ABSTRACT
Periodontitis, rheumatoid arthritis (RA), atherosclerosis (AS), and Alzheimer’s disease (AD) are examples of complex human diseases with chronic inflammatory components in their etiologies. The initial trigger of inflammation that progresses to these diseases remains unresolved. Porphyromonas gingivalis is unique in its ability to secrete the P. gingivalis-derived peptidyl arginine deiminase (PPAD) and consequently offers a plausible and exclusive link to these diseases through enzymatic conversion of arginine to citrulline. Citrullination is a post-translational enzymatic modification of arginine residues in proteins formed as part of normal physiological processes. However, PPAD has the potential to modify self (bacterial) and host proteins by deimination of arginine amino acid residues, preferentially at the C-terminus. Migration of P. gingivalis and/or its secreted PPAD into the bloodstream opens up the possibility that this enzyme will citrullinate proteins at disparate body sites. Citrullination is associated with the pathogenesis of multifactorial diseases such as RA and AD, which have an elusive external perpetrator as they show epidemiological associations with periodontitis. Therefore, PPAD deserves some prominence as an external antigen, in at least, a subset of RA and AD cases, with as yet unidentified, immune/genetic vulnerabilities.
Introduction
Investigating the effect of complex human diseases on specific organs has previously been the norm. However, new collaborative research assesses the knock on effect of diverse pathologies on conditions that develop because of the human ageing processes. Periodontitis (PD), rheumatoid arthritis (RA), atherosclerosis (AS), and Alzheimer’s disease (AD) are examples of complex diseases. There is a strong correlation of contributions from oral pathogens in their development without specific understanding of the mechanisms leading to the disease pathogenesis. The focus of this review is on the periodontal keystone pathogen Porphyromonas gingivalis [Citation1,Citation2], and its secreted peptidyl arginine deiminase (PPAD) enzyme in the development of the extraoral autoimmune and inflammatory diseases mentioned above [Citation3–Citation7] ().
Figure 1. Schematic to show additive effect from an oral condition such as periodontitis to the development of mixed pathologies through smoking, atherosclerosis, and rheumatoid arthritis with direct inflammatory mediator input from P. gingivalis infection to Alzheimer’s disease. The major arrows point to major risk factors with plausible effect on each condition. The three-way arrows provide explanation of the link with periodontitis
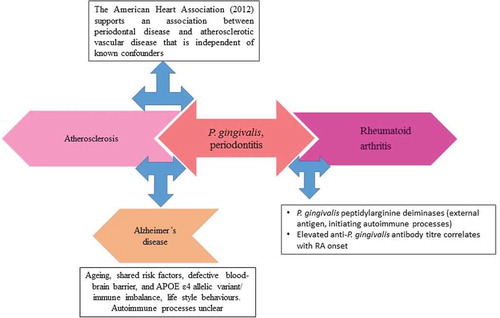
A chronic form of PD becomes prevalent in tricenarian/quadragenarian vulnerable hosts, whilst sporadic AD is common in octo-nonagenarians. P. gingivalis manipulates the host’s cellular immune responses and undermines host-microbe homeostasis [Citation5], thereby leading to dysbiosis in a previously symbiotic microbiome [Citation2]. Breakdown of cellular barriers allows dissemination of this pathogen to the rest of the body. Undoubtedly, human polymorphic genes do influence the susceptibility of the host to disease but they also affect the direction taken by the pathogens that use them for their survival and proliferation. Thus, P. gingivalis is a typical example of a pathogen that shows this trait by adapting to challenging inflammophilic environments of the host directed to kill it [Citation8]. The virulence and potential pathogenic effects of P. gingivalis are diverse and, through them, this bacterium can affect many different organs and diseases [Citation3,Citation6,Citation7,Citation9,Citation10]. The virulence factor under focus here is the enzyme P. gingivalis peptidyl-arginine deiminase (PPAD). This enzyme modifies both bacterial and host proteins by deimination of arginine residues in proteins and peptides, converting them to citrulline [Citation11–Citation13] (). Protein citrullination causes deregulation of the host’s inflammatory signalling network by altering the spatial arrangement of the original 3D-structure and function of the protein [Citation3,Citation6]. This may lead to exposure of damage- and/or pathogen associated molecular patterns (PAMP/DAMP) which can then be used by pattern recognition receptors (PRR), to provide entry and immune evasion [Citation14]. Subsequent immune responses directed against the bacterial antigens by the infected organ can lead to tissue damage. In some individuals, this can initiate autoimmune responses [Citation3,Citation6] (). At present, P. gingivalis is the only known pathogen that produces PPAD [Citation3]. This gives the bacterium prominence in causing both periodontitis and extraoral diseases, especially RA.
Figure 2. Chemical modification of arginine amino acid residue by P. gingivalis and host-mediated peptidylarginine deiminases resulting in the conversion of arginine on functional peptide to citrulline (defective protein). This posttranslational modification alters the spatial arrangement of the original 3D-structure and function of the protein peptide as indicated by arrows (arginine-peptide and citrulline-peptide). Ammonia released during the chemical reaction is beneficial for PPAD activation
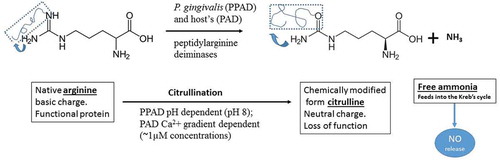
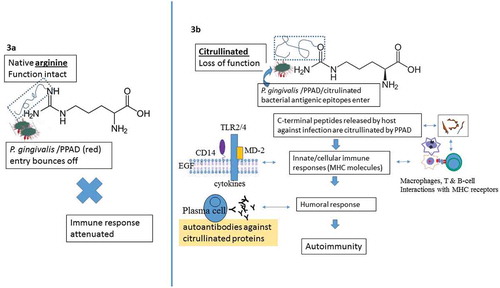
Figure 3. Schematic to illustrate immune events leading to autoimmunity resulting from PPAD-mediated citrullination of C-terminal arginine residues. These citrullinated peptides produced by the combined action of gingipains cleave polypeptides into fragments. This results in PPAD structurally becoming closer to gingipains on the bacterial surface membrane
The host also has intrinsic sources of citrullination due to genes coding for a family of enzymes called peptidyl arginine deiminases (PADs). Although the human peptidylarginine deiminase family contains five isotypes (PAD 1, 2, 3, 4/5 and 6) with tissue specific expression [Citation15], there is a paucity of information on the P. gingivalis PPAD(s) infecting different tissues and cells [Citation16]. Protein citrullination is important for many normal physiological processes such as epithelial terminal differentiation, regulation of gene expression, apoptosis, and inflammation [Citation14,Citation15,Citation17]. However, the posttranslational modification involving the citrullination process can affect the function of several signalling molecules as well as protein structures and functions. One such example is C5a anaphylatoxin. This is a glycoprotein with a number of arginine residues that are released following complement activation. Functional C5a induces vasodilation and chemotaxis of inflammatory cells in the site of injury. On citrullination by PPAD, C5a loses this function [Citation18]. It is not surprising therefore that an increased citrullination of cytoskeletal filaments and PAD enzymes have been found in numerous chronic inflammatory and autoimmune diseases like AD, RA, and multiple sclerosis (MS), respectively [Citation15,Citation19]. Owing to their similar etiologies, PPAD deserves some consideration as an extrinsic antigen in the pathogenesis of RA and MS, thus contributing to autoimmune processes. The physiological conditions and the specific arginine residues targeted for deimination by PPAD and/or host PADs remain differential. For example, PPAD requires a higher pH for its activity and preferentially targets carboxy-terminal and free arginine residues [Citation20] cleaved by arginine-gingipains (Rgps). The human PADs, on the other hand, exclusively citrullinate internal peptidyl arginine residues [Citation11,Citation21], which are activated following an influx of calcium ions from the extracellular environment or from the cytosol [Citation22]. Although both (host and bacterial) enzymes catalyse the same chemical reaction [Citation12] (), identification of either PPAD or PAD citrullinated arginine residues in cells presents technical challenges. This may limit progress in our ability to differentiate PPAD citrullinated arginine residues from those of the host. The aim of the present review is to discuss the possible importance of citrullination in the pathogenesis of PD, RA, AS, and AD, which are common, complex, chronic inflammatory diseases with unclear etiologies.
Periodontitis and citrullination
Periodontitis is an inflammatory oral disease affecting the tissues supporting teeth in their bony sockets and occurs in two forms, aggressive and chronic. If left untreated, periodontitis will lead to loss of teeth. Although, PD is not an autoimmune disease per se, P. gingivalis infection has the potential to induce autoimmune responses in oral tissues [Citation23]. Periodontitis affects approximately 65 million (47%) US adults, 30 years and older [Citation24]. By adopting a more resilient phenotype through selecting different signalling pathway molecules, in vitro studies demonstrate the survival ‘instincts’ of this pathogen under both poor and sufficient bioavailability of haemin [Citation25,Citation26]. This keystone pathogen with its companion species is associated with the initiation and progression of chronic periodontitis by secretion of several virulence factors including Rgps and PPAD in the periodontal pocket [Citation27,Citation28].
Citrullinated peptides initiated by P. gingivalis are produced by the combined action of Rgps that cleave polypeptides into fragments with C-terminal arginine, followed by citrullination with PPAD [Citation29]. Thus, citrullination of surface proteins depends on the action of Rgp proteases. The modification of C-terminal arginine residues is the result of PPAD becoming structurally closer to Rgps on the bacterial surface. This dual enzymatic action of modification was initially reported following production of citrullinated peptides derived from fibrinogen and a-enolase by PPAD [Citation30]. These two proteins are major auto-antigens in RA [Citation3]. The secreted PPAD may spread deeply within the connective tissue by shedding P. gingivalis outer membrane vesicles, or through tissue diffusion of the soluble enzyme [Citation13]. The soluble enzyme modifies the epidermal growth factor (EGF) located in the inflamed periodontium which subsequently interferes with EGF function by blocking the recognition between the epithelium and the EGF signalling pathway molecules [Citation13]. This is a mechanism for breaking local protective epithelial cell–periodontal tissue barriers and delaying the healing process [Citation13]. In addition, PPAD inhibits the ability of EGF to stimulate epidermal cell proliferation and migration and prevents epidermal growth factor receptor (EGFR)–EGF interaction-dependent stimulation by suppressing cytokine signalling 3 and interferon regulatory factor 1 signalling [Citation13]. Ammonia produced as a byproduct during deimination can promote the survival of P. gingivalis in the periodontal pocket [Citation12] and potentially optimize the pH-dependent function of Rgp and PPAD. In this way, Rgps and PPAD may inactivate hemagglutinins, promote ATP production, and exert negative effects on neutrophil functions [Citation11,Citation13,Citation27,Citation31].
P. gingivalis also citrullinates the proteins associated with its cell envelope [Citation3], thus generating in a PPAD-dependent manner, a pool of potent antigenic epitopes that can break the tolerance to specific citrullinated host peptides [Citation14]. The loss of tolerance can generate autoantibodies against citrullinated proteins (ACPAs). Increased levels of ACPAs are detected in patients with aggressive periodontitis [Citation32] compared to controls [Citation33]. ACPA-positive patients are also more inclined to have moderate to severe PD than ACPA-negative patients [Citation34]. P. gingivalis infection in PD implies the induction of autoimmune responses that are characteristic of RA. Shimada et al. [Citation35] found an association between anti-PPAD IgG and anti-cyclic citrullinated peptide (CCP) IgG responses, and proposed a role for PPAD in protein citrullination of patients with both PD and RA.
Rheumatoid arthritis and citrullination
RA is an autoimmune disorder that occurs when the host is unable to differentiate self from non-self-antigens. This can give rise to an immune system mistakenly attacking the host (self) tissues. RA manifests as painful and chronic inflammation of the joints. It also affects other areas of the body including skin, eyes, lungs, heart and blood vessels. Patients with RA have a higher frequency of morbidity and mortality from cardiovascular dysfunctions [Citation36]. Most data from the developed world estimates an RA prevalence between 0.5 and 1% [Citation37], with a mortality risk of 1.5–1.6 greater than that of the general population [Citation38]. Citrullinated protein and anti-citrullinated protein antibodies play important roles in RA development [Citation39]. The PAD4 gene encoding the PAD4 protein is one of the RA risk genes associated with protein citrullination [Citation40], and anti-PAD4 antibodies are specific markers of RA [Citation18]. Badillo-Soto et al. [Citation41] suggested that citrullination of synovial proteins is PAD2- and PAD4-dependent and both these enzymes have been detected in the RA synovium [Citation42]. These authors also found that RA patients have high titres of antibodies preferentially binding to fibrinogen citrullinated by PAD4. Seri et al. [Citation43] reported data from mice suggesting that PAD4 deficiency reduced the severity of arthritis in a glucose-6-phosphate isomerase-induced arthritis model. An explanation could be that chronic exposure to citrullinated proteins in the periodontal pocket may predispose susceptible individuals to generate ACPA in the synovia with subsequent development of RA. This is because ACPA titres in RA patients correlate with the presence of PD [Citation34].
Atherosclerosis and citrullination
Atherosclerosis occurs when the arteries become narrower and harden due to an excessive build-up of plaque within the lumen. The plaque reduces the blood flow around the body, causing ischaemia, which in turn may lead to cardiovascular complications. Atherosclerosis is common in the elderly and remains the major cause of death and disability in this group. The American Heart Association (AHA) recognizes that PD is independently associated with arteriosclerotic vascular disease (ASVD) [Citation44]. The connection between citrullinated peptides and the development of atherosclerosis remains unclear in comparison to RA. Citrullinated proteins are detected in atherosclerotic plaques [Citation45,Citation46], but their true relevance here has not been clarified. This observation has provided a rationale for using citrullinated histone seroreactivity as a biomarker for atherosclerosis [Citation45]. It may reveal citrullinated fibrinogen (cFb) within atherosclerotic plaque and therefore could underpin the reason for accelerated atherosclerosis in RA patients. Geraldino-Pardilla et al. [Citation46] reported that higher levels of ACPAs targeting citrullinated histone 2B were associated with higher coronary artery calcium (CAC) scores when compared with lower antibody levels, suggesting a potential role of seroreactivity to citrullinated histone in the pathogenesis of atherosclerosis [Citation46].
PPAD acting together with arginine-specific proteinases from P. gingivalis may promote the growth of this pathogen in the periodontal pocket [Citation11]. PPAD deiminates the guanidine group of C-terminal arginine residues on a variety of peptides, including the vasoregulatory peptide-hormone bradykinin yielding a citrulline residue and ammonia [Citation11]. Citrullinated bradykinin must be resistant to inactivation by ubiquitous Arg-specific tissue and cell-surface associated carboxypeptidases thus prolonging its vasodilatory activity. Such pathophysiological events may allow bacteria to penetrate the vasculature, and advance the development of cardiovascular disease [Citation47]. As P. gingivalis has a high dependency on environmental haem [Citation48], sourced from lysing erythrocytes, this activity implies host deprivation of its ample supply of oxygen. This would also promote hypoxic conditions as well as development of atherosclerotic plaque.
A recent report described citrullination that was unique to the cardiac proteome [Citation49]. Protein citrullination appeared to have caused important structural alterations in the cardiac sarcomere with subsequent detrimental consequences to the myocardium of patients who died of heart failure. This implied that pathogenic citrullination occurs in systemic diseases but further research is needed to understand its adverse role in heart-related tissues.
Citrullinated proteins such as fibrinogen and vimentin are associated with the CAC score. Sokolove et al. [Citation45] demonstrated fibrinogen and vimentin CAC scores in 134 patients with RA diagnosis. Previously mentioned PAD4 enzymes and citrullinated proteins have been detected within atherosclerotic plaques and ACPAs from atherosclerotic plaques in RA patients [Citation45]. In a cohort of 3,052 healthy males, Cambridge et al. [Citation50] monitored ACPAs for the development of coronary artery disease. Their results showed that 10.4% of the cases were ACPA positive compared to 3.8% of controls. Statistical significance remained after controlling for classical risk factors such as smoking and C-reactive protein (p = 0.02). If PD had been monitored in the same cohort this would have demonstrated a prominent role for P. gingivalis in the production of ACPAs. Strong staining for citrulline was detected in the myocardial interstitium of RA patients compared to rheumatoid disease and controls [Citation51] and staining for citrullination was higher in the myocardial interstitium of RA patients compared to other diseases. Since there is extensive citrullination in the normal myocardium of RA patients as well as in the atherosclerotic plaque, there is potential for protein citrullination to promote cardiovascular pathology within the population at large [Citation52]. In this context, serum antibodies to citrullinated proteins may be a risk factor for coronary heart disease.
Alzheimer’s disease and citrullination
AD is the most common form of dementia, constituting 60–80% of all dementias, and has two forms. The sporadic form is common with potential for the role for susceptibility genes and pathogens as well as co-morbidities, similar to those implicated in the aforementioned human complex diseases [Citation7]. Due to the rising number of dementia cases, and the paucity of adequate treatment for AD, there is a consensus for preventing the disease in at least a third of all sporadic AD cases by modifying behavioural life-styles [Citation10]. A person showing symptoms of AD can have difficulty in remembering words, suffer from depression and show behavioural changes and confusion. The benchmark of AD confirmation is the presence of two key neurohistological hallmarks namely beta amyloid (Aβ) plaques, and neurofibrillary tangles (NFTs) comprising hyper-phosphorylated tau protein in specific anatomical regions of the brain. All risk factors that apply to heart disease also apply to AD. An underlying feature of stroke pathology includes vascular infections where P. gingivalis is often identified [Citation53]. Increased evidence links peripheral infections with AD, and lipopolysaccharide (LPS) entry into the ageing brain generates cytokines via innate immune responses () [Citation9]. LPS ischaemia from atherosclerosis and hypoxia link P. gingivalis with early death of erythrocytes for the supply of haem. All are potential causes for the development of sporadic AD. P. gingivalis was shown to migrate from its oral location to the brain [Citation54,Citation55] where it invoked inflammatory responses typical of neurodegenerative diseases in mice with blood–brain barrier (BBB) damage [Citation55,Citation56]. Ishigami et al. [Citation57] identified glial fibrillary acidic protein (GFAP), a marker of astrocytes in the AD brain, to be a substrate for host PAD2, and suggested a role for the citrullinated GFAP in the progression of this neurodegenerative disease. GFAP deimination was characteristic for AD in humans and in experimental autoimmune encephalomyelitis (EAE) in mice where the BBB was breached [Citation58–Citation60]. Although not conclusively shown, citrullinated GFAP (dysfunctional protein) would link with defects in BBB integrity because the foot processes of astrocytes tightly cover capillary openings in the endothelial cell junctions to protect neurons from extrinsic insults. Another report implicated brain-reactive autoantibodies to AD in relation to BBB breakdown and to certain cytoskeletal proteins such as tubulin, GFAP, and S-100 [Citation61] (). The relevance of humoral responses in the pathophysiology of AD, is little understood; however, by analogy, RA patients having a high titre of antibodies in their serum suggests a strong possibility of PPAD/host PAD mediated loss of tolerance against citrullinated nerve tract covering myelin sheath proteins in AD.
Acharya et al. [Citation62] confirmed citrullination of pyramidal neuronal intracellular proteins in the AD hippocampus. Antibodies against myelin basic protein (MBP) are detectable in serum of patients with active demyelinating lesions in MS [Citation63]. Analogous to MS as an autoimmune condition, damaged myelin interacts with Aβ deposits in AD and antibodies to glial derived antigens are reported [Citation64]. Acharya et al. [Citation62] suggested that autoantibodies in AD associate with host PAD4 and protein citrullination, although it is not clear if this refers to the antibodies reported by Papuc et al. [Citation64]. Matsuomi et al. [Citation65] found an abnormal accumulation of citrullinated proteins and an increase of the PAD2 content in the hippocampi of AD patients. The most over-citrullinated proteins in AD were structural proteins such as vimentin, MBP and GFAP [Citation15]. Neurodegenerative processes following P. gingivalis infection in animal models are currently under-investigated and further research is essential to determine PD-related causal factors in AD.
Over expression of PADs and protein citrullination are abnormal features of neurodegeneration and inflammatory diseases [Citation66] and have actually been proposed as a possible cause of AD [Citation67]. During neurodegenerative processes, it has been hypothesized that a higher concentration of Ca++ activates citrullination [Citation15] but, P. gingivalis LPS and intact citrulline constitute potential contributory factors [Citation68].
PAD2 and PAD4 enzymes are detected in astrocytes and neurons, and there is a concomitant accumulation of citrullinated proteins within PAD4-expressing cells including neurons of the hippocampus and the cerebral cortex [Citation62,Citation65]. This implies that citrullination of neuronal cytoskeletal proteins may be toxic, because disease-associated neuronal loss appears to result in the release of their cellular contents into the cerebral parenchyma from which they enter the blood and lymph circulation. Some of them are able to elicit an immune response that results in the production of autoantibodies [Citation62]. Inhibition of PAD may therefore be worth serious therapeutic consideration in the treatment/prophylaxis of diseases where citrullination takes place.
Concluding remarks
Conversion of arginine to citrulline is a post-translational modification that occurs during normal physiological processes. Conversely, abnormal citrullination can lead to severe human diseases. Epidemiologically, there seems to be a correlation between citrullination caused by P. gingivalis and PD [Citation69,Citation70] and between P. gingivalis and RA [Citation14,Citation71–Citation73]. There is also an association between AS and RA [Citation74]. In reality, cardiovascular disease, including coronary heart disease is a significant cause of death in RA [Citation75] and AD patients [Citation76]. P. gingivalis secreting PPAD is related to both RA [Citation14] and AS [Citation77]. It seems plausible that PPAD through its ability to citrullinate proteins could contribute to PD, RA, AS, and AD through increased inflammation, although currently the anti-PPAD antibody response remains unique to RA. P. gingivalis infection may precede RA but whether it is a direct cause is controversial despite supporting data from studies on animals in the development and aggravation of experimental arthritis [Citation72]. Overall, citrullination may contribute to a better understanding of host proteins [Citation14]. There is great heterogeneity in the extracellular proteome and citrullinome of P. gingivalis [Citation78]. This adds to the well-known fact that different strains of P. gingivalis have different degrees of virulence. The suggestions outlined above would benefit from more scientific support and drawing firm conclusions on them at this time would be inappropriate. However, if future research shows them to be correct; it would emphasize the need for prevention and aggressive treatment of advancing periodontitis where P. gingivalis is a keystone pathogen, but not necessarily the only one. It is also clear that P. gingivalis as a keystone pathogen is only present at high prevalence rates in the host subset susceptible to PD. Therefore, although the P. gingivalis-PPAD-systemic disease axis is a compelling line of thought, this may not be true for all patients with RA or AD where P. gingivalis has been implicated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:1–8.
- Hajishengallis G, Darveau RP, Curtis MA. The keystone pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725.
- Wegner N, Lundberg K, Kinloch A, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54.
- Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol. 2015;7:29143.
- Olsen I, Lambris JD, Hajishengallis G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J Oral Microbiol. 2017;9:1340085.
- Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:606–620.
- Carter CJ, France J, Crean S, et al. The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to Alzheimer’s disease, diabetes and cardiovascular diseases. Front Aging Neurosci. 2017;9:408.
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257.
- Olsen I, Taubman MA, Singhrao SK. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer’s disease. J Oral Microbiol. 2016;8:33029.
- Harding A, Robinson S, Crean S, et al. Can better management of periodontal disease delay the onset and progression of Alzheimer’s disease? J Alzheimers Dis. 2017;58:337–348.
- McGraw WT, Potempa J, Farley D, et al. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 1999;67:3248–3256.
- Rodríguez SB, Stitt BL, Ash DE. Expression of peptidylarginine deiminase from Porphyromonas gingivalis in Escherichia coli: enzyme purification and characterization. Arch Biochem Biophys. 2009;488:14–22.
- Pyrc K, Milewska A, Kantyka T, et al. Inactivation of epidermal growth factor by Porphyromonas gingivalis as a potential mechanism for periodontal tissue damage. Infect Immun. 2013;81:55–64.
- Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014;16:408.
- György B, Tóth E, Tarcsa E, et al. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677.
- Marchant C, Smith MD, Proudman S, et al. Effect of Porphyromonas gingivalis on citrullination of proteins by macrophages in vitro. J Periodontol. 2013;84:1272–1280.
- Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213.
- Bielecka E, Scavenius C, Kantyka T, et al. Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J Biol Chem. 2014;289:32481–32487.
- Goulas T, Mizgalska D, Garcia-Ferrer I, et al. Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci Rep. 2015;5:11969.
- Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008;58:1958–1967.
- Zhao J, Zhao Y, He J, et al. Prevalence and significance of anti-peptidylarginine deiminase 4 antibodies in rheumatoid arthritis. J Rheumatol. 2008;35:969–974.
- Jones J, Causey CP, Knuckley B, et al. Protein arginine deiminase 4 (PAD4): current understanding and future therapeutic potential. Curr Opin Drug Discov Devel. 2009;12:616–627.
- Jeong E, Lee JY, Kim SJ, et al. Predominant immunoreactivity of Porphyromonas gingivalis heat shock protein in autoimmune diseases. J Periodontal Res. 2012;47:811–816.
- Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the USA: 2009 and 2010. J Dent Res. 2012;91:914–920.
- Al-Qutub MN, Braham PH, Karimi-Naser LM, et al. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 2006;74:4474–4485.
- Herath TD, Wang Y, Seneviratne CJ, et al. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity differentially modulates the expression of IL-6 and IL-8 in human gingival fibroblasts. J Clin Periodontol. 2011;38:694–701.
- Niederman R, Brunkhorst B, Smith S, et al. Ammonia as a potential mediator of adult human periodontal infection: inhibition of neutrophil function. Arch Oral Biol. 1990;35(Suppl):205S–209S.
- Takahashi N, Saito K, Schachtele CF, et al. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol Immunol. 1997;12:323–328.
- Quirke A-M, Lugli EB, Wegner N, et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 2014;73:263–269.
- Wegner N, Wait R, Sroka A, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672.
- Takahashi N. Oral microbiome metabolism: from “who are they?” to “what are they doing?”. J Dent Res. 2015;94:1628–1637.
- Hendler A, Mulli TK, Hughes FJ, et al. Involvement of autoimmunity in the pathogenesis of aggressive periodontitis. J Dent Res. 2010;89:1389–1394.
- Lappin DF, Apatzidou D, Quirke A-M, et al. Influence of periodontal disease, Porphyromonas gingivalis and cigarette smoking on systemic anti-citrullinated peptide antibody titres. J Clin Periodontol. 2013;40:907–915.
- Dissick A, Redman RS, Jones M, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81:223–230.
- Shimada A, Kobayashi T, Ito S, et al. Expression of anti-Porphyromonas gingivalis peptidylarginine deiminase immunoglobulin G and peptidylarginine deiminase-4 in patients with rheumatoid arthritis and periodontitis. J Periodontal Res. 2016;51:103–111.
- Solaro RJ. A sarcomeric protein tongue-twister: post-translation, citrullination/deimination and elimination of arginine residues. Cardiovasc Res. 2015;108:212–214.
- Bernatsky S, Dekis A, Hudson M, et al. Rheumatoid arthritis prevalence in Quebec. BMC Res Notes. 2014;7:937.
- Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(Suppl 51):S35–S61.
- Suzuki A, Yamada R, Yamamoto K. Citrullination by peptidylarginine deiminase in rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:323–339.
- Shoda H. Citrullination and rheumatoid arthritis. Nihon Rinsho. 2016;74: 902–906. (Article in Japanese, English summary).
- Badillo-Soto MA, Rodríguez-Rodríguez M, Pérez-Pérez ME, et al. Potential protein targets of the peptidylarginine deiminase 2 and peptidylarginine deiminase 4 enzymes in rheumatoid synovial tissue and its possible meaning. Eur J Rheumatol. 2016;3:44–49.
- Blachère NE, Parveen S, Frank MO, et al. High titer rheumatoid arthritis antibodies preferentially bind fibrinogen citrullinated by peptidyl arginine deiminase 4. Arthritis Rheumatol. 2016; DOI:10.1002/art.40035.
- Seri Y, Shoda H, Suzuki A, et al. Peptidylarginine deiminase type 4 deficiency reduced arthritis severity in a glucose-6-phosphate isomerase-induced arthritis model. Sci Rep. 2015;5:13041.
- Bale BF, Doneen AL, Vigerust DJ. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis. Postgrad Med J. 2016;ii . postgradmedj-2016-134279. DOI:10.1136/postgradmedj-2016-134279.
- Sokolove J, Brennan MJ, Sharpe O, et al. Citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013;65:1719–1724.
- Geraldino-Pardilla L, Giles JT, Sokolove J, et al. Association of anti-citrullinated peptide antibodies with coronary artery calcification in rheumatoid arthritis. Arthritis Care Res. (Hoboken). 2016; DOI:10.1002/acr.23106.
- Imamura T, Potempa J, Pike RN, et al. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect Immun. 1995;63:1999–2003.
- Butler CA, Dashper SG, Zhang L, et al. The Porphyromonas gingivalis ferric uptake regulator orthologue binds haemin and regulates haemin-responsive biofilm development. PLoS One. 2014;9:e111168.
- Fert-Bober J, Giles JT, Holewinski RJ, et al. Citrullination of myofilament proteins in heart failure. Cardiovasc Res. 2015;108:232–242.
- Cambridge G, Acharya J, Cooper JA, et al. Antibodies to citrullinated peptides and risk of coronary heart disease. Atherosclerosis. 2013;228:243–246.
- Giles JT, Fert-Bober J, Park JK, et al. Myocardial citrullination in rheumatoid arthritis: a correlative histopathologic study. Arthritis Res Ther. 2012;14:R39.
- Fert-Bober J, Sokolove J. Proteomics of citrullination in cardiovascular disease. Proteomics Clin Appl. 2014;8:522–533.
- Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. 2015;7:28788.
- Poole S, Singhrao SK, Chukkapalli S, et al. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE-/- mice brains. J Alzheimers Dis. 2015;43:67–80.
- Singhrao SK, Chukkapalli S, Poole S, et al. Chronic Porphyromonas gingivalis infection accelerates the occurrence of age-related granules in ApoE−/- mice brains. J Oral Microbiol. 2017;9:1:1270602.
- Rokad F, Moseley R, Hardy RS, et al. Cerebral oxidative stress and microvasculature defects in TNF-α expressing transgenic and Porphyromonas gingivalis-infected ApoE-/- mice. J Alzheimers Dis. 2017;60:359–369.
- Ishigami A, Masutomi H, Handa S, et al. Mass spectrometric identification of citrullination sites and immunohistochemical detection of citrullinated glial fibrillary acidic protein in Alzheimer’s disease brains. J Neurosci Res. 2015;93:1664–1674.
- Ishigami A, Ohsawa T, Hiratsuka M, et al. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J Neurosci Res. 2005;80:120–128.
- Nicholas AP, Sambandam T, Echols JD, et al. Expression of citrullinated proteins in murine experimental autoimmune encephalomyelitis. J Comp Neurol. 2005;486:254–266.
- Raijmakers R, Vogelzangs J, Croxford JL, et al. Citrullination of central nervous system proteins during the development of experimental autoimmune encephalomyelitis. J Comp Neurol. 2005;486:243–253.
- Levin EC, Acharya NK, Han M, et al. Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood-brain barrier breakdown. Brain Res. 2010;1345:221–232.
- Acharya NK, Nagele EP, Han M, et al. Neuronal PAD4 expression and protein citrullination: possible role in production of autoantibodies associated with neurodegenerative disease. J Autoimmun. 2012;38:369–380.
- Schirmer L, Srivastava R, Hemmer B. To look for a needle in a haystack: the search for autoantibodies in multiple sclerosis. Mult Scler. 2014;20:271–279.
- Papuć E, Kurys-Denis E, Krupski W, et al. Can antibodies against glial derived antigens be early biomarkers of hippocampal demyelination and memory loss in Alzheimer’s disease? J Alzheimers Dis. 2015;48:115–121.
- Masutomi H, Kawashima S, Kondo Y, et al. Induction of peptidylarginine deiminase 2 and 3 by dibutyryl cAMP via cAMP-PKA signaling in human astrocytoma U-251MG cells. J Neurosci Res. 2016; DOI:10.1002/jnr.23959.
- Jang B, Ishigami A, Maruyama N, et al. Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration. Prion. 2013;7:42–46.
- Ishigami A, Maruyama N. Importance of research on peptidylarginine deiminase and citrullinated proteins in age-related disease. Geriatr Gerontol Int. 2010;10(Suppl 1):S53–S58.
- Poole S, Singhrao SK, Kesavalu L, et al. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 2013;36:665–677.
- Gully N, Bright R, Marino V, et al. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS One. 2014;9:e100838.
- Harvey GP, Fitzsimmons TR, Dhamarpatni AA, et al. Expression of peptidylarginine deiminase-2 and −4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodontal Res. 2013;48:252–261.
- de Smit MJ, Brouwer E, Vissink A, et al. Rheumatoid arthritis and periodontitis; a possible link via citrullination. Anaerobe. 2011;17:196–200.
- de Smit MJ, Westra J, Brouwer E, et al. Periodontitis and rheumatoid arthritis: what do we know? J Periodontol. 2015;86:1013–1019.
- Gabarrini G, de Smit M, Westra J, et al. The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis. Sci Rep. 2015;5:13936.
- Spinelli FR, Pecani A, Conti F, et al. Post-translational modifications in rheumatoid arthritis and atherosclerosis: focus on citrullination and carbamylation. J Int Med Res. 2016;44(1S):81–84.
- Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307.
- Park J. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–145.
- Hussain M, Stover CM, Dupont A. P. gingivalis in periodontal disease and atherosclerosis – scenes of action for antimicrobial peptides and complement. Front Immunol. 2015;6:45.
- Stobernack T, Glasner C, Junker S, et al. Extracellular proteome and citrullinome of the oral pathogen Porphyromonas gingivalis. J Proteome Res. 2016;15:4532–4543.
