ABSTRACT
Salivary protein levels have been studied in periodontitis. However, there is lack of information on salivary cytokine levels in early gingival inflammation. The aim of this study was to determine salivary levels of vascular endothelial growth factor (VEGF), interleukin (IL)-8, monocyte chemoattractant protein (MCP)-1, IL-1β, and IL-1 receptor antagonist (IL-1Ra) in gingival inflammation. Twenty-eight systemically and orally healthy nonsmokers abstained from oral hygiene protocols for 10 days. After that, self-performed cleaning was resumed for 14 days. Plaque and gingival indexes were measured, and saliva samples were collected at days 1, 4, 7, 10, and 24. Salivary cytokines were detected with Luminex®-xMAP™. Salivary IL-1β, IL-1Ra, and VEGF levels decreased after 10 days’ development of experimental gingivitis and reached baseline levels at the end of the 2-week resolution period. Salivary IL-8 levels decreased and remained low during development and resolution of experimental gingivitis. Initial inflammation in gingival tissues is associated with a decrease in inflammatory cytokines in saliva. Further studies are needed to evaluate if inflammatory cytokines bind to their functional receptors within the gingival tissue during early gingivitis, which may limits their spillover to the gingival crevice and ultimately saliva.
Introduction
Gingivitis is the inflammatory gingival response against excessive bacterial biofilms [Citation1]. It is a reversible condition. However, if left undisturbed, the gingival inflammation becomes chronic and may ultimately lead to periodontitis, with degradation of the tooth-supporting tissues.
Early detection of inflammatory changes in the gingival tissues has major significance for the treatment and prognosis of periodontal diseases. While gingivitis is easily reversed in healthy individuals, the risk of disease progression to periodontitis in susceptible individuals is substantial. At present, the diagnosis of gingivitis relies on clinical signs of gingival inflammation (i.e. gingival edema, bleeding on probing, and increased crevicular fluid) the latter of which spill over to the saliva. Based on its content of various biological substances, saliva may possess the potential to serve as a diagnostic tool for oral diseases [Citation2]. Several salivary proteins, such as interleukin (IL)-1β, matrix metalloproteinase (MMP)-8, and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), have successfully been used in screening for periodontal disease activity [Citation3,Citation4]. However, as these proteins are associated with extensive collagen degradation and osteoclast activation [Citation5,Citation6], they do not reflect the initiation of gingivitis.
At the molecular level, initiation and resolution of gingival inflammation are regulated by host chemokines, cytokines, and to some extent pro-resolving lipid mediators [Citation7]. The initial steps in this inflammatory process are increased blood flow and vascularization (angiogenesis), increased vascular permeability, and migration of neutrophils and monocytes/macrophages to the site of infection [Citation6]. The hypothesis for this study was that early-stage gingivitis impacts salivary levels of specific chemokines and cytokines. Thus, the aim was to quantify selected chemokines and cytokines associated with early stages of gingivitis (i.e. vascular endothelial growth factor [VEGF], IL-8, monocyte chemoattractant protein [MCP-1], IL-1β, and IL-1 receptor antagonist [IL-1Ra]).
Materials and methods
Study population and its selection criteria
A total of 31 dental students from the University of Copenhagen were enrolled. The recruitment protocol was based on the following selection criteria: systemically and orally healthy nonsmokers aged ≥18 years who were willing to discontinue brushing their teeth and interdental cleaning for 10 days. All subjects with active caries lesions, gingivitis or periodontitis, hyposalivation, any prescribed medication within the previous 3 months, including antibiotics and/or professional dental cleaning, were excluded. The volunteers were informed about the objectives of this experiment prior to obtaining their written consent. The study was approved by the regional ethical committee of the Capital Region of Denmark (H-16016368–56386) and complied with the Declaration of Helsinki. Furthermore, it was reported to the Danish Data Authority (SUND-2016–58) and registered at clinicaltrials.gov (NCT02913235).
Study design and clinical recordings
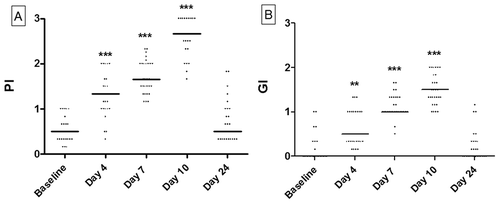
Oral health status was screened full-mouth and by use of bitewings radiographs. The total duration of this study was 24 days, out of which participants stopped all oral hygiene protocols for 10 days. Regular oral hygiene was then resumed for 14 days. Gingival examinations, including registrations of plaque and gingival inflammation, were performed at baseline and at days 4, 7, 10, and 24 by the same examiner (DB). Plaque index (PI) and gingival index (GI) were recorded and calculated based on four sites (buccal, lingual, mesial, and distal) of six preselected teeth (16, 12, 24, 44, 32, and 36) as previously described [Citation8,Citation9].
Saliva sampling
Stimulated saliva samples were collected, as previously described [Citation10]. At each visit, the samples were collected from the study population by the same examiner (DB) between 8:00am and 6:00pm. Great care was taken that all saliva sampling per subject was performed at the same time of the day. In brief, participants were instructed to avoid eating and drinking for at least 2 h before sampling. Chewing a sterile paraffin gum was used to stimulate saliva secretion, and participants spat into a sterile plastic cup until 1 mL of sample material was collected. Saliva samples were then divided into two aliquots and immediately placed on dry ice. Samples were stored at −80°C until further analysis.
Analysis of salivary biomarkers
After thawing, all saliva samples were centrifuged at 9300 g for 5 min. In salivary assays, the supernatant was used. Salivary concentrations of VEGF, IL-8, MCP-1, IL-1β, and IL-1Ra were detected by the Luminex® xMAP™ technique (Luminex Corporation, Austin, TX) with the commercially optimized Bio-Plex kits (pro-human cytokine group I assays; Bio-Rad, Santa Rosa, CA) according to the manufacturer’s instructions. The detection limit of the assay was 3.1 pg/mL for VEGF, 1.0 pg/mL for IL-8, 1.1 pg/mL for MCP-1, 0.6 pg/mL for IL-1β, and 5.5 pg/mL for IL-1Ra.
Statistical analyses
All data were checked for normal distribution. Data following a Gaussian distribution were compared using repeated measures of analysis of variance and repeated t-test, whereas nonparametric data were analyzed with Friedman’s test with Dunn’s multiple comparison tests. p-Values <0.05 were considered statistically significant. Correlations between cytokines were computed using Spearman’s signed rank test and corrected for multiple dependent comparisons. GraphPad Prism v5 (GraphPad Software, Inc., La Jolla, CA) was used as statistical software.
Results
Out of 31 recruited subjects, 29 (95.3%) completed the study. The mean age of the participants was 24.7 years (range 22–29 years), and the majority (82.8%) were female. The reason for the two dropouts was the use of antibiotics prescribed for bacterial infection unrelated to the oral cavity. Furthermore, as one saliva sample tube broke during centrifugation, the remaining samples from that participant were discarded. Consequently, samples from 28 participants were included in further analyses.
Clinical data
PI and GI values increased significantly during the experimental gingivitis period (at days, 4, 7, and 10). After 2 weeks of resolution (day 24), PI and GI scores were not significantly different from baseline scores ().
Early gingivitis impacts levels of salivary cytokines
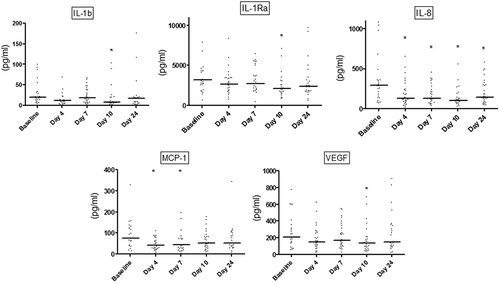
In all samples, salivary concentrations of tested cytokines were above the detection limits of the test kit. Salivary levels of IL-1β, IL-1Ra, IL-8, MCP-1, and VEGF are presented in . Salivary IL-1β, IL-1Ra, and VEGF were all significantly reduced after 10 days (p < 0.05) compared to baseline, whereas MCP-1 levels were reduced at day 4 and day 7, respectively (p < 0.05). Salivary IL-8 was significantly reduced at day 4 and stayed low, even after resolution of experimental gingivitis (p < 0.05; ).
Figure 2. Salivary levels of interleukin (IL)-1β, IL-1Ra, IL-8, monocyte chemoattractant protein (MCP-1), and vascular endothelial growth factor (VEGF). Dot-plot of salivary levels of IL-1β, IL-1Ra, IL-8, MCP-1, and VEGF recorded at baseline and at days 4, 7, 10, and 24. Horizontal lines: median value. *p < 0.05.
Correlation of salivary cytokines
Correlation of baseline concentrations and levels recorded at days 4, 7, 10, and 24 of each cytokine is presented in . A strong correlation was observed between baseline samples and samples collected 14 days after resolution of experimental gingivitis (day 24) of IL-1β (R = 0.66, p < 0.001), IL-1Ra (R = 0.85, p < 0.001), IL-8 (R = 0.79, p < 0.001), and VEGF (R = 0.80, p < 0.001).
Table 1. Correlation coefficients (R) for salivary IL-1β, IL-1Ra, IL-8, MCP-1, and VEGF levels between the visits at baseline and at day 4, 7, 10, and 24
Correlation between salivary IL-1β, IL-1Ra, IL-8, MCP-1, and VEGF is detailed in . While highly significant correlations were observed between salivary levels of all cytokines investigated, the strongest correlations were observed between IL-1β and IL-8 (R = 0.86) and IL-1Ra and VEGF (R = 0.92).
Table 2. Correlation coefficients (R) between salivary levels of IL-1β, IL1-Ra, IL-8, MCP-1, and VEGF
No significant correlation was observed at the individual level between clinical parameters (PI and GI) and salivary levels of IL-1β, IL-1Ra, IL-8, MCP-1, and VEGF (p > 0.05).
Discussion
The purpose of the present investigation was to characterize salivary levels of five selected inflammatory cytokines associated with gingivitis in order to test the hypothesis that early-stage gingival inflammation impacts salivary concentrations of specific cytokines. The main finding was that early-stage experimental gingivitis was associated with a significant decrease in salivary concentrations of all cytokines investigated.
Salivary levels of the cytokines tested were altered at different time points. MCP-1 and IL-8 were significantly decreased already at day 4, whereas VEGF, IL-1β, and IL-1Ra were significantly decreased after 10 days of oral hygiene discontinuation compared to baseline levels. These findings probably reflect the different roles of the chemokines and cytokines investigated in early inflammation. IL-8 and MCP-1 are chemoattractants, which promote the migration of neutrophils and monocytes/macrophages to the site of the infectious challenge [Citation11–Citation13]. Thus, the significant decrease in IL-8 and MCP-1 at day 4 is consistent with the clinical recordings, which showed moderate plaque formation but minimal signs of gingival inflammation. On the contrary, VEGF and IL-1β are cytokines that orchestrate inflammatory changes of the gingival tissues such as angiogenesis and edema [Citation14–Citation16]. It is therefore interesting that VEGF and IL-1β were not significantly decreased until 10 days after oral hygiene discontinuation, when the majority of the participants had developed moderate to severe gingivitis.
Other experimental gingivitis studies have demonstrated decreased or steady gingival crevicular fluid IL-8 levels [Citation11,Citation13], which is consistent with data from the present investigation. On the other hand, elevated IL-1β, steady IL-1Ra, and decreased MCP-1 levels have been observed in gingival crevicular fluid samples in experimental gingivitis [Citation17–Citation19], which conflicts with data from this report. Furthermore, in one investigation, salivary IL-1Ra levels were found to be associated with increased pocket-depth measurements in an experimental gingivitis model, while no association was observed between IL-1Ra and PI or GI [Citation20]. These discrepancies might reflect differences between studies in terms of sample material used (saliva vs. crevicular fluid) and duration of the experimental gingivitis protocol (10 vs. 21 days). Notably, the discontinuation of oral hygiene employed in this investigation lasted only 10 days, and data suggest that in the initial phases of inflammation, preserved gingival tissue integrity supports the binding of the chemokines and cytokines investigated to limit their release into gingival crevicular fluid and saliva.
Major intra-individual differences in salivary levels of each cytokine were noted. This finding probably reflects the fact that while some individuals developed pronounced generalized gingivitis, others only showed modest clinical signs of gingival inflammation during the 10-day period of oral hygiene cessation. This is consistent with clinical data from other experimental gingivitis studies in which substantial variations in gingival inflammation were also recorded [Citation11,Citation21,Citation22]. However, a good correlation between salivary levels of each cytokine recorded suggests their possible use in screening for early signs of gingival inflammation. Obviously, this is especially relevant in individuals susceptible to periodontitis.
Some potential limitations apply to the results of this study. Stimulated saliva samples were used for cytokine analysis, which means that salivary levels of the cytokines investigated were possibly diluted as a consequence of the sampling strategy [Citation23]. However, salivary levels of each cytokine were above the detection level in all samples. Collection of unstimulated saliva samples might be influenced by tongue and cheek movements, which may impact salivary secretion and protein concentration between subjects. Consequently, stimulated saliva samples are suggested as a valid alternative to unstimulated saliva samples [Citation24]. Partial recordings were used for calculation of PI and GI, which may have influenced correlation analysis between clinical parameters and salivary cytokine levels. Thus, future experimental gingivitis studies could employ full-mouth registration of plaque accumulation and gingival inflammation.
In conclusion, salivary levels of inflammatory chemokines and cytokines were significantly decreased during initiation and development of gingivitis. Preserved gingival tissue integrity at early phases of gingivitis may explain why pro-inflammatory cytokines are not released into gingival crevicular fluid and saliva. Further studies in susceptible groups, including smokers, diabetics, and patients with aggressive forms of periodontitis, are needed to evaluate the potential of salivary concentrations of inflammatory cytokines to be used for screening of early signs of gingival inflammation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Daniel Belstrøm
Daniel Belstrøm is assistant professor at Section of Periodontology and Oral Microbiology, Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen. DDS from University of Copenhagen, Denmark in 2009, and PhD in 2014 from University of Copenhagen. Author of 25 publications in international and national scientific peer-reviewed journals and board member of Danish Academy of Periodontology.
Christian Damgaard
Christian Damgaard is assistant professor at Section of Periodontology, Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen. DDS from University of Copenhagen, Denmark in 2011. PhD in 2015 from University of Copenhagen and The Forsyth Institute, Cambridge, MA. Author of 14 publications in international and national scientific peer-reviewed journals and board member of Danish Academy of Periodontology.
Eija Könönen
Eija Könönen is professor and chair of periodontology at the University of Turku, Finland, since 2007. She completed her dental undergraduate and specialist (periodontology) education and received her PhD (oral microbiology) at the University of Helsinki, Finland, being there an adjunct professor of oral microbiology. She has also worked at the National Public Health Institute, Helsinki, as a senior investigator and the head of the Anaerobe Reference Laboratory. She has over 130 international scientific articles/book chapters; most publications deal with anaerobic bacteria.
Mervi Gürsoy
Mervi Gürsoy is an associate professor in periodontology and works as university teacher at the Department of Periodontology, Institute of Dentistry, University of Turku (Finland). She was awarded her DDS degree by the University of Helsinki (1999), and then her PhD in periodontology (2012) and her qualification as a specialist in periodontology (2013) from the University of Turku. She publishes articles in the field of periodontology and oral microbiology.
Palle Holmstrup
Palle Holmstrup is professor and Section Head (Periodontology, Oral Microbiology, Surgery, Pathology, Physiology, Radiology and Community Dentistry) at School of Dentistry, Faculty of Health and Medical Sciences, University of Copenhagen. DDS from the Royal Dental College of Copenhagen in 1971. PhD in 1976 and employed at the University Hospital of Copenhagen with Board Certification in Oral Surgery 1983. Dr.Odont. 1985. Honorary Doctor, Sahlgrenska Academy, University of Gothenburg in 2005. Advisor of 23 PhD projects. Editor-in-chief of Oral Health and Preventive Dentistry 2002-14 and of Acta Odontologica Scandinavica from 2014. More than 400 guest lectures, seminars and courses in Scandinavia and abroad. Around 280 publications (Oral Medicine and Periodontology) in international and national scientific journals and book chapters. H-index: 56 (Google Scholar), 39 (Web of Science).
Ulvi Kahraman Gürsoy
Ulvi K. Gürsoy received his DDS from Gazi Un., Turkey in 1998 and accomplished his PhD in periodontology in Cumhuriyet Un., Turkey in 2004. He started his post-doctoral training in Helsinki Un., Finland and National Public Health Institute, Helsinki, Finland in 2005. In 2009, he was appointed as university teacher in Institute of Dentistry, University of Turku, Finland. He received his docentship in the same university in 2010 and his university lecturer position in 2012. His main research interests are the oral biofilm-host cell interactions and analyses of salivary components in diagnosis of periodontitis.
References
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):1–6.
- Gursoy UK, Kononen E. Editorial: use of saliva in diagnosis of periodontitis: cumulative use of bacterial and host-derived biomarkers. Front Cell Infect Microbiol. 2016;6:196.
- Gursoy UK, Kononen E, Pradhan-Palikhe P, et al. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010;37(6):487–493.
- Jaedicke KM, Preshaw PM, Taylor JJ. Salivary cytokines as biomarkers of periodontal diseases. Periodontol 2000. 2016;70(1):164–183.
- Syndergaard B, Al-Sabbagh M, Kryscio RJ, et al. Salivary biomarkers associated with gingivitis and response to therapy. J Periodontol. 2014;85(8):e295–e303.
- Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 2011;90(2):143–153.
- Cekici A, Kantarci A, Hasturk H, et al. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64(1):57–80.
- Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–187.
- Silness J, Loe H. Periodontal diseases in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135.
- Bardow A, Lykkeaa J, Qvist V, et al. Saliva composition in three selected groups with normal stimulated salivary flow rates, but yet major differences in caries experience and dental erosion. Acta Odontol Scand. 2014;72(6):466–473.
- Dommisch H, Staufenbiel I, Schulze K, et al. Expression of antimicrobial peptides and interleukin-8 during early stages of inflammation: an experimental gingivitis study. J Periodontal Res. 2015;50(6):836–845.
- Garlet GP, Martins W Jr., Ferreira BR, et al. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003;38(2):210–217.
- Deinzer R, Weik U, Kolb-Bachofen V, et al. Comparison of experimental gingivitis with persistent gingivitis: differences in clinical parameters and cytokine concentrations. J Periodontal Res. 2007;42(4):318–324.
- Cetinkaya BO, Keles GC, Ayas B, et al. The expression of vascular endothelial growth factor in a rat model at destruction and healing stages of periodontal disease. J Periodontol. 2007;78(6):1129–1135.
- Wang D, Zhang S, Li L, et al. Structural insights into the assembly and activation of IL-1beta with its receptors. Nat Immunol. 2010;11(10):905–911.
- Keles GC, Cetinkaya BO, Eroglu C, et al. Vascular endothelial growth factor expression levels of gingiva in gingivitis and periodontitis patients with/without diabetes mellitus. Inflamm Res. 2010;59(7):543–549.
- Offenbacher S, Barros S, Mendoza L, et al. Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. J Clin Periodontol. 2010;37(4):324–333.
- Trombelli L, Scapoli C, Carrieri A, et al. Interleukin-1 beta levels in gingival crevicular fluid and serum under naturally occurring and experimentally induced gingivitis. J Clin Periodontol. 2010 1;37(8):697–704.
- Scott AE, Milward M, Linden GJ, et al. Mapping biological to clinical phenotypes during the development (21 days) and resolution (21 days) of experimental gingivitis. J Clin Periodontol. 2012;39(2):123–131.
- Morelli T, Stella M, Barros SP, et al. Salivary biomarkers in a biofilm overgrowth model. J Periodontol. 2014;85(12):1770–1778.
- Schincaglia GP, Hong BY, Rosania A, et al. Clinical, immune, and microbiome traits of gingivitis and peri-implant mucositis. J Dent Res. 2017;96(1):47–55.
- Meyer S, Giannopoulou C, Courvoisier D, et al. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res. 2016. DOI:10.1111/clr.12912
- Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241–248.
- Jasim H, Olausson P, Hedenberg-Magnusson B, et al. The proteomic profile of whole and glandular saliva in healthy pain-free subjects. Sci Rep. 2016;6:39073.