ABSTRACT
The effect of fluoride concentrations in dental products could be different depending on the Streptococcus mutans strain. The aim of this study was to investigate the effect of different fluoride concentrations corresponding to dental products on biofilm formation and metabolic activity of S. mutans strains. Seven S. mutans strains (UA159, A32-2, NG8, 10449, UA130, LM7, and OMZ175) were inoculated into 96-well microtiter plates and were tested with various concentrations of sodium fluoride (0.0, 1.0, 1.56, 3.13, 6.25, 12.5, 25, 50, 100, 125, 175, 225, 275, 625, 1,250, 2,250, and 5,500 ppm) for inhibition of biofilm formation and bacterial metabolic activity by recording absorbance values followed by scanning electron microscope (SEM) images. Data were analyzed by one-way analysis of variance and Tukey’s tests (α = 5%). Significantly more (p≤0.05) biofilm mass in the presence of fluoride was produced by A32-2 and NG8. UA130, LM7, and OMZ175 were more sensitive to increased fluoride and demonstrated few bacterial cells and extracellular polysaccharide (EPS) production at 100 ppm in SEM images. All strains were unable to produce significant biofilm at concentrations >225 ppm. Patients with tolerantS. mutans strains would potentially benefit less from the inherent antibacterial effect of fluoride.
Introduction
Fluoride is the most effective agent used in controlling dental caries [Citation1]. The mechanisms of action of fluoride include a decrease in demineralization and a corresponding enhancement of remineralization of dental hard tissues [Citation2]. However, a third well-documented mechanism involves the antimicrobial properties of fluoride against cariogenic bacteria [Citation3,Citation4]. This mode of action of fluoride is considered somewhat less relevant from a clinical point of view [Citation2]. However, the understanding of the effect of fluoride against oral bacteria is an important goal in understanding the complex processes that take place in the oral cavity. Several mechanisms have been suggested for the antibacterial action of fluoride, including inhibition of bacterial enzymes such as enolase [Citation5–Citation7] and the reduction of the production of intracellular and extracellular polysaccharides (EPS) [Citation8,Citation9]. However, bacteria can develop tolerance to the effect of fluoride, as described recently by Liao et al. [Citation10].
Among the cariogenic bacteria, Streptococcus mutans is considered the main culprit [Citation11]. Numerous strains of this microorganism have been isolated [Citation12], and the growth susceptibility of these strains to antimicrobial agents, including fluoride, varies [Citation13–Citation16]. Furthermore, the effect of fluoride concentrations corresponding to various commonly used oral-health products on S. mutans strains might be of direct clinical relevance, especially in patients with a high risk of caries. These individuals are encouraged to use multiple forms of oral-care products that contain different concentrations of fluoride. To the authors’ knowledge, the effect of fluoride concentrations corresponding to different oral-care products has not been explored previously. It was hypothesized that different S. mutans strains would have different susceptibilities to fluoride. Thus, the main objective of this experiment was to investigate the effect of different fluoride concentrations, including levels corresponding to specific dental products, on the biofilm formation and metabolic activity in vitro of seven strains of S. mutans.
Materials and methods
Bacterial strains and media
Seven S. mutans strains – UA159 (American Type Culture Collection [ATCC] 700610), UA130 (ATCC 700611), 10449 (ATCC 25175), A32-2 (isolated in this lab) [Citation17], NG8 (serotype c), LM7 (serotype e), and OMZ175 (serotype f) – were used in this study. Mitis Salivarius Sucrose Bacitracin agar plates were used to grow the strains initially, and tryptic soy broth with 1% sucrose (TSBS; Difco Laboratories, Detroit, MI) was used as the main culture medium. Growth conditions were set at 5% CO2 and 37°C unless otherwise stated.
Fluoride concentrations
Different concentrations of sodium fluoride (NaF) were added to the TSBS broth in order to achieve the desired levels of fluoride within the culture medium. The fluoride concentrations used in this experiment were 5,500 (similar to the fluoride content in dental varnish after a 1:3 dilution), 2,250 (fluoride content in topical dental gel after a 1:3 dilution), 1,250 (fluoride content in prescription toothpaste after a 1:3 dilution), 625, 275 (fluoride content in regular toothpaste after a 1:3 dilution), 225 (fluoride content in mouth rinse after a 1:3 dilution), 175, and 125 ppm. Additional fluoride levels (100, 50, 25, 12.5, 6.25, 3.13, and 1.56 ppm) were obtained by serial dilution in deionized water. The 1:3 dilution has been used previously to simulate concentrations of oral products when applied in the oral cavity [Citation18,Citation19]. In addition, to represent the recommended fluoride level for caries prevention in drinking water, a 1.0 ppm fluoride concentration was prepared. Also, 0.12% chlorhexidine (CHX) and 0 ppm fluoride were used as positive and negative controls, respectively.
Biofilm assay
Sterile 96-well flat-bottom polystyrene microtiter plates (Fisher Scientific, Pittsburgh, PA) were utilized. In each well, 290 µL of TSBS containing the mentioned fluoride concentrations was placed in triplicates followed by inoculation with 10 µL of S. mutans (corresponding to an inoculum size of 104 cells/well) from an overnight TSBS culture. Wells without bacteria received 300 µL of TSBS. Each S. mutans strain had its own microtiter plate with specific controls (TSBS alone and TSBS supplemented with 0.12% CHX). The microtiter plates were incubated for 24 h at 37°C in 5% CO2 without agitation.
After incubation, planktonic bacteria were removed by pipetting. The wells were gently washed twice with saline, and the biofilms in the microtiter plates were fixed by adding 100 µL of a 10% formaldehyde solution before being left overnight at room temperature. The formaldehyde was removed from the wells. The wells were then washed twice, and 100 µL of 0.1% crystal violet was added. The plates were kept at room temperature for 1 h [Citation20,Citation21]. The crystal violet solution was removed, and the wells were washed three times before 250 µL of undiluted isopropanol was placed in each well for 1 h to release the crystal violet. The absorbance of each well was read at 490 nm using a spectrophotometer (Molecular Devices, Inc., Sunnyvale, CA) with isopropanol as the blank.
Scanning electron microscopy
For qualitative examination of the formed biofilms, sterile microscopic slides with four wells (Lab-Tek Chamber slides; Thermo Fisher Scientific, Rochester, NY) were used to grow bacterial biofilms from the seven S. mutans strains by placing 680 µL of TSBS containing 0, 50, and 100 ppm of fluoride on the surface of the slide and inoculating with 20 µL of an overnight bacterial culture. Slides were incubated in a humid chamber for 24 h at 5% CO2 and 37°C. After incubation, the medium was removed and the biofilms gently washed twice in saline. Then, 700 µL of 1% glutaraldehyde solution was added and left overnight. To prepare for imaging, wells were washed for 5 min with deionized water (700 µL) and dehydrated using serial ethanol washes. Next, hexamethyldisilazane (HMDS) solution was added for 15 min, and the slides were placed in a desiccator for 3 days. After desiccation, slides were mounted on aluminum stubs with carbon tape, and their sides were painted with conductive colloidal silver paint. The samples were analyzed under high vacuum with 20 kV of accelerating voltage at a working distance of 10 mm (JEOL 5310 LV, Japan) at 1,000, 3,000, and 7,000× magnification. Three microscopic fields/slide were examined.
Determination of bacterial metabolic activity
To investigate the effect of different fluoride concentrations up to 100 ppm on viable sessile cells, a sodium 3ʹ-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT) assay was utilized [Citation22]. Biofilms from the seven S. mutans strains were allowed to form in 96-well microtiter plates for 24 h by adding 10 µL of overnight cultures to 290 µL of 1% TSBS and incubating in 5% CO2 at 37°C. Biofilms were washed three times with sterile 0.9% NaCl to remove nonadherent cells, and 300 µL of each fluoride concentration in TSBS, TSBS control, or TSBS containing 0.12% CHX was added to designated wells (in triplicates) and incubated at 37°C for 24 h. After incubation, the treated biofilms were washed three times, and the metabolic activity of the biofilms was determined by the addition of XTT solution. After 2 h of incubation at room temperature in the dark, XTT solution was transferred into a new microtiter plate, and the absorbance at 490 nm was determined.
Statistical tests
All experiments were conducted three times. Absorbance values from biofilm and metabolic activity assays were compared using a univariate model. Further, for each fluoride level, one-way analysis of variance followed by Tukey’s post hoc test was done at a 0.05 significance level. Inter- and intra-strain comparisons were also conducted. All tests were done using SPSS Statistics for Windows v16.0 statistical software (SPSS, Inc., Chicago, IL).
Results
Biofilm formation
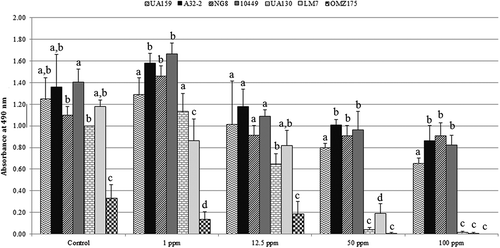
There was a clear visual difference in the amount of biofilm formed by each strain in the control wells without fluoride. Overall, the amount of biofilm produced by OMZ175 without fluoride was significantly less compared to the other strains. CHX at 0.12% significantly inhibited biofilm formation of all seven strains. Quantitatively, three distinct patterns between the various strains treated with different fluoride concentrations were recorded (). The first group of strains (group A; UA159, A32-2, NG-8, and 10449) demonstrated high biofilm absorbance values in fluoride concentrations up to 6.25 ppm. Then, a gradual decline was noted as the fluoride content within the culture medium increased. At 100 ppm, a marked decrease in biofilm absorbance was recorded until the values approached 0 between 125 and 175 ppm. The second group of strains (group B; UA130 and LM7) exhibited a lower overall biofilm mass compared to strains in group A and remained relatively unaffected by increased fluoride concentrations up to 25 ppm. However, at 50 ppm, a sudden decrease in biofilm was recorded. The last group containing only OMZ175 strain (group C) demonstrated the lowest biofilm absorbance values until reaching 0 at the 25 ppm fluoride level.
Figure 1. Biofilm absorbance values for seven Streptococcus mutans strains grown at different fluoride concentrations. Each data point represents the mean of triplicates from three independent experiments. Data points were connected for illustrative purposes only.
To make statistical testing more manageable and to indicate distinct differences between the tested strains, detailed biofilm results and discussion for five fluoride levels (0, 1, 12.5, 50, and 100 ppm) are presented. exhibits biofilm absorbance values with statistical comparisons between the seven strains at the above-mentioned fluoride concentrations. A relatively similar statistical pattern to the one mentioned above can be observed. Statistical testing with strains from group A mostly associated with significantly higher (p < 0.05) absorbance values compared to strains from groups B and C. Overall, values for A32-2 and NG8 were consistently higher compared to all other strains. On the other hand, OMZ175 was consistently the lowest.
Scanning electron microscopy
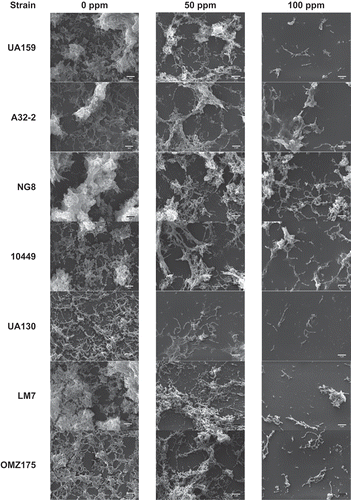
presents selected scanning electron microscope (SEM) images at 0, 50, and 100 ppm concentrations at a 3,000× magnification. Biofilm cells were clearly distinguishable from extracellular polysaccharide (EPS) in these images. At 0 ppm, group A strains, along with LM7, demonstrated the highest aggregation of EPS. At 50 ppm, there was a reduction in the amount of EPS, especially in group B and C strains. That reduction was more pronounced at the 100 ppm level, with UA159, UA130, and OMZ175 exhibiting almost no EPS production. However, A32-2 and NG8 were still able to produce some EPS at that fluoride level.
Bacterial metabolic activity
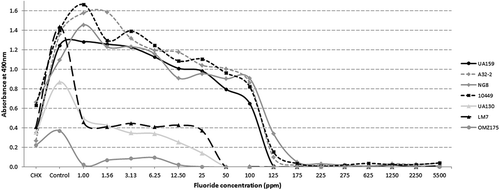
indicates the results of statistical comparisons for the metabolic activity assay. Overall, bacterial metabolic activity increased with increased fluoride concentrations, except for UA159 and OMZ175. A32-2 and NG8 were associated with higher metabolic activity values, especially at 50 and 100 ppm fluoride levels.
Table 1. Mean absorbance values of metabolic activity of the seven Streptococcus mutans strains grown at 0, 1, 12.5, 50, and 100 ppm of sodium fluoride and 0.12% chlorhexidine (CHX)
Discussion
Fluoride is one of the most important agents for controlling dental caries [Citation23]. Although the reported mechanism of action of fluoride to reduce dental caries is mainly by increasing mineral uptake by enamel and decreasing demineralization [Citation2], a third mode of action is by affecting bacterial metabolism [Citation3,Citation24–Citation26]. The main objective of this study was to investigate the effect of different fluoride concentrations, corresponding to different oral-care products, on the metabolic activity and biofilm formation of seven strains of S. mutans. The concentrations tested were based on a 1:3 dilution that has been used previously [Citation19].
The effect of fluoride against cariogenic bacteria could be attributed to the inhibition of bacterial metabolic enzymes, such as enolase, that decreases sucrose metabolism by affecting phosphoenolpyruvate (PEP) levels [Citation26]. This will deprive the cell from glucose and can affect its metabolic activity, growth, and multiplication [Citation5]. Subramaniam and Nandan observed a significant reduction in S. mutans colony counts when they administered a mouth rinse containing a combination of fluoride, xylitol, and triclosan [Citation6]. This effect was further demonstrated in this study where biofilm synthesis of all strains was greatly reduced at 100 ppm () and was almost nonexistent at concentrations >225 ppm, which is the level of an over-the-counter fluoride rinse (). However, the strains used by Subramaniam and Nandan were different from the ones used in the current investigation. Still, the current findings are in agreement with results by Maltz and Emilson [Citation27] that reported a bactericidal effect of NaF salts at high concentrations (12,500 ppm), even though the effect recorded in the present study was in relation to 5,500 ppm representing a fluoride varnish.
Another mechanism of fluoride is through inhibition of ATPase production causing intracellular cytoplasmic acidification via the inhibition of proton outflux from the bacterial cell [Citation26,Citation28]. This will lead to inhibition of acid production and limit the pH drop within bacterial biofilms [Citation25]. In an experiment to investigate the effect of NaF on acid production by S. mutans [Citation7], investigators found that 50 ppm of NaF almost totally inhibited the pH drop within the medium, and some effect was observed in concentrations as low as 6.25 ppm. Furthermore, rinsing with a NaF solution demonstrated a significant decrease in pH drop in clinical trials [Citation29,Citation30]. In one clinical study, there was a significant difference in acid production in biofilms from children in areas with a 1.8 ppm fluoride level in the water supply compared to areas with a 1.0 ppm level [Citation7].
A third suggested antimicrobial mechanism is via the fluoride effect on the production of intracellular polysaccharide [Citation31] and EPS [Citation8]. Sodium fluoride at a concentration of 70 ppm affected the amount of EPS produced, as well as changed the composition of the polymer. Although the fluoride concentrations used may appear high, they were diluted concentrations based on actual fluoride concentrations found in commercial products. Bowen and Hewitt [Citation8] suggest that high concentrations of ionic fluoride could occur in plaque biofilm after topical application of fluoride-containing products that could potentially affect the cariogenicity of the biofilm. A recent study indicated that fluoride at low concentrations was able to decrease the secretion of glucosyltransfrase, consequently reducing EPS synthesis [Citation9]. The present results are in agreement with these findings, and SEM images demonstrated a marked decrease in EPS production as fluoride concentration increased. However, the amount of baseline EPS was visually different between the different strains. Group A strains exhibited the largest amount of polymer production. However, within this group, some strains (UA159 and 10449) were more sensitive to increased fluoride levels, and their EPS production was more affected compared to others within the same group (A32-2 and NG8).
Since it has been confirmed that fluoride levels in biofilms after exposure to topical products are elevated [Citation29,Citation32,Citation33], the rationale was to test different levels of NaF concentrations, some of them corresponding to the diluted levels after the use of oral-care products. Toothpaste-slurry mixtures could contain concentrations up to 250 ppm of NaF after brushing that taper to slightly higher than baseline 60 min after brushing [Citation34]. This is the case with toothpastes that contain concentrations up to 1,500 ppm of NaF. However, a 22,500 ppm–containing fluoride varnish should contain a higher fluoride level. Basically, higher concentrations (625–5,500) were used to simulate these conditions. At these levels, the amount of biofilm production was almost non-existent. As mentioned previously, a bactericidal effect of NaF salt was earlier observed at 12,500 ppm [Citation27]. Results from the metabolic activity assay indicated a less predictable pattern, however, with some strains demonstrating an increase in bioactivity while others exhibited a reduction (). A similar pattern was observed in a previous study where UA159 exhibited increased metabolic activity at high concentrations (sub-MBC) of nicotine owing to tolerance to the chemical [Citation21]. Nevertheless, inherent tolerance to antimicrobials was observed in these data, with NG8 demonstrating the highest tolerance to fluoride and CHX. OMZ175 exhibited very low tolerance to fluoride, and all strains responded significantly more to CHX compared to fluoride, indicating much lower antibacterial properties for fluoride.
For this study, a mix of reference strains and clinical isolates were chosen based on serotypes. Some of these strains (UA159, A32-2, and NG8) were isolated from individuals at high risk of caries, and one of these strains (A32-2) was isolated in the authors’ lab. Overall, the OMZ175 strain demonstrated the least amount of biofilm formation, corresponding with the lowest levels of ESP production at 100 ppm. Although UA130 and LM7 exhibited more cellular quantities relative to OMZ175, their potential cariogenicity could not be compared to group A strains that were associated with both a higher number of cells and amount of extracellular matrix formation. Future investigations aiming to test the effect of fluoride on S. mutans virulence should use strains from group A, since they are more resistant to NaF salts.
In summary, fluoride levels owing to diluted oral-care products (5,500–225 ppm fluoride) were able to inhibit biofilm formation in vitro. Inhibition of biofilm production and metabolic activity at lower concentrations were dependent on the strain of bacteria. The fluoride concentration in drinking water (1.0 ppm) showed some effect on group B and C strains.
Understanding the physiological differences between cariogenic strains of S. mutans is very important in developing individualized strategies for clinical practice, since it was previously reported that differences in S. mutans strains were linked to patients’ caries activity [Citation35]. Knowledge of differences between S. mutans strains could help deliver a more tailored treatment plan based on the particular organism present in a specific patient. For example, more fluoride resistant strains such as NG8 and A32-2 require a more potent approach, since these strains are able to produce EPS even at high fluoride levels. Furthermore, determining the sensitivity of different strains of S. mutans to oral agents will allow a more complete understanding of their biological processes.
Acknowledgments
This article was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah. The authors therefore acknowledge with thanks the DSR for the technical and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zero DT. Dental caries process. Dent Clin North Am. 1999;43:1–7.
- Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials–fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–362.
- Hamilton IR. Biochemical effects of fluoride on oral bacteria. J Dent Res. 1990;69 Spec:No:660-7; discussion 82-3.
- Pandit S, Kim JE, Jung KH, et al. Effect of sodium fluoride on the virulence factors and composition of Streptococcus mutans biofilms. Arch Oral Biol. 2011;56:643–649.
- Kanapka JA, Hamilton IR. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971;146:167–174.
- Subramaniam P, Nandan N. Effect of xylitol, sodium fluoride and triclosan containing mouth rinse on Streptococcus mutans. Contemp Clin Dent. 2011;2:287–290.
- Jenkins GN, Edgar WM. The distribution and metabolic effects of human plaque fluorine. Arch Oral Biol. 1969;14:105–119.
- Bowen WH, Hewitt MJ. Effect of fluoride on extracellular polysaccharide production by Streptococcus mutans. J Dent Res. 1974;53:627–629.
- Koo H, Sheng J, Nguyen PT, et al. Co-operative inhibition by fluoride and zinc of glucosyl transferase production and polysaccharide synthesis by mutans streptococci in suspension cultures and biofilms. FEMS Microbiol Lett. 2006;254:134–140.
- Liao Y, Chen J, Brandt BW, et al. Identification and functional analysis of genome mutations in a fluoride-resistant Streptococcus mutans strain. Plos One. 2015;10:e0122630.
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380.
- Kuramitsu HK. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–176.
- Li YH, Bowden GH. The effect of environmental pH and fluoride from the substratum on the development of biofilms of selected oral bacteria. J Dent Res. 1994;73:1615–1626.
- Nassar HM, Li M, Gregory RL. Effect of honey on Streptococcus mutans growth and biofilm formation. Appl Environ Microbiol. 2012;78:536–540.
- Ronanki S, Kulkarni S, Hemalatha R, et al. Efficacy of commercially available chlorhexidine mouthrinses against specific oral microflora. Indian J Dent Res. 2016;27:48–53.
- Jain I, Jain P, Bisht D, et al. Use of traditional Indian plants in the inhibition of caries-causing bacteria–Streptococcus mutans. Braz Dent J. 2015;26:110–115.
- Gregory RL, El-Rahman AM, Avery DR. Effect of restorative treatment on mutans streptococci and IgA antibodies. Pediatr Dent. 1998;20:273–277.
- Hara AT, Gonzalez-Cabezas C, Creeth J, et al. Interplay between fluoride and abrasivity of dentifrices on dental erosion-abrasion. J Dent. 2009;37:781–785.
- Nassar HM, Lippert F, Eckert GJ, et al. Dentifrice fluoride and abrasivity interplay on artificial caries lesions. Caries Res. 2014;48:557–565.
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–1500.
- Huang R, Li M, Gregory RL. Effect of nicotine on growth and metabolism of Streptococcus mutans. Europ J Oral Sci. 2012;120:319–325.
- Roehm NW, Rodgers GH, Hatfield SM, et al. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265.
- Adair SM. Evidence-based use of fluoride in contemporary pediatric dental practice. Pediatric Dent. 2006;28:133-42; discussion 92-8.
- Van Loveren C. Antimicrobial activity of fluoride and its in vivo importance: identification of research questions. Caries Res. 2001;35(Suppl 1):65–70.
- Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;26:493–510.
- Jenkins GN. Review of fluoride research since 1959. Arch Oral Biol. 1999;44:985–992.
- Maltz M, Emilson CG. Susceptibility of oral bacteria to various fluoride salts. J Dent Res. 1982;61:786–790.
- Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008;20:17–21.
- Geddes DA, McNee SG. The effect of 0.2 per cent (48 mM) Naf rinses daily on human plaque acidogenicity in situ (stephan curve) and fluoride content. Arch Oral Biol. 1982;27:765–769.
- Woolley LH, Rickles NH. Inhibition of acidogenesis in human dental plaque in situ following the use of topical sodium fluoride. Arch Oral Biol. 1971;16:1187–1194.
- Weiss S, King WJ, Kestenbaum RC, et al. Influence of various factors on polysaccharide synthesis in S. mitis. Ann N Y Acad Sci. 1965;131:839–850.
- Duckworth RM, Morgan SN, Murray AM. Fluoride in saliva and plaque following use of fluoride-containing mouthwashes. J Dent Res. 1987;66:1730–1734.
- Vogel GL, Mao Y, Chow LC, et al. Fluoride in plaque fluid, plaque, and saliva measured for 2 hours after a sodium fluoride monofluorophosphate rinse. Caries Res. 2000;34:404–411.
- Bruun C, Givskov H, Thylstrup A. Whole saliva fluoride after toothbrushing with NaF and MFP dentifrices with different F concentrations. Caries Res. 1984;18:282–288.
- Oishi Y, Watanabe K, Kumada H, et al. Purification and characterization of a novel secondary fimbrial protein from Porphyromonas gulae.. J Oral Microbiol. 2012;4. DOI:10.3402/jom.v4i0.19076.