ABSTRACT
Background: Xerostomia is a very relevant and frequent complication of radiotherapy, causing the irradiated oral mucosa to be affected by bacterial, fungal and viral infections.
Objective: The objective of this study was to evaluate a possible relationship between oral shedding of human herpesviruses and xerostomia in patients with squamous cell carcinoma of head and neck submitted to radio/chemotherapy.
Methods: In this study, oral rinse samples were collected weekly from 20 patients during radiotherapy. The samples were submitted to PCR and enzymatic digestion for detection of human herpesviruses. Xerostomia was evaluated according to the Seminars in Radiation Oncology criteria.
Results: There was a higher frequency of grade 1 xerostomia (51.4%), observed first in the 1st week of radiotherapy. In the 4th week of radiotherapy, all patients presented some degree of xerostomia. Analysis of herpesviruses showed oral shedding of EBV, HHV-6 and HHV-7 in all weeks. Considering all the periods, the highest frequency was in patients with EBV excretion (55.0%), which was significantly higher than that of other viruses.
Conclusion: We observed that oral shedding of herpesviruses was not affected by xerostomia as there was a progression in their excretion, even with the evolution of xerostomia. This suggested that there is a local replication in the oral cavity that is not completely dependent of salivary excretion.
Introduction
Radiotherapy (RT) is an antineoplastic treatment capable of providing high growth control rates for stage 1 (80%) and stage 2 (60%–70%) tumours while preserving important anatomical structures [Citation1,Citation2]. Despite its effectiveness, the RT for head and neck tumours is accompanied by a number of complications arising from the involvement of radiosensitive tissues located close to the tumour [Citation3–Citation5].
Xerostomia is a relevant complication of RT caused by possible destruction of the glandular parenchyma, thus diminishing the production of saliva. The reduction of salivary flow causes discomfort to patients, and in the most severe cases, interferes with chewing, swallowing and speech. Clinical symptoms such as oral mucosal burning, dry lips, lip ridges, altered tongue surface, difficult adaptation of dental prosthesis, as well as denture stomatitis, are commonly reported by patients with xerostomia [Citation6].
A study by Caielli et al. demonstrated that, in association with the reduction of salivary flow, xerostomia can also be characterised by salivary thickening and pH change during the treatment weeks. According to the authors, serous acini are the first to undergo alterations resulting from radiotherapy, followed by mucous membranes and duct cells. There is a consensus that changes in the salivary flow will happen when salivary glands receive more than 50 Gy of radiation, and below that, they can be transitory and limited [Citation7]. Xerostomia is commonly reported by patients after the first week of RT, and an increase in its severity is observed along the weeks of RT [Citation8]. The sensation of dry mouth may extend for long periods after the end of RT [Citation9].
The irradiated oral mucosa can be affected by bacterial, fungal and viral infections as described by different authors, exacerbating the manifestation of RT adverse effects [Citation10–Citation13]. The association between viral infections and oral complications from RT are poorly reported in the literature, and the existing results are contradictory. Some authors suggest that RT may cause a transient state of immunosuppression, thus contributing to reactivation of herpesviruses [Citation14]. Until this moment, there have been few studies in the literature relating oral shedding of human herpesviruses to xerostomia in patients undergoing RT associated with chemotherapy [Citation15–Citation17].
The aim of this study was to evaluate the oral shedding of human herpesviruses, namely, HSV-1 and 2 (herpes simplex viruses 1 and 2), EBV (Epstein-Barr virus), CMV (cytomegalovirus), VZV (varicella-zoster virus), HHV-6, HHV-7 and HHV-8 (human herpesviruses 6, 7 and 8), and their possible relationship with xerostomia in patients undergoing RT associated with chemotherapy for squamous cell carcinoma in the head and neck region.
Material and methods
This study was approved by the Research Ethics Committee of the University of São Paulo School of Medicine according to protocol number 910.924 (CAAE 37922114.9.0000.0065).
In our study, we have analysed 158 oral rinse samples which were collected weekly from 20 patients admitted to the Division of Dentistry of the São Paulo Institute of Cancer (ICESP) for RT for squamous cell carcinoma in head and neck. Patients were selected if they met the eligibility criteria as follows: diagnosis of head and neck squamous cell carcinoma, treatment protocol with three-dimensional RT (3D-RT) with a total dose of 60 Gy associated with chemotherapy with cisplatin 100 mg/m2, no surgery for tumour resection, dental treatment before starting RT, oral hygiene orientation and low-laser level therapy during RT to prevent and treat oral mucositis. The medical history of every patient was obtained through records for collection of data on gender, age, tumour location and the TNM classification of malignant tumours.
The treatment consisted of 3D-RT with a total dose of 60 Gy, divided into 30 fractions daily for five consecutive days a week for approximately six weeks. Chemotherapy was performed with cisplatin 100 mg/m2 in three cycles every 21 days (days 1, 22 and 43), starting on day one of RT.
The clinical evaluation of xerostomia was conducted by the same dentist, beginning at the screening visit and then throughout the six weeks of RT (after each RT session) and in the follow-up visit (1 month after the end of RT treatment). The evaluation was performed according to criteria set by the Seminars in Radiation Oncology () [Citation18].
Table 1. Classification of xerostomia (Seminars in Radiation Oncology, ref 18)
The serology for human herpesviruses was conducted at the Laboratory of Virology, Institute of Tropical Medicine of Sao Paulo, by using ELISA kits (AbCam®, Cambridge, UK) for HSV-1, HSV-2, CMV, VZV and EBV on three different occasions: screening visit, last RT session and 30 days after the end of RT.
Oral rinse samples were collected from patients by instructing them to perform a mouthwash with 5 ml of distilled water for 30 s. The samples were stored at −80°C after being simultaneously collected during evaluation of xerostomia, i.e. at the screening visit, in the six weeks of RT and on the follow-up visit.
From these samples, according to a previous work of ours [Citation13,Citation19], we extracted DNA by using a semi-automatic method (NucliSENS® easyMAG® system, BioMérieux, Durham, NC) before amplification with PCR by using two sets of primers (i.e. HSVP1/P2 and VZVP1/P2) which correspond to a DNA sequence alignment from eight human herpesvirus described by Johnson [Citation20] (). The first set, HSVP1/P2, amplifies the subtypes HSV-1, HSV-2, EBV, CMV and HHV-8 and the second set, VZVP1/P2, amplifies the subtypes VZV, HHV-6 and HHV-7. The positive samples were submitted to enzymatic digestion with BamHI and BstUI restriction enzymes (New England Biolabs) for specific determination of each one of the eight herpesviruses, also described in a previous work of ours [Citation13,Citation19].
Table 2. Sequence alignment for herpesviruses DNA
All the patients were daily submitted to prophylactic low-level laser therapy after each RT session, starting on the first day, as described previously [Citation13]. In addition to laser therapy, the patients were instructed about oral hygiene and also received artificial saliva and analgesic medication when necessary.
The results were presented in absolute and relative frequencies. For comparison of the frequencies of xerostomia grades in relation to the RT time points, we have used a chi-square test. McNemar´s test was applied for a paired analysis in relation to virus type at each time point. To compare the frequency of negative cases of herpesviruses at the moment of screening within the 1-month follow-up, we used Fisher´s Exact test. In order to assess whether there was a correlation between presence/grade of xerostomia and oral shedding of different herpesviruses, we have used Spearman’s test. The statistical software used was Biostat 5.0® (Belém, Pará, Brazil) at a significance level of 5%.
Results
Fifty patients diagnosed for squamous cell carcinoma of the head and neck undergoing RT combined with chemotherapy were evaluated. Only 25 patients (50%) started RT, whereas the others were excluded for different reasons, namely: changes or suspension in the RT protocol, patients moving to other cities, patients refusing to participate in the study during sample collection and death. Five patients did not complete the RT, either because of treatment suspension due to toxicity (three patients) or because of death (two patients). Therefore, 20 patients completed the RT and met the eligibility criteria of our study. The general characteristics of these patients were: 19 males and one female, age between 39 and 72 years old (mean = 54.4 years). The tumour sites were: six in the oropharynx and soft palate, four in the oropharynx and base of the tongue, four in the base of the tongue, two in the floor of the mouth, one in the oropharynx and retromolar trigone, one in the retromolar trigone, one in the gums and one in the jugal mucosa. In relation to the TNM classification, we have observed three patients rated as T4N3M0, 10 as T4N2M0, two as T4N2M0, one as T3N0M0, one as T2N2M0, two as T2N0M0 and one as T1N1M0. All these characteristics were also described in a previous published work of our group [Citation13].
Xerostomia
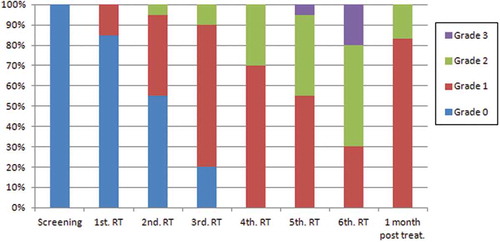
Grade 1 of xerostomia had the highest frequency (51.4%), which was statistically different compared to grade 2 (21%; chi-square test, p < 0.001) and grade 3 (3.6%, chi-square test, p < 0.001), by analysing the frequencies of xerostomia observed during and one month post-RT.
In the fourth week of RT, all patients presented some degree of xerostomia, but it was in the sixth week that the most severe degrees were observed ().
Considering the degrees of xerostomia which cause dietary changes (grades 2 e 3), a significantly higher frequency of these xerostomia grades was observed in the last 3 weeks of RT () (chi-square test, p < 0.001).
In the follow-up visit (i.e. one month after the end of RT), 10 patients (55%) presented a decrease in the xerostomia degree, but without complete remission. Fifteen patients (83.3%) presented grade 1 and three patients (16.0%) grade 2 xerostomia ().
Human herpesviruses
Serology
By analysing the IgG serology of these patients, which corresponds to previous contact with virus, it was possible to observe that the totality of patients (20 patients) presented IgG-antibodies to HSV1-HSV2, EBV and VZV in all three study periods: the screening visit, the last RT session and the follow-up visit. Only one patient was IgG negative for CMV in the three analysed periods.
Considering the IgM serology, which corresponds to recent viral contact or possible viral reactivation, one patient showed IgM-antibodies to HSV1-HSV2 and another one IgM-antibodies to CMV in all three study periods. Another patient presented IgM-antibodies to CMV detection in the last period, that is, 1 month after the end of RT, thus indicating a possible reactivation of this virus.
Oral shedding of human herpesviruses
By analysing the oral shedding results for human herpesvirus in saliva, the results showed 18 patients (90%) with oral shedding of EBV, 14 (70%) with HHV-7, seven (35%) with HSV-1 and three (15%) with HHV-6. Possible co-detections, with 15 patients (75%) presenting shedding of two or more herpesviruses, were also observed. Four patients (20%) presented oral shedding of EBV only and one (5%) presented negative results for all human herpesviruses. By analysing all the patients together, the EBV oral shedding frequency was significantly higher compared to that of other herpesviruses (chi-square test, p < 0.001).
Considering the oral shedding of human herpesviruses and the weeks of RT, the results showed oral shedding of EBV, HHV-6 and HHV-7 in all weeks of RT (). The oral shedding of HSV-1 was not observed in the screening visit (). shows the virus expression for each patient in accordance with the time points. The frequency of patients positive for EBV increased mainly from the second week, but the difference of this frequency in relation to the baseline (‘before RT’) was not significant (McNemar´s test, p = 0.070). The expression of the other virus types exhibited a great variance in the time points, but without significant differences in relation to the baseline ().
Table 3. Oral shedding for herpesviruses EBV, HHV-7, HSV-1 and HHV-6 in each patient in relation to the period before radiotherapy (RT) and each week during RT
Figure 2. Frequency of oral shedding of human herpesviruses in the screening, along the weeks of RT, and one month after RT
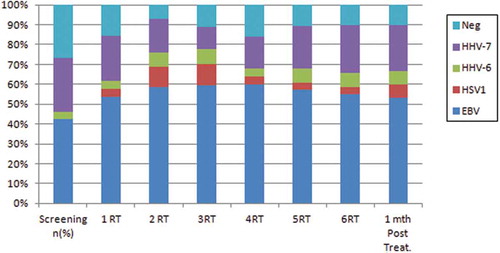
Analysis of the weeks of RT showed an increase in the number of patients with EBV oral shedding (85%) in the second week (). There was no statistically significant difference in the EBV oral shedding frequencies observed in the first three weeks and those observed in the last three weeks of RT.
During the RT, negative samples for herpesvirus oral shedding were observed in all weeks ( and ). In the screening visit, a higher number of patients were observed (seven patients, 35%). A statistically significant difference was found between the frequency of negative cases in the screening visit and that of the follow-up visit (1 month after RT) as there was a decrease in the number of negative cases () (11%; Fisher’s Exact test, p = 0.043).
Oral shedding of human herpesviruses and xerostomia
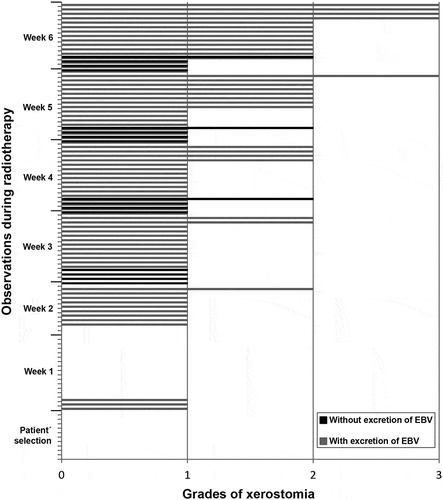
A correlation analysis between xerostomia and frequency of oral shedding of different viruses was performed. As for EBV, no significant correlation was found between frequency of xerostomia and excretion of EBV, not even when the most severe degrees of xerostomia (grade ≥ 2) were considered. shows the correlation between frequency of xerostomia and EBV excretion. It is noted that, in the majority of the cases, patients with and without excretion of EBV exhibited grade 1 xerostomia.
Figure 3. Frequency of EBV oral shedding in accordance with degrees of xerostomia observed in the screening and in each week of RT
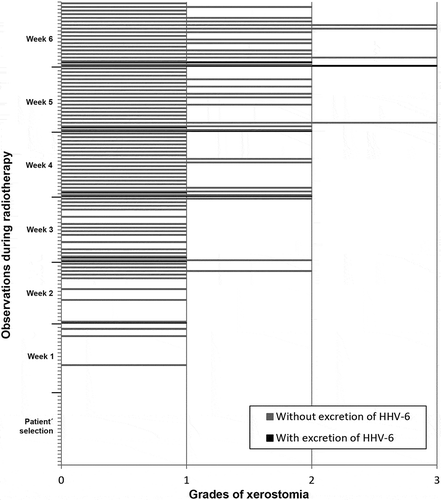
In the analysis of the correlations between excretion of HHV-7, HSV-1 and HHV-6 and presence/degree of xerostomia, there was no significant relationship. , and show the correlation between frequency of xerostomia and excretion of these viruses. The low number of cases in which HHV-7, HSV-1 and HHV-6 were excreted in association with a certain degree of xerostomia probably did not allow us to determine any correlation between these two variables.
Figure 4. Frequency of HHV-7 oral shedding in accordance with degrees of xerostomia observed in the screening and in each week of RT
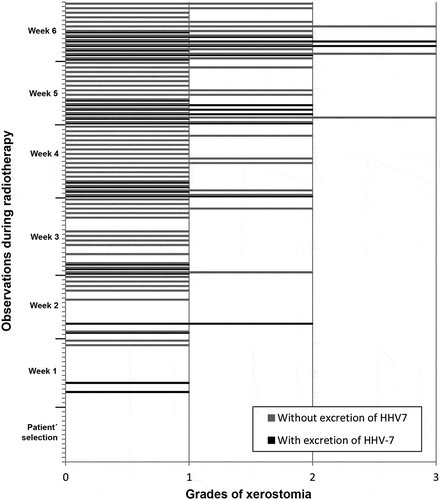
Figure 5. Frequency of HSV-1 oral shedding in accordance with degrees of xerostomia observed in the screening and in each week of RT
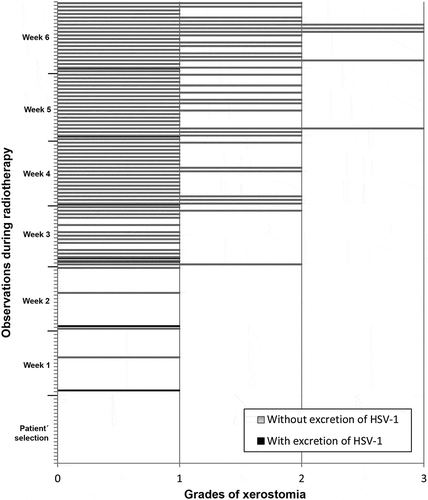
Figure 6. Frequency of HHV-6 oral shedding in accordance with degrees of xerostomia observed in the screening and in each week of RT
By analysing the degree of xerostomia and presence of virus co-detection, we did not found any significant correlation between the xerostomia grade ≥ 2 and presence of herpesvirus co-detection along the RT weeks.
Discussion
The RT used as a treatment for squamous cell carcinoma of the head and neck is widely known, as well as its oral complications. Patients undergoing RT may develop dysphagia, xerostomia, dysgeusia, mucositis and opportunistic infections (bacterial, fungal and viral) during treatment [Citation12,Citation21–Citation23].
Different authors report a variety of oral complications resulting from radiotherapy. Among them, a systematic review by Trotti et al. showed that xerostomia was frequently found [Citation24]. In the study by Cardoso et al. the authors observed that 100% of the patients irradiated with doses superior or equal to 60 Gy presented xerostomia [Citation25], which was also observed in our study as all patients presented some degree of xerostomia. According to studies by Mosel et al. and Trotti et al. the sensation of dry mouth is usually observed in the third or fourth session of radiotherapy due to the lower secretion of saliva by the glands involved in the radiation field [Citation12,Citation24]. This dry mouth sensation impairs greatly the quality of life of the patients, interfering not only with chewing and swallowing, but also with speech, which limits their social life. In our study, we have observed the first reports of xerostomia in the first week of RT, with three patients (15%) presenting xerostomia grade 1. The authors also reported that xerostomia can be transient or permanent, depending on the irradiated field [Citation12,Citation24,Citation25]. In our study, despite the short period of control after RT, 100% of the patients still had some degree of xerostomia in the follow-up visit, that is, one month after RT. Moreover, all the patients presented some degree of xerostomia in the fourth week of RT which persisted until the follow-up visit, meaning a possible permanent damage to the parenchyma of the salivary glands.
According to studies by Nicolatou-Galitis et al. and Correia et al. the great difficulty in comparing studies on xerostomia induced by head and neck RT and its possible correlation with HSV-1 is the lack of standardised samples. This may happen due to inclusion of different tumour types, patients with different protocol treatments for RT and/or chemotherapy, collection of oral rinsing samples performed at distinct moments, and lack of serological data [Citation16,Citation17,Citation26].
In the present study, all the patients included were diagnosed with squamous cell carcinoma of head and neck and whose treatment protocol was RT, at a total dose of 60 Gy, associated with chemotherapy (Cisplatin 100 mg/m2). They had been also submitted to dental treatment before starting RT and chemotherapy. All the patients were treated with preventive and curative laser therapy for oral mucositis. The differential factor of our work was the weekly collection of oral rinsing samples during the whole period of RT, thus enabling a profile of the salivary excretion to be traced for different herpesviruses over time.
Our results have shown that five patients (25%) were positive for HSV-1 throughout the RT and two patients (10%) in the follow-up visit (1 month after treatment), with a rate of excretion of 35%. Of the total of patients followed up, none of them presented positive sample for HSV-1 before the beginning of RT treatment. However, there was no correlation between HSV-1 oral shedding and onset or worsening of xerostomia as well as no correlation with RT times either. A possible hypothesis for this lack of correlation with the degrees of xerostomia may be the control of the oral excretion of HSV-1 by the application of low-level laser, with similar results to the control of herpes labialis lesions [Citation27–Citation33].
It is known that HHV-6 and HHV-7 herpesviruses can be detected in healthy individuals who are asymptomatic [Citation34] as exanthema subitum can be caused by a primary infection by HHV-6 and, less frequently, by HHV-7 [Citation35,Citation36]. The rate of oral excretion of HHV-6 in the healthy population is low (i.e. about 10%), while oral shedding rates of HHV-7 can vary from 12.6% to 90% depending on the population group [Citation34,Citation37]. HHV-6 and HHV-7 can be observed not only in healthy patients, but also in immunocompromised ones. A study by Pinheiro et al. evaluated the oral desquamation in children with positive HIV status, reporting that 68% of them presented HHV-6 and 18% presented HHV-7 in the oral cavity [Citation36]. HHV-6 oral shedding seems to be higher and continuous in children compared to adults [Citation38]. In our study, HHV-7 was the virus with the second highest frequency of oral shedding, responding for 70% of the positive samples (p = 0.001), whereas HHV-6 presented 15% of the oral shedding frequency. There was no statistical difference between HHV-6 and HSV-1 as well as no correlation between these viruses and xerostomia degrees or RT times.
In our study, EBV was the virus with the highest frequency of excretion during the study period, with 90% of the patients presenting positive sample. There was statistically significant difference compared to all other excreted viruses (p < 0.001). Ambinder et al. and Van der Beek et al. stated that 25%–98% of the saliva samples of immunosuppressed patients were positive for EBV as a result of chemotherapy [Citation14,Citation39]. According to the authors, all cancer patients presented some degree of immunosuppression, which may explain the higher rates of EBV excretion. It is widely reported in the literature that oncological patients present immunosuppression at different degrees, which may explain the higher rates of EBV excretion, as previously observed in other immunosuppressed groups for several reasons [Citation19,Citation40].
In another study by Pasoto et al. the authors analysed serum samples from 100 patients with Sjögren’s syndrome, a chronic autoimmune disease affecting exocrine glands (salivary and lachrymal) and which leads to xerostomia and keratitis sicca. As a result, they could assess the frequency of EBV antibodies in Sjögren’s patients, which were higher compared to healthy individuals. The authors concluded that EBV virus in the salivary glands, in its latent form, can occasionally resume its replicating form and become sub-clinically reactivated, suggesting that it could be a potentially aetiological candidate for triggering autoimmune diseases [Citation41].
In our study, we have sought to evaluate whether the decrease in salivary excretion leads to a decrease in the oral shedding of human herpesviruses in the oral cavity. Our results showed that the xerostomia caused by RT does not affect the excretion of human herpesviruses, since it was possible to observe an increase in oral shedding of these herpesviruses in the oral cavity, even in the weeks when xerostomia was more severe. A hypothesis for this fact is that RT may have caused an alteration in the inflammatory microenvironment of the oral mucosa, favouring a possible local replication of herpesviruses in the oral cavity and so increasing the presence of them in this region.
In conclusion, the oral shedding of human herpesviruses is not affected by the presence of xerostomia because there was a progression in relation to the excretion of herpesviruses even with evolution of xerostomia, suggesting a local viral replication in the oral cavity without being completely dependent of salivary excretion.
Acknowledgments
We would like to thank the volunteer participants for their time and dedication to this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Michelle Palmieri
Michelle Palmieri graduated in Dentistry, from University of Sao Paulo School of Dentistry (2003). She is Specialist in Oral and Maxillofacial Surgery and assistant professor at Oral and Maxilofacial Surgery Section at FUNDECTO - USP, since 2006. She earned her Master degree in Oral and Maxillofacial Pathology from University of Sao Paulo School of Dentistry in 2016. She is studying for her PhD degree in Oral and Maxillofacial Pathology from University of São Paulo School of Dentistry. She publishes articles in international and national journals, in the field of Oral and Maxillofacial Surgery and Oral Pathology.
Mariana Ornaghi
Mariana Ornaghi graduated in Dentistry, from Fundacao Herminio Ometto, UNIARARAS (2017). She participated in the program “Iniciacao Cientifica” from University of Sao Paulo School of Dentistry in 2016.
Victor Adriano de Oliveira Martins
Victor Adriano de Oliveira Martins graduated in Dentistry, from University of Sao Paulo School of Dentistry (2016). He participated in the program “Iniciacao Cientifica” in 2015 supported by FAPESP. He is Specialist in Oral and Maxillofacial Surgery from University of Sao Paulo School of Medicine. He is studying for his PhD degree in Rheumatology from University of Sao Paulo School of Medicine.
Luciana Correa
Luciana Correa is Associate Professor at Department of Stomatology, General Pathology, in the School of Dentistry, University of São Paulo since 2004. She has worked with oral mucosa repair since 2001, a period when she concluded her Doctorate in Oral Pathology at School of Dentistry, University of São Paulo. Her main research lines are focused on cell death pathways activated during injuries in the oral mucosa, mainly those provoked by chemotherapy and radiotherapy agents. In her researches, she also investigates the effect of photobiomodulation with low level laser therapy and photodynamic therapy on the process of tissue regeneration during these injuries.
Thais Bianca Brandao
Thaís Bianca Brandão is a Dentist and the Coordinator of Dental Oncology Service, Instituto do Câncer do Estado de São Paulo (ICESP), Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil. She received the Master in Science Degree in Prosthodontics (2011) from the University of São Paulo, Brazil and the PhD Title in Oral Medicine (2017) from the University of Campinas, Brazil. Her research interests are focuses in ‘maxillofacial rehabilitation of cancer patients’, ‘head and neck cancer’, and ‘oral toxicities of cancer treatment’.
Ana Carolina do Prado Ribeiro
Ana Carolina Prado-Ribeiro works as a Dentist and a Researcher at the Dental Oncology Service, Instituto do Câncer do Estado de São Paulo (ICESP), Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil. She is also a Lecturer atUniversidade Brasil, Fernandópolis, São Paulo, Brazil. She received the Master in Science Degree in Oral Medicine (2008) from Sao Paulo State University, Brazil, and the PhD Title in Oral Pathology (2011) from The University of Campinas, Brazil. Her main research interests are ‘head and neck cancer’ and ‘oral toxicities of cancer treatment’.
Laura Masami Sumita
Laura Massami Sumita is Research Associate at Department of Virology, University of Sao Paulo Institute of Tropical Medicine. She received her Master degree in Medical Sciences from the University of São Paulo Medical School in 2009. She participated in 36 publications in international and national scientific peer-reviewed journals.
Tania Regina Tozetto-Mendoza
Tania Regina Tozetto-Mendoza is Research Associate at the Department of Virology, University of São Paulo Institute of Tropical Medicine, since 2000. She received her Master (1997) and PhD (2013) degrees in Tropical Disease and International Health from University of São Paulo. She is supervisor of the Program of Specialists in Laboratorial Techniques in Virology and Tropical Hematology of Clinics Hospital of the University of São Paulo School of Medicine. She publishes articles in the field of molecular, cellular and serological diagnosis and her main research interest is in human herpesviruses.
Claudio Sergio Pannuti
Claudio Sergio Pannuti, PhD and MD, is Senior Professor at the Department of Virology, University of São Paulo Institute of Tropical Medicine. He is former Director of the Institute of Tropical Medicine of São Paulo and Head of the Virology Laboratory. He has over 140 publications in international and national peer-reviewed journals.
Paulo Henrique Braz-Silva
Paulo Henrique Braz-Silva is Assistant Professor at Division of Pathology, Department of Stomatology, University of Sao Paulo School of Dentistry and at the Department of Virology, University of Sao Paulo Institute of Tropical Medicine since 2014. He earned his Doctor of Dental Surgery (DDS) degree in 2003, and a Master degree in Oral and Maxillofacial Pathology in 2005 from University of São Paulo School of Dentistry. PhD in Oral and Maxillofacial Pathology from University of São Paulo School of Dentistry, and in Cellular and Molecular Biology from University of Nice Sophia Antipolis, France, in 2009. He has conducted researchers in viruses oral shedding in immunocompromised patients. He has over 40 publications in international and national peer-reviewed journals.
References
- Halperin EC, Perez CA, Brady LW. The discipline of radiation oncology. In: David E. Wazer (Ed.). Principles and practice of radiation oncology. Philadelphia (PA): Lippincott Willians & Wilkins; 2004. p. 4,6,10.
- Bhide SA, Nutting CM. Advances in radiotherapy for head and neck cancer. Oral Oncol. 2010;46:439–9.
- Johansson J, Blomquist E, Montelius A, et al. Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother Oncol. 2004;72:129–138.
- Fang FM, Chien CY, Tsai WL, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy - a longitudinal study. Int J Radiat Oncol Biol Phys. 2008;72:356–364.
- Parvathaneni U, Laramore GE, Liao JJ. Technical advances and pitfalls in head and neck radiotherapy. J Oncol. 2012;2012:597467.
- van der Laan HP, Bijl HP, Steenbakkers RJ, et al. Acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiother Oncol. 2015;115(1):56–62.
- Radvansky LJ, Pace MB, Siddiqui A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am J Health Syst Pharm. 2013;70(12):1025–1032.
- Mossman K, Shattzman A, Chencharick J. Longterm effects of radiotherapy on taste and salivary function in man. Int J RadiatOncolBiolPhys. 1982;8(6):991–997.
- Niedermeier W, Matthaeus C, Meyer C, et al. Radiation-induced hyposalivation and its treatment with oral pilocarpine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(5):541–549.
- Melkos AB, Massenkeil G, Arnold R, et al. Dental treatment prior to stem cell transplantation and its influence on the post transplantation outcome. Clin Oral Investig. 2003;7:113–115.
- Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients with cancer. Dent Clin North Am. 2008;52:1–17.
- Mosel DD, Bauer RL, Lynch DP, et al. Oral complications in treatment of cancer patients. Oral Diseases. 2011;17:550–559.
- Palmieri M, Martins VA, Sumita LM, et al. Oral shedding of human herpesviruses in patients undergoing radiotherapy/chemotherapy treatment for head and neck squamous cell carcinoma. Clin Oral Investig. 2017;21:2291–2301.
- Van der Beek MT, Laheij AM, Raber-Durlacher JE, et al. Viral loads and antiviral resistance of herpesviruses and oral ulcerations in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2012;47:1222–1228.
- Redding SW, Luce EB, Boren MW. Oral herpes simplex virus infection in patients receiving head and neck radiation. Oral Surg Oral Med Oral Pathol. 1990;69:578–580.
- Nicolatou-Galitis O, Athanassiadou P, Kouloulias V, et al. Herpes simplex virus-1 (HSV-1) infection in radiation-induced oral mucositis. Support Care Cancer. 2006;14:753–762.
- Correia AVL, Coelho MRCD, de Oliveira Mendes C, et al. Seroprevalence of HSV1-2 and correlation with aggravation of oral mucositis in patients with squamous cell carcinoma of the head and neck region submitted to antineoplasic treatment. Support Care Cancer. 2015;23:2105–2111.
- Camphausen K. Seminars in radiation oncology. Semin Radiat Oncol. 2009;19:141.
- De Santana Sarmento DJ, Tozetto-Mendoza TR, Masami Sumita L, et al. Oral shedding of human herpesviruses in renal transplant patients. Clin Oral Investig. 2017 Jul 1 [Epub ahead of print]. DOI:10.1007/s00784-017-2166-3
- Johnson G, Nelson S, Petric M, et al. Comprehensive PCR-based assay for detection and species identification of human herpesviruses. J Clin Microbiol. 2000;38:3274–3279.
- Andrews N, Griffiths C. Dental complications of head and neck radiotherapy: part 1. Aust Dent J. 2001;46:88–94.
- Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury- pathogenesis, measurement, epidemiology, and consequences for patients. Cancer Supp. 2004;100:1995–2025.
- Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck. 2004;26:77–84.
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262.
- Carneiro-Neto JN, de-Menezes JD, Moura LB, et al. Protocols for management of oral complications of chemotherapy and/or radiotherapy for oral cancer: systematic review and meta-analysis current. Med Oral Patol Oral Cir Bucal. 2017;22(1):e15–e23.
- Nicolatou-Galitis O, Dardoufas K, Markoulatos P, et al. Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J Oral Pathol Med. 2001;30:471–480.
- Carvalho RR, de Paula Eduardo F, Ramalho KM, et al. Effect of laser phototherapy on recurring herpes labialis prevention: an in vivo study. Lasers Med Sci. 2010;25:397–402.
- Bello-Silva MS, de Freitas PM, Aranha AC, et al. Low- and high-intensity lasers in the treatment of herpes simplex virus 1 infection. Photomed Laser Surg. 2010;28:135–139.
- Eduardo C, Bezinelli LM, Eduardo F, et al. Prevention of recurrent herpes labialis outbreaks through low-intensity laser therapy: a clinical protocol with 3-year follow-up. Lasers Med Sci. 2012;27(5):1077–1083.
- Muñoz Sanchez PJ, Capote Femenías JL, Díaz Tejeda A, et al. The effect of 670-nm low laser therapy on herpes simplex type 1. Photomed Laser Surg. 2012;30(1):37–40.
- de Paula Eduardo C, Aranha AC, Simões A, et al. Laser treatment of recurrent herpeslabialis: a literature review. Lasers Med Sci. 2014;29:1517–1529.
- Dougal G, Lee SY. Evaluation of the efficacy of low-level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin Exp Dermatol. 2003;38:713–718.
- Stona P, da Silva VE, Dos Santos Pires L, et al. Recurrent labial herpes simplex in pediatric dentistry: low-level laser therapy as a treatment option. Int J Clin Pediatr Dent. 2014;7(2):140–143.
- Biganzoli P, Ferreyra L, Sicilia P, et al. IgG subclasses and DNA detection of HHV-6 and HHV-7 in healthy individuals. J Med Virol. 2010;82:1679–1683.
- Ward KN. The natural history and laboratory diagnosis of human herpesviruses-6 and −7 infections in the immunocompetent. J Clin Virol. 2005;32:183–193.
- Pinheiro RS, Ferreira DC, Nóbrega F, et al. Current status of herpesvirus identification in the oral cavity of HIV-infected children. Rev Soc Bras Med Trop. 2013;46(1):15–19.
- Magalhães IM, Martins RV, Cossatis JJ, et al. Detection of human herpesvirus 6 and 7 DNA in saliva from healthy adults from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2010;105:925–927.
- Matrajt L, Gantt S, Mayer BT, et al. Virus and host-specific differences in oral human herpesvirus shedding kinetics among Ugandan women and children. Sci Rep. 2017;7:13105.
- Ambinder RF, Wingard JR, Burns WH, et al. Detection of Epstein-Barr virus DNA in mouthwashes by hybridization. J Clin Microbiol. 1985;21:353–356.
- Braz-Silva PH, Ortega KL, Rezende NP, et al. Detection of Epstein-Barr virus (EBV) in the oral mucosa of renal transplant patients. Diagn Cytopathol. 2006;34:24–28.
- Passoto SG, Natalino RR, Chakkour HP, et al. EBV reactivation serological profile in primary Sjögren’s syndrome: an underlying trigger of active articular involvement? Rheumatol Int. 2013;33(5):1149–1157.