or interaction.
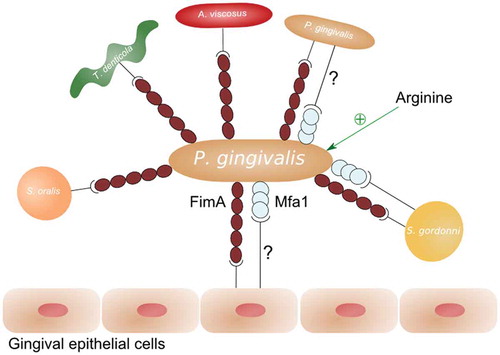
Little is known about the role of Mfa1 in P. gingivalis biofilm formation. Short fimbriae are involved in P. gingivalis co-aggregation with S. gordonii [Citation24]. On the other hand, Mfa1-deficient mutants were reported to display enhanced auto-aggregation [Citation18]. The latter is contradicted by an earlier report showing that short fimbriae are required for P. gingivalis auto-aggregation [Citation25]. Supporting these findings, elevated expression of short fimbriae in a ClpXP-deficient strain results in increased P. gingivalis biofilm formation [Citation26].
Recent studies have illustrated the importance of extracellular arginine in fimbriae-mediated biofilm formation. P. gingivalis is unable to form microcolonies with Streptococcus cristatus as a result of a downregulation of fimA expression by streptococcal ArcA, which catalyzes the hydrolysis of arginine to citrulline [Citation27]. Similarly, ArcA from Streptococcus intermedius represses FimA and Mfa1 production in P. gingivalis [Citation28]. Finally, the addition of arginine promotes P. gingivalis biofilm formation [Citation29].
In addition to fimbriae, surface-associated LPS have been shown to mediate P. gingivalis biofilm formation. LPS typically consist of three parts: a lipid A moiety that tethers LPS to the outer membrane, a core oligosaccharide, and an O-antigen polysaccharide [Citation30]. The absence of GalE, which is involved in the synthesis of sugar nucleotides in the Leloir pathway, results in shortened LPS O-antigens and significantly increases P. gingivalis biofilm formation [Citation31].
Furthermore, surface-attached internalines, which are involved in protein–protein interactions, have been shown to play a role in the initial attachment of the bacteria [Citation32]. Indeed, inactivation of the internalin family protein InlJ reduces P. gingivalis monospecies biofilm formation and enhances mixed-species P. gingivalis–S. gordonii biofilm formation [Citation32].
Last, P. gingivalis is known to produce a capsular polysaccharide, which encases the cell surface, thereby masking surface components such as LPS and surface proteins. Interestingly, loss of capsule production positively affects biofilm formation of P. gingivalis [Citation33]. On the other hand, it was reported that P. gingivalis capsules mediate co-aggregation between P. gingivalis and Fusobacterium nucleatum [Citation34].
Role of quorum sensing in biofilm formation
Quorum sensing is a bacterial communication mechanism in which the expression of genes is coordinated through the accumulation of specific signaling molecules [Citation35]. P. gingivalis utilizes the LuxS/Autoinducer-2 (AI-2) quorum sensing system [Citation36,Citation37]. luxS encodes the AI-2 synthase, which cleaves S-ribosylhomocysteine into 4,5-dihydroxy-2,3-pentanedione (DPD). Subsequently, DPD undergoes spontaneous derivatizations, forming signaling molecule AI-2 [Citation38]. This quorum sensing system has been shown to play a role in interspecies communication of P. gingivalis. More specifically, AI-2 was demonstrated to be necessary for the formation of P. gingivalis–S. gordonii mixed biofilms, and AI-2 produced by S. gordonii is able to complement a luxS mutation in P. gingivalis [Citation39]. Similarly, the biovolume of Filifactor alocis–P. gingivalis mixed-species biofilms was significantly reduced with a P. gingivalis luxS-mutant, indicating a role for AI-2 in the interaction between P. gingivalis and F. alocis [Citation40]. In addition, it was shown that AI-2 from Actinobacillus actinomycetemcomitans is capable of complementing a luxS mutation in P. gingivalis [Citation41]. Finally, it was demonstrated that AI-2 from F. nucleatum induced both P. gingivalis monospecies biofilm formation and F. nucleatum–P. gingivalis mixed-species biofilm formation [Citation42].
Role of heme and heme uptake systems in biofilm formation
Iron is an essential growth factor for most bacteria. Unlike many other microorganisms, P. gingivalis does not produce siderophores to sequester and transport iron. Instead, the bacterium utilizes specific proteases such as gingipains and surface-associated proteins to acquire iron from host heme [Citation43].
Proteolytic gingipains Kgp and Rgp play an important role in the acquisition of iron by releasing heme from hemoglobin [Citation43]. In addition, it was found that Kgp suppresses P. gingivalis auto-aggregation, and Rgp mediates microcolony formation and restrains the biovolume [Citation18]. Furthermore, gingipains are involved in adherence of P. gingivalis to epithelial cells and in co-aggregation of P. gingivalis with T. denticola, Prevotella intermedia, and S. aureus [Citation44–Citation46].
Surface-expressed hemagglutinins mediate the acquisition of heme through erythrocyte binding [Citation47]. Furthermore, the heme-binding outer-membrane-associated lipoprotein HmuY and its cognate receptor HmuR are involved in the capture and internalization of heme [Citation48]. Deletion of hmuY or the hemagglutinin gene hagC results in reduced levels of biofilm, suggesting a role in biofilm formation [Citation49,Citation50]. In addition, the hemin-associated transcriptional regulator Har, which controls the expression of hmuY and mfa1, was found to be a positive regulator of biofilm formation [Citation51]. This study also demonstrated that heme-limitation per se decreases P. gingivalis biofilm formation and development [Citation51].
Role of nutritional interactions in biofilm formation
Nutritional interactions have been described to play a role in the co-existence of P. gingivalis and T. denticola. A study revealed that P. gingivalis produces isobutyric acid, which enhances growth of T. denticola, while T. denticola produces succinate that positively affects growth of P. gingivalis [Citation52]. This may explain the finding that P. gingivalis and T. denticola show enhanced planktonic and biofilm growth when they are cultured together in comparison to mono-species growth [Citation52,Citation53].
Treatment of P. gingivalis–related infections
P. gingivalis is one of the most prevalent bacteria in periodontitis, a chronic inflammatory disease of the oral cavity [Citation8]. This disease is characterized by destruction of the supporting structures of the teeth (i.e. the gingiva, the periodontal ligament, and the alveolar bone) and can eventually lead to loss of teeth [Citation54]. Furthermore, periodontitis has recently been associated with an increased risk for delivery of premature labor and low-birth-weight infants [Citation55]. The prevalence of periodontitis is high, with the moderate form affecting up to 46% and the severe form 8.9% of the US population [Citation56]. Smoking and diabetes are known major risk factors for periodontitis [Citation57].
P. gingivalis is also recognized as a keystone pathogen in peri-implantitis, a periodontal disease characterized by inflammation of the hard and soft tissues surrounding dental implants. When left untreated, peri-implantitis can result in loss of the dental implant [Citation9,Citation58]. Studies have reported a considerably high prevalence of peri-implantitis, with estimations ranging from 20% to 56% of the patients, depending on the time frame under investigation [Citation59,Citation60]
Current strategies
Treatment procedures of P. gingivalis–mediated diseases such as periodontitis and peri-implantitis focus on the eradication of oral pathogens at the site of infection, usually by surface debridement procedures followed by adjunctive therapies, including the use of antiseptics or/and antibiotics [Citation61–Citation66].
The antiseptic chlorhexidine has been widely used in dental practice because of its broad-spectrum antimicrobial activity [Citation67]. Local application of chlorhexidine can be done in the form of gels, chips, mouthwashes, or films [Citation68–Citation71]. Despite its widespread use, some limitations have been reported, including brown discoloration of the teeth, alteration in taste, supraginigival calculus formation, and, more rarely, oral mucosal erosion and parotid swelling. Additionally, chlorhexidine is characterized by a bitter taste, contributing to patient non-compliance [Citation72–Citation74].
Several antibiotic classes have also been suggested for the treatment of P. gingivalis–related infections, including tetracyclines (tetracycline hydrochloride, minocycline, doxycycline), macrolides (erythromycin), lincosamides (clindamycin), ß-lactams (ampicillin, amoxicillin), and nitroimidazoles (metronidazole) [Citation64,Citation75–Citation77]. These antibiotics can be administered by either local or systemic routes. Systemically administered antibiotics can penetrate the periodontal tissues and reach deep periodontal pockets via serum. In this way, antibiotics can target oral pathogens that are inaccessible for cleaning instruments or locally applied antibiotics [Citation78]. However, this application route requires good patient compliance, can cause side effects, and can facilitate antibiotic resistance [Citation66,Citation79–Citation81]. Local administration has the advantage that higher therapeutic concentration of antibiotics can be delivered inside the pocket, avoiding some of the side effects of systemic administration.
In the last few years, concerns have been raised about the efficacy of the aforementioned antimicrobials in treatment of oral biofilm-related infections. First, compared with planktonic cells, P. gingivalis cells residing in biofilms are less susceptible to antimicrobials such as chlorhexidine, minocycline, metronidazole, amoxicillin, doxycycline, cefuroxime, ampicillin, and ofloxacin [Citation82–Citation87]. More specifically, biofilms can be up to 500 times less sensitive to antibiotics [Citation87]. Second, several studies have examined the antibiotic susceptibility of subgingival microflora isolated from patients suffering from periodontitis and peri-implantitis. This is illustrated by the finding that 74.2% of patients with periodontitis and 71.7% of patients with peri-implantitis harbor pathogens resistant to at least one standard antibiotic [Citation80,Citation81]. A recent study also reported that 25.49%, 23.52%, and 21.56% of the P. gingivalis strains isolated from patients with periodontitis are resistant to amoxicillin, clindamycin, and metronidazole, respectively [Citation88]. Similarly, periodontitis isolates of P. gingivalis were demonstrated to be resistant against penicillin, amoxicillin, erythromycin, azithromycin, clindamycin, and tetracycline [Citation89].
The current data concerning biofilm-mediated resistance in dental practice, together with the emerging global threat of antimicrobial resistance, have prompted researchers to search for new antimicrobial agents targeting oral pathogens. Newly identified antibacterial agents that show activity against P. gingivalis biofilms are discussed below.
New antibacterial agents
Quorum sensing inhibitors
Quorum sensing inhibitors have been presented as promising alternatives for the treatment of biofilm-related infections, as they do not affect growth and thus have a low potential for resistance development [Citation90]. In this respect, quorum sensing inhibitors (5Z)-4-bromo-5-(bromomethylene)-2(5 H)-furanone (2 mM) and D-ribose (50 mM) have been shown to reduce both P. gingivalis monospecies and F. nucleatum and P. gingivalis mixed-species biofilm development [Citation42]. Furthermore, these agents are not toxic for human monocytic cells and human gingival fibroblasts at tested concentrations, and do not stimulate production of proinflammatory factors. In addition, these inhibitors remain active against P. gingivalis under in vivo conditions, making them suitable candidates for further development into anti–P. gingivalis drugs [Citation91].
Antimicrobial peptides
Antimicrobial peptides are widely proposed as a new source of future antibiotics, as they often have broad-spectrum activity and a low tendency for resistance development [Citation92]. An overview of the currently known antimicrobial peptides that show antibiofilm activity against P. gingivalis is given in .
Table 1. Overview of antimicrobial peptides effective against Porphyromonas gingivalis biofilm formation.
The shortened alanine-substituted peptide AS10, derived from the cathelicidin-related antimicrobial peptide, which was identified in the islets of Langerhans of the murine pancreas, was reported to inhibit P. gingivalis biofilm formation [Citation93]. Agents derived from lactoferrin, an iron-binding host defense antimicrobial protein present in saliva and gingival crevicular fluids, also exhibit antibiofilm activity against P. gingivalis [Citation94]. In addition, Nal-P-113, which is a β-naphthylalanine-substituted derivative of the saliva protein histatin 5, has an effect on P. gingivalis biofilm formation under both in vitro and in vivo conditions, without significant cytotoxicity [Citation95]. Furthermore, a recent study demonstrated that the newly designed peptide Pac-525 has the ability to kill bacteria within P. gingivalis biofilms formed on titanium surfaces at a concentration that does not exert cytotoxic effects against eukaryotic cells [Citation96].
The adhesion of P. gingivalis to primary colonizing bacteria such as S. gordonii has been recognized as an important step in the initial formation of subgingival biofilms. P. gingivalis is known to adhere to S. gordonii through interaction of the short fimbrial antigen Mfa1 with a specific region of the streptococcal SspB polypeptide (designated BAR). In this regard, a recent study has designed the BAR peptide, a synthetic peptide comprising the BAR sequence, which has been reported to inhibit adherence of P. gingivalis to S. gordonii, thereby preventing the formation of P. gingivalis–associated biofilms [Citation97].
Natural sources
Plant-derived antibacterial agents
To overcome the alarming scarcity of new antibiotic classes, several recent studies have focused on finding new antibiotics from unexplored natural sources [Citation98]. In this context, plants have proved to be a good new source for finding new antibacterial agents. This is not surprising, as plants are frequently exposed to bacterial infections and thus have developed various defense mechanisms to combat bacterial pathogens. gives an overview of new antibiotics derived from plants that affect P. gingivalis biofilm formation.
Table 2. Overview of plant-derived compounds effective against P. gingivalis biofilm formation.
The non-dialyzable material fraction of cranberry juice rich in proanthocyanidins and A-type cranberry proanthocyanidins extracted from cranberry juice concentrate were shown to exhibit activity against P. gingivalis biofilms [Citation99,Citation100]. The latter fraction was also found to affect adherence to oral epithelial cells negatively and have anti-inflammatory activities by inhibiting the secretion of interleukin-8 and chemokine ligand 5 [Citation100]. Of note, the activity of these A-type cranberry proanthocyanidin can be increased by combination therapy with Licochalcone A, a major chalcone in licorice root [Citation109].
A number of prenylated flavonoids isolated from Epimedium species were reported to inhibit biofilm formation by P. gingivalis and to interfere with Rgp and Kgp gingipain activity [Citation101].
Lacinartin derived from Citrus fruits and Tea catechin derived from Camellia sinensis have been demonstrated to inhibit biofilm formation of P. gingivalis biofilms and to desorb pre-formed biofilms [Citation102,Citation103]. Furthermore, Lacinartin negatively affected adherence to epithelial cells.
Extracted oils obtained from plants also possess activity against P. gingivalis biofilms. Indeed, essential oils extracted from medicinal and aromatic plants such as Aloysia gratissima, Coriandrum sativum L., Muhlenbergia glomerata, Cyperus articulatus, Lippia sidoides, and from shiitake have been reported to inhibit P. gingivalis biofilm formation [Citation104,Citation105]. Additionally, carvacrol, a monoterpene phenol present in the volatile oils of Thymus vulgaris, Carum copticum, and oreganum species, inhibits P. ginigvalis biofilm formation on titanium implant material [Citation106].
Recent studies revealed the antibiofilm effects of roselle calyx extract and capsaicin, which is the active compound of Capsicum plants (chili peppers) against P. gingivalis [Citation107,Citation108]. The latter also reduces the viability of pre-formed biofilms and has an inhibitory effects on both inflammatory cytokine secretion and in vitro osteoclastogenesis.
Saccharides of marine origin
In recent years, the marine environment has been explored as a source for finding new natural antibacterial agents. In this context, OligoG, which is an oligosaccharide derived from brown algae alginate, was found to reduce biofilm formation of P. gingivalis drastically [Citation110]. Furthermore, treatment of titanium surfaces with triclosan combined with OligoG significantly decreases P. gingivalis attachment to titanium surfaces compared with treatment of the surfaces with triclosan alone.
Chitosan, which is a natural linear polysaccharide derived from chitin present in the exoskeleton of marine crustaceans, has also been reported to have antibiofilm activities against P. gingivalis [Citation111].
Sugar alcohols
Sugar alcohols are commonly used in place of sucrose as non-cariogenic sweeteners. However, little is known about their activity against periodontal bacteria [Citation112]. A recent study reported that the sugar alcohol erythritol effectively inhibits P. gingivalis biofilm formation and reduces P. gingivalis accumulation onto S. gordonii substrata [Citation113]. The authors concluded that erythritol acts via several pathways, including suppression of growth resulting from DNA and RNA depletion, attenuated extracellular matrix production, and alterations of dipeptide acquisition and amino acid metabolism.
Other compounds
Screening of compound libraries has resulted in the identification of new antibacterial agents that show activity against P. gingivalis. We screened a compound library in search for new molecules that exhibit activity against the opportunistic pathogen Pseudomonas aeruginosa [Citation114]. From this screening, a dichlorocarbazol derivative was identified as a new antibacterial agent with broad-spectrum activity, including activity against P. gingivalis biofilms. In another study, a library of small molecules based on 2-aminoimidazole and 2-aminobenzimidazole scaffolds was screened with the aim of identifying compounds that could inhibit co-colonization of P. gingivalis and S. gordonii. In this screening, three small molecules derived from oroidin and containing 2-aminoimidazole or 2 aminobenzimidazole moieties were identified. These compounds inhibit co-colonization by reducing expression of both mfa1 and fimA fimbrial genes in P. gingivalis [Citation115].
Drug repurposing has increasingly been applied over the last years as a strategy to uncover new antibiotics. This strategy has some advantages over de novo drug discovery, including known toxicological and pharmacological profiles, thereby accelerating the drug-development process significantly [Citation116]. In this context, we recently screened a drug-repositioning library (NIH Clinical Collection) to identify new compounds that show activity against P. gingivalis [Citation117]. The screen led to the discovery of three new molecules that show antibiofilm activity against P. gingivalis: zafirlukast, an anti-asthmatic drug, toremifene, an anti-cancer drug, and N-(4-Hydroxyphenyl)arachidonylamide (AM404), an active metabolite of paracetamol [Citation117–Citation119]. The anthelmintic drug oxantel, which is typically used for the treatment of intestinal worms, has also been proven to inhibit biofilm formation by P. gingivalis significantly by inhibition of fumarate reductase. Furthermore, oxantel is more effective than the conventional antibiotic metronidazole in inhibiting P. gingivalis biofilms [Citation120]. In a follow-up study, it was demonstrated that oxantel can disrupt the development of polymicrobial biofilms composed of P. gingivalis, Tannerella forsythia, and T. denticola in a concentration-dependent manner [Citation121].
Antibacterial coatings
The coating of titanium surfaces with antibacterial agents has recently been explored as a new strategy for the prevention of peri-implant infections [Citation122]. A number of studies have investigated the potential of antibacterial peptides to be used in coating applications. Indeed, coatings that are functionalized with GL13K (derived from the human salivary protein Parotid Secretory Protein [BPIFA2]), histatin-5 (belonging to a family of peptides secreted by the major salivary glands), lactoferricin (generated by gastric pepsin cleavage of lactoferrins), and synthetic peptide Tet213 have been demonstrated to strongly reduce P. gingivalis biofilm formation [Citation123–Citation125].
The antibiofilm activity of titanium surfaces coated with silver has also been explored. As such, titanium surfaces coated with silver-hydroxyapatite/titania nanocomposites have been shown to act in both a bactericidal and anti-adhesive way against P. gingivalis [Citation126]. In addition, the potential of silver- and gallium-doped phosphate-based glasses to inhibit growth of P. gingivalis–S. gordonii dual-species biofilms has been demonstrated [Citation127]. Furthermore, a follow-up study showed that the gallium-doped phosphate-based glasses remain active against P. gingivalis under in vivo conditions [Citation128].
In addition, bifunctional coatings with both antibacterial and pro-osteodifferentiation capabilities have been developed. Simvastatin is known to increase the osteogenic capability of mesenchymal stem cells, while metronidazole is an antimicrobial agent that has excellent activity against strict anaerobic bacteria. Integration of these drugs into a calcium phosphate coating for titanium surfaces prevents the growth of P. gingivalis and increases osteogenic cell differentiation [Citation129].
Concluding remarks
P. gingivalis is a notorious pathogen in the development of periodontitis and peri-implantitis. As these infections are biofilm-related, conventional antimicrobials often fail to eradicate the entire biofilm, which results in chronic infections and necessitates surgical removal of infected areas. Thus, there exists a need for the development of new antibacterials to combat biofilm-related infections.
In recent years, a significant number of new compounds with antibiofilm activity against P. gingivalis have been identified. Unfortunately, to our knowledge, only one compound has progressed to clinical trials: the antibacterial peptide lactoferrin [Citation94]. Different factors may explain the limited availability of new antibacterial drugs. For example, in spite of the promising results of the above-mentioned antibacterial peptides, there are still some challenges to their applications, such as potential toxicity, susceptibility to proteases, and high production costs [Citation130]. As for the natural products interfering with P. gingivalis biofilm formation, limited information is currently present on their mode of action and their cytotoxicity. In addition, the active concentrations of some plant-derived compounds are up to 1,000 times higher than conventional antibiotics, indicating limited antibacterial activity [Citation83,Citation84,Citation87]. Regarding the surface coating strategies to prevent biofilm formation on implants, there still exists a great discrepancy between the suggested strategies and their clinical applications [Citation131]. Furthermore, potential limitations of these coatings such as toxicity and hampered antibacterial activity under in vivo conditions should be tackled in future studies [Citation131,Citation132].
Thus, further mode-of-action studies, comprehensive toxicity analyses, and in vivo tests will be necessary to reveal fully the potential of newly discovered antibacterial agents to be used in the treatment of oral infections. In addition, a broader knowledge of the regulatory and molecular mechanisms behind P. gingivalis biofilm formation may further accelerate the development of future strategies for treatment of P. gingivalis–associated infections.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Evelien Gerits
Dr. Evelien Gerits recently obtained her PhD and is now working as a Junior safety and regulatory affairs officer.
Natalie Verstraeten
Dr. Natalie Verstraeten is an early career scientist with an interest in antimicrobial strategies.
Jan Michiels
Prof. Jan Michiels is a Professor in molecular microbiology with a focus on antibiotic-tolerance or persistence, evolutionary dynamics of adaptation to complex phenotypes and bacterium-plant interactions.
References
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1–11.
- De La Fuente-Núñez C, Reffuveille F, Fernández L, et al. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol. 2013;16:580–589.
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138.
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122.
- Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437.
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732.
- Kolenbrander PE, Palmer RJ, Periasamy S, et al. Oral multispecies biofilm development and the key role of cell–cell distance. Nat Rev Microbiol. 2010;8:471–480.
- How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53.
- Mahato N, Wu X, Wang L. Management of peri-implantitis: a systematic review, 2010-2015. Springerplus. 2016;5:105.
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263.
- Holt SC, Kesavalu L, Walker S, et al. Virulence factors of Porphyromonas gingivalis. Periodontol. 2000;1999(20):168–238.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633.
- Lo AW, Seers CA, Boyce JD, et al. Comparative transcriptomic analysis of Porphyromonas gingivalis biofilm and planktonic cells. BMC Microbiol. 2009;9:18.
- Enersen M, Nakano K, Amano A. Porphyromonas gingivalis fimbriae. J Oral Microbiol. 2013;5:1–10.
- Hamada N, Watanabe K, Sasakawa C, et al. Construction and characterization of a fimA mutant of Porphyromonas gingivalis. Infect Immun. 1994;62:1696–1704.
- Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006;85:392–403.
- Kuboniwa M, Amano A, Inaba H, et al. Homotypic biofilm structure of Porphyromonas gingivalis is affected by FimA type variations. Oral Microbiol Immunol. 2009;24:260–263.
- Kuboniwa M, Amano A, Hashino E, et al. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 2009;9:105.
- Goulbourne PA, Ellen RP. Evidence that Porphyromonas (Bacteroides) gingivalis fimbriae function in adhesion to Actinomyces viscosus. J Bacteriol. 1991;173:5266–5274.
- Hashimoto M, Ogawa S, Asai Y, et al. Binding of Porphyromonas gingivalis fimbriae to Treponema denticola dentilisin. FEMS Microbiol Lett. 2003;226:267–271.
- Lamont RJ, Bevan CA, Gil S, et al. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993;8:272–276.
- Maeda K, Nagata H, Yamamoto Y, et al. Glyceraldehyde-3-Phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect Immun. 2004;72:1341–1348.
- Narimatsu M, Noiri Y, Itoh S, et al. Essential role for the gtfA gene encoding a putative glycosyltransferase in the adherence of Porphyromonas gingivalis. Infect Immun. 2004;72:2698–2702.
- Park Y, Simionato MR, Sekiya K, et al. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2004;73:3983–3989.
- Lin X, Wu J, Xie H. Porphyromonas gingivalis minor fimbriae are required for cell-cell interactions. Infect Immun. 2006;74:6011–6015.
- Capestany CA, Tribble GD, Maeda K, et al. Role of the Clp system in stress tolerance, biofilm formation, and intracellular invasion in Porphyromonas gingivalis. J Bacteriol. 2008;190:1436–1446.
- Wang B, Wu J, Lamont RJ, et al. Negative correlation of distributions of Streptococcus cristatus and Porphyromonas gingivalis in subgingival plaque. J Clin Microbiol. 2009;47:3902–3906.
- Christopher AB, Arndt A, Cugini C, et al. A streptococcal effector protein that inhibits Porphyromonas gingivalis biofilm development. Microbiology. 2010;156:3469–3477.
- Cugini C, Stephens DN, Nguyen D, et al. Arginine deiminase inhibits Porphyromonas gingivalis surface attachment. Microbiology. 2013;159:275–285.
- Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res. 2005;84:584–595.
- Nakao R, Senpuku H, Watanabe H. Porphyromonas gingivalis galE is involved in lipopolysaccharide O-antigen synthesis and biofilm formation. Infect Immun. 2006;74:6145–6153.
- Capestany CA, Kuboniwa M, Jung I-Y, et al. Role of the Porphyromonas gingivalis InlJ protein in homotypic and heterotypic biofilm development. Infect Immun. 2006;74:3002–3005.
- Davey ME, Duncan MJ. Enhanced biofilm formation and loss of capsule synthesis: deletion of a putative glycosyltransferase in Porphyromonas gingivalis. J Bacteriol. 2006;188:5510–5523.
- Rosen G, Sela MN. Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol Lett. 2006;256:304–310.
- Saini R, Saini S, Sharma S. Biofilm: a dental microbial infection. J Nat Sci Biol Med. 2011;2:71–75.
- Chung WO, Park Y, Lamont RJ, et al. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J Bacteriol. 2001;183:3903–3909.
- Burgess NA, Kirke DF, Williams P, et al. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology. 2002;148:763–772.
- Schauder S, Shokat K, Surette MG, et al. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2016;4:463–476.
- McNab R, Ford SK, El-Sabaeny A, et al. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J Bacteriol. 2003;185:274–284.
- Wang Q, Wright CJ, Dingming H, et al. Oral community interactions of Filifactor alocis in vitro. Plos One. 2013;8:e76271.
- Fong KP, Chung WO, Lamont RJ, et al. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect Immun. 2001;69:7625–7634.
- Jang YJ, Choi YJ, Lee SH, et al. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol. 2013;58:17–27.
- Olczak T, Simpson W, Liu X, et al. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 2005;29:119–144.
- Zhu Y, Dashper SG, Chen -Y-Y, et al. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. Plos One. 2013;8:e71727.
- Kamaguchi A, Nakayama K, Ichiyama S, et al. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. 2003;47:485–491.
- Chen T, Nakayama K, Belliveau L, et al. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect Immun. 2001;69:3048–3056.
- Han N, Whitlock J, Progulske-Fox A. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect Immun. 1996;64:4000–4007.
- Olczak T, Sroka A, Potempa J, et al. Porphyromonas gingivalis HmuY and HmuR: further characterization of a novel mechanism of heme utilization. Arch Microbiol. 2008;189:197–210.
- Connolly E, Millhouse E, Doyle R, et al. The Porphyromonas gingivalis haemagglutinins HagB and HagC are major mediators of adhesion and biofilm formation. Mol Oral Microbiol. 2015. DOI:10.1111/omi.12151
- Olczak T, Wójtowicz H, Ciuraszkiewicz J, et al. Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol. 2010;10:134.
- Butler CA, Dashper SG, Zhang L, et al. The Porphyromonas gingivalis ferric uptake regulator orthologue binds hemin and regulates hemin-responsive biofilm development. Plos One. 2014;9:e111168.
- Grenier D. Nutritional interactions between two suspected periodontopathogens, Treponema denticola and Porphyromonas gingivalis. Infect Immun. 1992;60:5298–5301.
- Yamada M, Ikegami A, Kuramitsu HK. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol Lett. 2005;250:271–277.
- Philstrom BL. Periodontal risk assessment, diagnosis and treatment planning. Periodontol 2000. 2001;25:37–58.
- Saini R, Saini S, Saini SR. Periodontitis: A risk for delivery of premature labor and low-birth-weight infants. J Nat Sci Biol Med. 2010;1:40–42.
- Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622.
- Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31.
- Ata-Ali J, Candel-Marti ME, Flichy-Fernández AJ, et al. Peri-implantitis: associated microbiota and treatment. Med Oral Patol Oral Cir Bucal. 2011;16:e937–e943.
- Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35:286–291.
- Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23:67–76.
- Prakasam A, Elavarasu SS, Natarajan RK. Antibiotics in the management of aggressive periodontitis. J Pharm Bioallied Sci. 2012;4:S252–S255.
- Herrera D, Sanz M, Jepsen S, et al. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29:136–159.
- Xajigeorgiou C, Sakellari D, Slini T, et al. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. J Clin Periodontol. 2006;33:254–264.
- Van Winkelhoff AJ. Antibiotics in the treatment of peri-implantitis. Eur J Oral Implantol. 2012;5:43–50.
- Prathapachandran J, Suresh N. Management of peri-implantitis. Dent Res J (Isfahan). 2012;9:516–521.
- Quirynen M, Teughels W, Van Steenberghe D. Microbial shifts after subgingival debridement and formation of bacterial resistance when combined with local or systemic antimicrobials. Oral Dis. 2003;9:30–37.
- Balagopal S, Arjunkumar R. Chlorhexidine: the gold standard antiplaque agent. J Pharm Sci Res. 2013;5:270–274.
- Paolantonio M, Perinetti G, D’Ercole S, et al. Internal decontamination of dental implants: an in vivo randomized microbiologic 6-month trial on the effects of a chlorhexidine gel. J Periodontol. 2008;79:1419–1425.
- Soskolne WA, Chajek T, Flashner M, et al. An in vivo study of the chlorhexidine release profile of the PerioChipTM in the gingival crevicular fluid, plasma and urine. J Clin Periodontol. 1998;25:1017–1021.
- McBain AJ, Bartolo RG, Catrenich CE, et al. Effects of a chlorhexidine gluconate-containing mouthwash on the vitality and antimicrobial susceptibility of in vitro oral bacterial ecosystems. Appl Environ Microbiol. 2003;69:4770–4776.
- Senel S, Ikinci G, Kaş S, et al. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193:197–203.
- Eley BM. Antibacterial agents in the control of supragingival plaque – a review. Br Dent J. 1999;186:286–296.
- Kolahi J, Soolari A. Rinsing with chlorhexidine gluconate solution after brushing and flossing teeth: a systematic review of effectiveness. Quintessence Int. 2006;37:605–612.
- Van Strydonck DA, Slot DE, Van der Velden U, et al. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol. 2012;39:1042–1055.
- Kapoor A, Malhotra R, Grover V, et al. Systemic antibiotic therapy in periodontics. Dent Res J (Isfahan). 2012;9:505–515.
- Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29:325–345.
- Keestra JA, Grosjean I, Coucke W, et al. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated aggressive periodontitis: a systematic review and meta-analysis. J Periodontal Res. 2015;50:689–706.
- Slots J, Ting M. Systemic antibiotics in the treatment of periodontal diseases. Periodontol. 2002;28:106–176.
- Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2016;71:82–112.
- Rams TE, Degener JE, van Winkelhoff AJ. Antibiotic resistance in human peri-implantitis microbiota. Clin Oral Implants Res. 2014;25:82–90.
- Rams TE, Degener JE, van Winkelhoff AJ. Antibiotic resistance in human chronic periodontitis microbiota. J Periodontol. 2014;85:160–169.
- Yamaguchi M, Noiri Y, Kuboniwa M, et al. Porphyromonas gingivalis biofilms persist after chlorhexidine treatment. Eur J Oral Sci. 2013;121:162–168.
- Noiri Y, Okami Y, Narimatsu M, et al. Effects of Chlorhexidine, Minocycline, and Metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J Periodontol. 2003;74:1647–1651.
- Larsen T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol Immunol. 2002;17:267–271.
- Asahi Y, Noiri Y, Igarashi J, et al. Synergistic effects of antibiotics and an N-acyl homoserine lactone analog on Porphyromonas gingivalis biofilms. J Appl Microbiol. 2012;112:404–411.
- Shen Y, Stojicic S, Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod. 2011;37:657–661.
- Maezono H, Noiri Y, Asahi Y, et al. Antibiofilm effects of azithromycin and erythromycin on Porphyromonas gingivalis. Antimicrob Agents Chemother. 2011; 55:5887–5892.
- Ardila CM, Granada MI, Guzmán IC. Antibiotic resistance of subgingival species in chronic periodontitis patients. J Periodontal Res. 2010;45:557–563.
- van Winkelhoff AJ, Herrera Gonzales D, Winkel EG, et al. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol. 2000;27:79–86.
- Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245.
- Cho YJ, Song HY, Ben Amara H, et al. In vivo inhibition of Porphyromonas gingivalis growth and prevention of periodontitis with quorum-sensing inhibitors. J Periodontol. 2016;2:1075–1082.
- Lohan S, Bisht GS. Recent approaches in design of peptidomimetics for antimicrobial drug discovery research. Mini Rev Med Chem. 2013;13:1073–1088.
- De Brucker K, Delattin N, Robijns S, et al. Derivatives of the mouse cathelicidin-related antimicrobial peptide (CRAMP) inhibit fungal and bacterial biofilm formation. Antimicrob Agents Chemother. 2014;58:5395–5404.
- Wakabayashi H, Yamauchi K, Kobayashi T, et al. Inhibitory effects of lactoferrin on growth and biofilm formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob Agents Chemother. 2009;53:3308–3316.
- Wang H-Y, Cheng J-W, Yu H-Y, et al. Efficacy of a novel antimicrobial peptide against periodontal pathogens in both planktonic and polymicrobial biofilm states. Acta Biomater. 2015;25:150–161.
- Li J, Wang X, Wang L, et al. High in vitro antibacterial activity of Pac-525 against Porphyromonas gingivalis biofilms cultured on titanium. Biomed Res Int. 2015;2015:909870–909878.
- Daep CA, James DM, Lamont RJ, et al. Structural characterization of peptide-mediated inhibition of Porphyromonas gingivalis biofilm formation. Infect Immun. 2006;74:5756–5762.
- Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discov. 2013;12:371–387.
- Labrecque J, Bodet C, Chandad F, et al. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J Antimicrob Chemother. 2006;58:439–443.
- La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 2010;54:1778–1784.
- Kariu T, Nakao R, Ikeda T, et al. Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. J Periodontal Res. 2016. DOI:10.1111/jre.12372
- Marquis A, Genovese S, Epifano F, et al. The plant coumarins auraptene and lacinartin as potential multifunctional therapeutic agents for treating periodontal disease. BMC Complement Altern Med. 2012;12:80.
- Asahi Y, Noiri Y, Miura J, et al. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J Appl Microbiol. 2014;2014(116):1164–1171.
- Bersan SMF, Galvão LCC, Goes VFF, et al. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement Altern Med. 2014;14:451.
- Solmaz G, Ozen F, Ekinci Y, et al. Inhibitory and disruptive effects of shiitake mushroom (Lentinula edodes) essential oil extract on oral biofilms. Jundishapur J Microbiol. 2013;6:1–6.
- Ciandrini E, Campana R, Federici S, et al. In vitro activity of Carvacrol against titanium-adherent oral biofilms and planktonic cultures. Clin Oral Investig. 2014;18:2001–2013.
- Sulistyani H, Fujita M, Miyakawa H, et al. Effect of roselle calyx extract on in vitro viability and biofilm formation ability of oral pathogenic bacteria. Asian Pac J Trop Med. 2016;9:119–124.
- Zhou Y, Guan X, Zhu W, et al. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur J Clin Microbiol Infect Dis. 2014;33:211–219.
- Feldman M, Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol. 2012;113:438–447.
- Roberts JL, Khan S, Emanuel C, et al. An in vitro study of alginate oligomer therapies on oral biofilms. J Dent. 2013;41:892–899.
- Costa EM. Antimicrobial effect of chitosan against periodontal pathogens biofilms. SOJ Microbiol Infect Dis. 2014;2:1–6.
- Van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 2004;38:286–293.
- Hashino E, Kuboniwa M, Alghamdi SA, et al. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol Oral Microbiol. 2013;28:435–451.
- Liebens V, Gerits E, Knapen WJ, et al. Identification and characterization of an anti-pseudomonal dichlorocarbazol derivative displaying anti-biofilm activity. Bioorg Med Chem Lett. 2014;24:5404–5408.
- Wright CJ, Wu H, Melander RJ, et al. Disruption of heterotypic community development by Porphyromonas gingivalis with small molecule inhibitors. Mol Oral Microbiol. 2014;29:185–193.
- Padhy BM, Gupta YK. Drug repositioning: re-investigating existing drugs for new therapeutic indications. J Postgrad Med. 2011;57:153–160.
- Gerits E, Van Der Massen I, Vandamme K, et al. In vitro activity of the antiasthmatic drug zafirlukast against the oral pathogens Porphyromonas gingivalis and Streptococcus mutans. FEMS Microbiol Lett. 2017;364:fnx005.
- Gerits E, Defraine V, De Cremer K, et al. Repurposing toremifene for the treatment of oral bacterial infections. Antimicrob Agents Chemother. 2017;61:e01846-e01816.
- Gerits E, Spincemaille S, De Cremer K, et al. Repurposing AM404 for the treatment of oral infections by Porphyromonas gingivalis. Clin Exp Dental Res. Forthcoming.
- Dashper S, Ang CS, Liu SW, et al. Inhibition of Porphyromonas gingivalis biofilm by oxantel. Antimicrob Agents Chemother. 2010;54:1311–1314.
- Dashper S, O’Brien-Simpson N, Liu SW, et al. Oxantel disrupts polymicrobial biofilm development of periodontal pathogens. Antimicrob Agents Chemother. 2014;58:378–385.
- Busscher HJ, van der Mei HC, Subbiahdoss G, et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med. 2012;4:153rv10.
- Holmberg KV, Abdolhosseini M, Li Y, et al. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013;9:8224–8231.
- Yoshinari M, Kato T, Matsuzaka K, et al. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling. 2010;26:103–110.
- Shi J, Liu Y, Wang Y, et al. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci Rep. 2015;5:16336.
- Mo AC, Xu W, Xian SQ, et al. Antibacterial activity of silver-hydroxyapatite/titania nanocomposite coating on titanium against oral bacteria. Key Eng Mater. 2007;330-332:455–458.
- Valappil SP, Coombes M, Wright L, et al. Role of gallium and silver from phosphate-based glasses on in vitro dual species oral biofilm models of Porphyromonas gingivalis and Streptococcus gordonii. Acta Biomater. 2012;8:1957–1965.
- Sahdev R, Ansari TI, Higham SM, et al. Potential use of gallium-doped phosphate-based glass material for periodontitis treatment. J Biomater Appl. 2015;30:85–92.
- Liu Y, Zhang X, Liu Y, et al. Bi-functionalization of a calcium phosphate-coated titanium surface with slow-release simvastatin and metronidazole to provide antibacterial activities and pro-osteodifferentiation capabilities. Plos One. 2014;9:e97741.
- Seo M-D, Won H-S, Kim J-H, et al. Antimicrobial peptides for therapeutic applications: a review. Molecules. 2012;17:12276–12286.
- Romanò CL, Scarponi S, Gallazzi E, et al. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res. 2015;10:157.
- Klibanov AM. Permanently microbicidal materials coatings. J Mater Chem. 2007;17:2479–2482.
