ABSTRACT
Background: Streptococcus mutans orchestrates the development of a biofilm that causes dental caries in the presence of dietary sucrose, and, in the bloodstream, S. mutans can cause systemic infections. The development of a cariogenic biofilm is dependent on the formation of an extracellular matrix rich in exopolysaccharides, which contains extracellular DNA (eDNA) and lipoteichoic acids (LTAs). While the exopolysaccharides are virulence markers, the involvement of genes linked to eDNA and LTAs metabolism in the pathogenicity of S. mutans remains unclear. Objective and Design: In this study, a parental strain S. mutans UA159 and derivative strains carrying single gene deletions were used to investigate the role of eDNA (ΔlytS and ΔlytT), LTA (ΔdltA and ΔdltD), and insoluble exopolysaccharides (ΔgtfB) in virulence in a rodent model of dental caries (rats) and a systemic infection model (Galleria mellonella larvae). Results: Fewer carious lesions were observed on smooth and sulcal surfaces of enamel and dentin of the rats infected with ∆lytS, ∆dltD, and ΔgtfB (vs. the parental strain). Moreover, strains carrying gene deletions prevented the killing of larvae (vs. the parental strain). Conclusions: Altogether, these findings indicate that inactivation of lytST and dltAD impaired S. mutans cariogenicity and virulence in vivo.
Streptococcus mutans is considered a primary agent for dental caries development since it metabolizes dietary sugars into organic acids and produces exopolysaccharides, which trap acids in microniches within biofilms and at the interface between biofilms and tooth surfaces, causing tooth demineralization [Citation1,Citation2]. In addition, S. mutans can also cause systemic infections when it reaches the bloodstream (bacteremia) and persist in tissues such as the heart (endocarditis) [Citation3–Citation5]. In both cases, S. mutans uses its ability to form biofilms and to withstand environmental stresses (oxidative, nutritional, among others). Biofilms are communities of microbial cells structured and organized in a dynamic environment, covered and embedded within a three-dimensional (3D) extracellular matrix [Citation6,Citation7]. The matrix of cariogenic biofilms formed by S. mutans is rich in exopolysaccharides and contain extracellular DNA (eDNA) and lipoteichoic acids (LTAs) that interact with exopolysaccharides [Citation8]. While exopolysaccharides are proven cariogenic factors [Citation2], it remains to be determined how genes associated with eDNA and LTA metabolism affect the occurrence of dental caries and systemic infection.
The presence of carbohydrates (sucrose and starch) contributes to the formation of the matrix in cariogenic biofilms. The matrix provides stability and structural integrity by allowing bacteria to adhere to tooth surfaces and provide protection against harmful environmental stimuli or other attacks [Citation9]. The adhesion mechanism is guided by the glucosyltransferase (Gtfs) exoenzymes, which synthesize exopolysaccharides [Citation9]. Furthermore, when carbohydrates are present in the oral cavity, acid production in the biofilm increases [Citation10]. Organic acids can be retained in low pH niches within the biofilm because the exopolysaccharide-rich matrix prevents neutralization of these acids by saliva [Citation11]. S. mutans encodes three different Gtfs, namely: GtfB, which produces water-insoluble exopolysaccharides (glucans); GtfC, which produces both water-soluble and -insoluble exopolysaccharides (glucans); and GtfD, which produces soluble exopolysaccharides (glucans); these enzymes are also known as Gtf-I, Gtf-SI and Gtf-S, respectively [Citation9].
In addition to exopolysaccharide production, S. mutans can release eDNA and LTA that will interact with exopolysaccharides [Citation8,Citation12]. Sucrose and starch induce the expression of gtfB [Citation13,Citation14], the two-component system lytST (associated with cell lysis and cell-wall remodeling) [Citation13,Citation14], and the operon dltABCD (related to LTA metabolism) [Citation13,Citation15]. This augmented expression increases the abundance of exopolysaccharides, eDNA and LTA that enable the construction of a bulky and sturdy extracellular matrix in S. mutans biofilms [Citation8,Citation14,Citation16].
eDNA can be released during cell lysis and via microvesicles [Citation17]. Reduction in lytST gene expression results in a decrease in eDNA presence in the matrix during the biofilm development process [Citation14]. LTA is a cell wall polymer of Gram-positive bacteria, consisting of 1,3-polyglycerol-phosphate bound to the cell membrane by a lipoprotein, which during the remodeling of the cell wall or cell division, can be released into the extracellular environment [Citation18,Citation19]. LTA plays a significant role in the colonization of Gram-positive bacteria, contributing to the formation and pathogenicity of biofilms, and is associated with adherence and colonization of oral streptococci [Citation20,Citation21]. D-alanine promotes adhesion and biofilm development [Citation22,Citation23] and the dltABCD (D-alanyl-LTA) genes metabolize these residues during LTA synthesis [Citation24]. Of note, activation of the dltA and dltD genes occurs during the early stages of matrix formation in mixed-species cariogenic biofilms [Citation15].
In an in vitro study the S. mutans deletion strains ∆lytS, ∆lytT, ∆dltA, and ∆dltD contributed to an increased amount of extracellular eDNA and LTA in the matrix of biofilms, compared to either the parental S. mutans strain, UA159, or the ∆gtfB strain [Citation8]. The presence of eDNA and LTA in the matrix resulted in an increased amount of soluble and insoluble exopolysaccharides, indicating that these biofilms could be more cariogenic (with a potential to cause increased caries) [Citation8]. Thus, the evaluation of these genes associated with the essential components of the matrix (exopolysaccharides, eDNA and LTA) may help to clarify their contribution to the pathogenicity of S. mutans. Moreover, it is still unknown how these deletion strains behave when subjected to a systemic infection model. Thus, it is essential to clarify the functions of lytST and dlt genes to better understand the biology of S. mutans and to direct therapies to control biofilm formation by this species. The purpose of this research was to analyze the virulence of the lytST and dltAD genes of S. mutans in the development and severity of carious lesions (in a rodent model of dental caries) and virulence in a systemic infection model (Galleria mellonella).
Materials and methods
Bacterial strains
Streptococcus mutans UA159, serotype c (ATCC 700,610), a proven cariogenic organism, was the parental strain used in this study. Mutant strain derivatives of UA159 were created carrying deletions in lytS, lytT, dltA and dltD. The deletion strains for lytS, lytT [Citation25], dltA, and dltD [Citation26] came from the collection of mutant strains created in the Quivey laboratory (kindly provided by Dr. Robert G. Quivey Jr, Center for Oral Biology, University of Rochester, Rochester, NY). In addition, a strain defective in gtfB was used as a control, because it displays impaired biofilm formation in vitro [Citation11,Citation27], and reduced pathogenicity in vivo [Citation28]. The gtfB mutant was kindly provided by Dr. Robert A. Burne (Department of Oral Biology, University of Florida, Gainesville, FL). The strains were stored at – 80°C in tryptic soy broth containing 20% glycerol and were plated on blood agar plates or BHI agar plates with recommended antibiotic before use in the following assays.
Rat model of dental caries
The animal experiment was reviewed and approved by the Ethical Committee on Animal Research at the University of Campinas (CEUA – Ethics Committee on Animal Use/UNICAMP, Campinas, SP, Brazil, process number 4463–1/2017) and was performed according to methods previously described [Citation29,Citation30]. An overview of the experimental design is shown in . A total of 56 SPF (Specific Pathogen Free) female Wistar rats, aged 19 days were provided by CEMIB/UNICAMP (Multidisciplinary Center for Biological Research, Campinas, SP, Brazil). From the arrival of the animals until the end of the experiment, the animals were kept in a room of the vivarium for research using Genetically Modified Organisms (GMOs), authorized and approved by the GMO Biosafety Committee from FOP/UNICAMP (A001-2017).
On the day after the arrival of the animals, they were randomized to form four groups composed of 14 animals, using ear tags. The four groups were color-coded, so the investigators handling the animals were blinded to the strain that would be used for infection. The black group was infected with the parental strain, UA159 (positive control strain). The red group was infected with ΔgtfB (a reduced-caries-causing control to which the caries-causing potential of the tested strains was compared). The blue and green groups were infected with ΔlytS and ΔdltD, respectively.
Each animal was weighed (COMTEC 426–1070) and the data recorded. Initial screening was performed to verify that the animals were not colonized with mutans streptococci. A sterile cotton bud (Labor Import), wetted in saline solution (0.89% NaCl), was used to swab the rat mouths. Each oral swab was struck on a Mitis Salivarius agar plate without antibiotic (MSA) and on a Mitis Salivarius agar plate containing 0.2 U of bacitracin per ml (MSB). The plates were incubated (37°C, 95% (v/v) air/5% (v/v) CO2, 48 h) and the morphologies of colonies were visually evaluated to ensure that the animals were free of mutans streptococci colonization.
Biofilm and inocula preparation for caries animal study
The four strains (parental strain UA159 and deletion strains ΔgtfB, ΔlytS, and ΔdltD) were grown in biofilm cultures on glass rods in the presence of sucrose. It has been shown that using biofilm cultures as the inoculum in the rat mouth enhances their ability to colonize tooth surfaces [Citation31,Citation32]. On day one of biofilm growth, 50 µL of each strain was transferred to a tube containing TY (2.5% tryptone plus 1.5% yeast extract, Difco) and 2% sucrose (w/v) (Dinamica®/1894–1), antibiotic was added to media for cultures of the deletion strains. After incubation at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere for 24 h (day two of biofilm growth), each rod was transferred to new tubes with fresh media containing 2% sucrose. These cultures were incubated under the same conditions described above, and the rods transferred to fresh media again on day three of biofilm growth. On day four of biofilm growth, biofilms were scraped off the rods and transferred to a new tube containing fresh media, then incubated as above. After 18 h, these cultures were plated on MSB agar plates and incubated for 48 h, after which time the plates were stored at 4°C to be utilized as inocula to infect the animals.
On the day before the arrival of the animals, starter cultures were prepared by inoculating 10 mL of TY liquid medium containing 1% glucose (w/v; Synth) with colonies from the plates described above. Two tubes were prepared for each strain and incubated at 37°C in a 95% air/5% CO2 atmosphere. After 18 h, the starter cultures were diluted in TY liquid medium containing 1% glucose (1:20 dilution). The diluted cultures were allowed to grow to an O.D.540nm ~ 0.5. Each culture was then transferred to a 50 mL tube containing 14 swabs. These tubes were maintained on ice until shortly before inoculating the animals' mouths, at which time the cultures were warmed to room temperature.
Infection and maintenance of animals
Rats were infected with the cultures described above by oral swabbing four times, on four consecutive days (as outlined in ). After these four infection procedures, a confirmatory screening was performed to verify the presence of the S. mutans strains in the mouth of each rat, similar to the procedure used in the initial screening. Immediately after the confirmatory screening, the animals were transferred to suspended cages (two animals per cage), where they remained for five weeks until sacrificed.
After the first infection, the rats’ diet was replaced with Diet 2000 (containing 56% sucrose [Table S1; Citation33]). The animals were given sterile, distilled, deionized, water containing 5% sucrose, ad libitum. The ad libitum cariogenic diet used during the five-week experimental period is an established method for allowing the development of carious lesions on the dental surfaces [Citation9]. The animals were weighed weekly (Figure S1). In the second and fourth weeks, the vitamin Vitagold Potenciado (Fabiani Saúde Animal, São Paulo, Brazil) was added to the drinking water (20 mL per 10 L of water) to prevent hair loss.
Recovery of jaws from animals
The animals were euthanized by anesthesia using Ketamine (Dopalen 1,000mg/10 mL – Ceva ®) (300 mg/kg) and Xylazine (Anasedan 2,000 mg/10 mL – Ceva ®) (30 mg/kg), followed by decapitation.
The lower left jaw of each animal was aseptically dissected and was suspended in 5 mL of sterile 0.89% NaCl solution. The jaws were sonicated twice, with a 10-s interval between probe sonication for 30 s at 7 W (Vibracell, Sonics and Material Inc., Newtown, CT). Aliquots of each biofilm suspension were used for 10-fold serial dilutions. The dilutions 1:10, 1:100 and 1:1,000 and undiluted suspension were seeded on blood agar medium (for total cultured microbiota) and MSB agar medium (for S. mutans), and the plates were incubated for 48 h at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere. The blood agar plates were incubated for another 24 h at 37°C. Colonies were counted and used to calculate CFU in 5 mL of biofilm suspension (CFU/biofilm). All jaws were stored at −20°C until manual dissection for caries scoring.
Caries scoring
The teeth were prepared for caries scoring according to Larson’s modification of Keyes system [Citation34]. One calibrated examiner performed the caries score of the codified jaws. The smooth surface caries scoring (buccal, morsal, proximal, lingual and palatinal) was done using a stereoscopic Zeiss magnifying lens (Stemi SV 6).
Before performing caries scoring on sulcal surfaces, the samples were prepared as follows: The jaws were painted with transparent nail polish to strengthen the structure and left to dry for 24 h. Murexide (SIGMA M-2628) dye solution (0.24 mg/mL) was used to stain the jaw and left to dry for 18 h. A sagittal cut was made in the jaws with a carbide disk coupled to a micromotor (mc52 – dent) to allow for better visualization of the sulcal surface. Caries scoring was performed on the sulcal surfaces using a stereoscopic Zeiss magnifying lens (Stemi SV 6).
Infection study in the invertebrate model G. mellonella
Five deletion strains (∆gtfB, ∆lytT, ∆lytS, ∆dltA and ∆dltD) and the parental strain (UA159) of S. mutans were used to examine pathogenicity in the G. mellonella model of infection. The strains were cultured as described in Bacterial Strains. Starter cultures of each of the six strains were prepared in 10 mL of TY liquid medium containing 1% glucose (w/v) and incubated at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere. After 18 h, the cultures were diluted in TY liquid medium containing 1% glucose (1:20 dilution) and grown to an O.D.540nm ~ 0.5.
Cells were collected by centrifugation (6,500 x g/4°C/15 min, Hettich Zentrifugen, rutin 420R) and pellets were washed twice with 3 mL of sterile 0.89% NaCl solution. Cell pellets were resuspended in 3 mL 0.89% NaCl. Briefly, a 100 μL aliquot of each strain suspension was transferred to a tube containing 900 μL of 0.89% NaCl solution to perform 10-fold serial dilutions. The 10–7 dilution was plated on blood agar (in triplicate), and the plates were incubated at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere. After 48 h, the colonies were counted and used for the calculation of CFU. A heat-killed control for each strain was incubated for 40 min at 85°C.
The systemic infection of G. mellonella was performed as described previously [Citation35–Citation37]. The larvae were stored at 32°C, in the dark, in a greenhouse (SOLAB BODSL-200/364, Solab, Piracicaba, Brazil) until the experiment. For each bacterial strain tested, a group of 10 larvae, varying from 200 to 300 mg and without signs of melanization, were selected for inoculation with the six strains of S. mutans (parental UA159, ΔgtfB, ΔlytT, ΔlytS, ΔdltA and ΔdltD) and the heat-inactivated control strains (all infections were performed in triplicate).
Five μL of S. mutans from each strain containing 1–1.5 × 108 CFU was injected intra-hemocoel via the last left proleg using a Hamilton syringe (Hamilton, Reno NV) [Citation38]. After injection, the larvae were placed in Petri dishes for incubation at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere. The appearance of melanization and larval survival were recorded three times per day according to the G. mellonella Health Index Scoring System [Citation39]. The larvae were classified as dead when they displayed no movement in response to touch.
Tolerance to oxidative stress by hydrogen peroxide (H2O2)
Planktonic cultures and biofilms of the six strains of S. mutans (parental UA159, ΔgtfB, ΔlytT, ΔlytS, ΔdltA and ΔdltD) were subjected to an H2O2 challenge, as oxidative stress is part of the innate immune system of G. mellonella [Citation38,Citation40]. The planktonic cultures were grown as described above for the infection of larvae. Cultures were exposed to 0.2% H2O2 for 0, 5, 15, 30 and 45 min. At the end of the exposure time, aliquots (100 µL) were used for 10-fold serial dilutions that were plated on blood agar plates.
The biofilm cultures were grown on vertically placed hydroxyapatite disks (1.25 cm diameter, Chromatography products Clarkson, Inc., South Williamsport, PA), coated with filter-sterilized clarified human whole saliva (sHA). The saliva and pellicle preparation were performed as described before [Citation8]. The saliva collection and use were approved by the Institutional Ethics Committee on Human Research at UNESP (CAAE: 31717914.3.0000.5416). Biofilm formation was performed as described by Castillo Pedraza et al. [Citation8]. Briefly, the strains were grown in TY broth containing 1% glucose (w/v) at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere until reaching late exponential growth phase (OD540nm ~1.0).
Next, each strain was inoculated individually (106 CFU mL−1) in 2.8 mL of TY with 0.1% sucrose (w/v) and 25% saliva (v/v) and incubated at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere. The beginning of this incubation was considered day 0 (biofilm age 0 h). At 19 h, the culture medium was replaced by transferring the custom-made disk holder with nascent biofilms to wells containing fresh medium (TY + 0.1% sucrose and 25% saliva) using forceps. After 29 h of biofilm growth, biofilms were transferred to TY containing 0.5% sucrose, (w/v) 1% starch (w/v), and 25% saliva to increase the amount of carbohydrate serving as substrates for microbial metabolism and matrix accumulation. The culture medium was then changed twice daily (8 am and 6 pm) until the end of the experimental period. Thus, the biofilms were kept immersed in 0.1% sucrose/25% saliva for 10 h, and in 0.5% sucrose/1% starch/25% saliva for 14 h. Biofilms were grown up to 67 h, and during all ages were incubated with the same initial environment as 0 h (at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere).
At 67 h biofilms were removed from the culture medium, rinsed (three times) with 0.89% NaCl solution, and transferred to wells of a 24-well plate containing 0.2% H2O2 and incubated for 15, 30 and 45 min. At time 0 min, biofilms were incubated without H2O2. At the end of the exposure time, the disks were transferred to tubes containing 2 mL 0.89% NaCl solution and sonicated for 10 min (in an ultrasonic bath) to harvest biofilms from the disks. The disks were scraped with a sterile spatula to remove residual biofilm. The biofilms were homogenized with a probe for 30 s at 7 W (Sonicator model Q125, QSonica). From these biofilm suspensions, aliquots (100 µL) were used for 10-fold serial dilutions that were plated on blood agar plates.
For both planktonic and biofilm cultures, the plates were incubated for 48 h at 37°C in a 95% (v/v) air/5% (v/v) CO2 atmosphere and the colonies were counted to obtain CFU/mL (planktonic cultures) or CFU/biofilm (biofilms). The population recovered at 0 min was set at 100% per strain for planktonic and biofilm cultures.
Statistical analyses
The virulence potential of the different deletion strains and the parental strain of S. mutans were analyzed using Prism 7 software (GraphPad Software, Inc.). The microbial population data (total culturable microbiota, S. mutans, and percentage of S. mutans) recovered from rats were evaluated with analysis of variance (ANOVA) one-way followed by Tukey’s test (α = 0.05). The development of carious lesions was evaluated by two-way ANOVA, followed by the Tukey’s test (α = 0.05) using as factors ‘S. mutans strain’ and ‘type of caries lesion on smooth and sulcal surfaces’. The amount of CFU inoculated per strains of S. mutans in G. mellonella larvae was analyzed by one-way ANOVA, followed by the Tukey’s test (α = 0.05). The virulence potential of S. mutans in the invertebrate model was assessed using the Kaplan-Meier survival curve, and estimates of survival differences were compared using the log-rank test. Oxidative stress tolerance (H2O2) was evaluated using ANOVA two-way followed by Tukey’s test (α = 0.05) using as factors ‘S. mutans strain’ and ‘exposure time’ for both planktonic cultures and biofilms.
Results
Loss of lytS and dltD reduced caries formation in a rat model
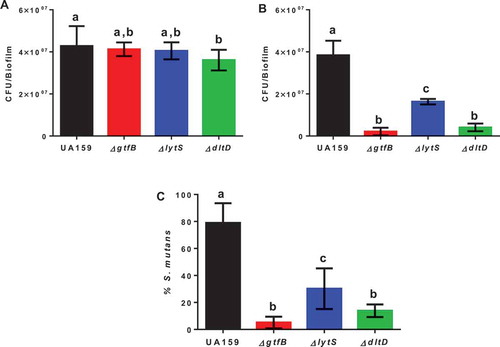
Total cultivable microbiota recovered from rats used in a model for caries formation revealed that rats infected with the parental strain UA159 and the ΔgtfB and ΔlytS strains, did not show significant differences between them, but the ΔdltD strain had fewer CFU/mL, compared to the parental strain UA159 (p = 0.0224, one-way ANOVA, followed by the Tukey’s test; ). Infection with the ΔgtfB strain, our reduced caries control, resulted in a lower S. mutans population, compared to the other strains tested (parental strain UA159 and ΔlytS strain) except for the ΔdltD strain (no significant difference between ∆gtfB and ∆dltD; ). The proportion of S. mutans in the total microbiota was higher for the parental strain UA159 compared to all deletion strains tested (p < 0.0001, one-way ANOVA, followed by the Tukey’s test; ). Moreover, the percentage of S. mutans for ΔgtfB was lower than for ΔlytS (p = 0.0027), and there was no difference between ΔgtfB and ΔdltD strains (p = 0.2036). Therefore, there was a successful implantation of S. mutans in the oral microbiota of all rats evaluated.
Figure 2. Microbial population recovered from the animals at the end of the caries study. The total cultivable microbiota (a) and S. mutans strains (b) derived from rats infected by parental strain UA159 and deletion strains ΔgtfB, ΔlytS and ΔdltD. (c) The percentage of S. mutans (%) in the total cultivable microbiota. Bars with the same letters indicate no statistical difference between the different strains in each graph (p > 0.05; one-way ANOVA, followed by Tukey’s test). The data represented are the means, and the error bars correspond to the standard deviation (n = 14 per group).
All strains tested caused dental caries on smooth and sulcal surfaces (). There were significant changes in smooth carious lesion scores for rats infected with the deletion strains, compared to UA159 (); however, there was not a significant difference among the mutant strains. Animals infected with the ΔlytS strain exhibited a greater number of smooth surface carious lesions on enamel (E) and dentin (Ds and Dm) than rats infected with ΔgtfB and ΔdltD (). None of the mutant strains tested caused severe dentinal caries (Dx) on smooth and sulcal surfaces, though the rats infected with the ΔlytS strain displayed a significantly higher number of slight dentinal caries (Ds) on smooth surfaces than the ΔdltD strain (). These data suggest that lytS and dltD could play an important role in the development of caries on smooth surfaces. This observation has already been recorded for smooth surface lesions in animals infected with a ∆gtfB strain [Citation28].
Figure 3. Influence of S. mutans strains on development and severity of dental caries on smooth (a) and sulcal (b) surfaces. The data displayed are the means, and the error bars correspond to the standard deviation (n = 14 per group). Bars with the same letters, by type of caries lesion, indicate no statistically significant differences between the strains (p > 0.05; two-way ANOVA, followed by the Tukey’s test). In the Dx category for both smooth and sulcal caries, no bars, other than S. mutans UA159, are represented, since the three deletion strains ΔgtfB, ΔlytS and ΔdltD did not yield this type of lesion. E: Enamel; Ds: light dentin caries; Dm: moderate dentin caries; and Dx: severe dentinal caries.
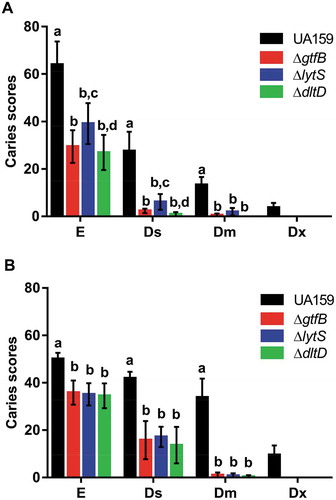
Sulcal carious lesions were reduced in rats infected with mutant strains, compared to infection with UA159. Moreover, there was no difference between the mutant strains in severity, but their scores were lower than those from UA159 infected animals (). Importantly, animals infected with the deletion strains also did not display Dx lesions on sulcal surfaces, suggesting that the products of the lytS, dltD and gtfB genes could also play an essential role in the development of caries on sulcal surfaces. Further, the loss of lytS, dltD and gtfB not only influenced the development of cariogenic biofilms in vitro [Citation8] but also in vivo.
Strains carrying gene deletions gtfB, lytT, lytS, dltA, and dltD prevented the killing of G. mellonella larvae
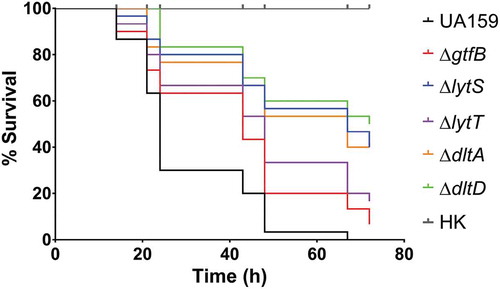
The deletion strains were then tested in an in vivo model of infection, G. mellonella. Using similar inocula (Figure S2), all the deletion strains were able to survive longer in the larvae than the parent strain, UA159, which killed all larvae after 67 h of infection (; Table S2). In addition, only 7% of the larvae inoculated with ∆gtfB survived at 72 h, demonstrating higher systemic virulence potential of this strain when compared to the other deletion strains. Specifically, the larvae inoculated with ∆lytT (16.6%), ∆lytS, (40%), ∆dltA (40%) and ∆dltD (50%) showed a higher percentage of survival at 72 h after inoculation. Thus, the ∆dltD strain was significantly less able to cause larvae killing, compared to the other deletion strains tested (). These results indicate that the products of the genes examined in this study may play a role in influencing how S. mutans thrives in systemic infections.
Figure 4. Survival of G. mellonella larvae after infection with S. mutans parental UA159 and deletion strains ΔgtfB, ΔlytT, ΔlytS, ΔdltA and ΔdltD. Virulence of S. mutans strains displayed by Kaplan-Meier survival curves. Estimates of differences in survival were compared using the Matel-Cox Log-rank test. All larvae survived the heat-killed (HK) control of all strains tested, therefore, the data shown for HK are representative results from one strain.
S. mutans strains’ tolerance to hydrogen peroxide
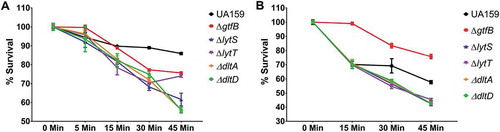
To further examine the mechanism by which the gene deletion strains were able to survive in the G. mellonella larvae, the ability of the strains, grown in planktonic or biofilm cultures, to survive exposure to H2O2 was determined. In planktonic cultures, the parent strain exhibited the greatest ability to survive a peroxide challenge following 45 min of exposure. There was a gradual decrease in the survival percentage of S. mutans in planktonic cultures () and biofilms () of ∆lytT, ∆lytS, ∆dltA, and ∆dltD strains after exposure to H2O2, except for the planktonic culture of ∆lytT that increased its population when it passed from 30 to 45 min. However, the ∆gtfB strain was significantly better able to withstand a peroxide challenge when grown in a biofilm culture. This finding suggests that a decreased amount of insoluble exopolysaccharides found in the extracellular matrix of ∆gtfB biofilm cultures [Citation7] did not facilitate H2O2 killing, and that other factor(s) may be playing a role (e.g., cell membrane composition and turnover). The ability of the ∆gtfB strain to persist in biofilm cultures exposed to H2O2 is borne out in the results observed in our in vivo G. mellonella infection model ().
Figure 5. S. mutans parental UA159 and deletion strains ΔgtfB, ΔlytT, ΔlytS, ΔdltA and ΔdltD tolerance to hydrogen peroxide. The graphs show the different survival percentages of the strains population from planktonic culture (a) and biofilms (b) after longitudinal exposure to H2O2. The data shown are the means, and the error bars correspond to the standard deviation (n = 6 per group).
Discussion
The present study demonstrated that the lytST and dltAD genes affect S. mutans virulence in a rodent model of caries and a systemic infection assay. All rats infected with the S. mutans parental strain UA159 and deletion strains developed carious lesions. Greater numbers of enamel and dentinal lesions were measured in teeth of animals infected with the parental UA159 strain on the smooth and sulcal surfaces of the animals’ teeth. However, in the animals infected with the deletion strains ΔgtfB, ΔlytS and ΔdltD, caries lesions were less severe; moreover, these deletion strains did not cause severe dentinal caries. Thus, the absence of the products encoded by gtfB, lytS and dltD resulted in decreased cariogenicity of S. mutans. In addition, loss of the gtfB, lytS, lytT, dltA and dltD gene products prevented the killing of G. mellonella larvae after systemic infection with S. mutans (compared to the UA159 parental strain), indicating that these five gene products may also influence systemic infections, by reducing the virulence of the organism. This reduction in systemic infection may be in part due to the absence of these genes made the deletion strains (except ΔgtfB) more susceptible to oxidative stress, which is part of the innate immune system of G. mellonella.
The two in vivo models were selected with distinct purposes; the rodent model was to assess the cariogenic potential of strains with deletion of specific genes, and the invertebrate model as an incipient model to evaluate whether the deleted genes could impair the systemic infection potential of S. mutans. Specifically, because the deletion strains caused fewer caries lesions with lower severity (vs. the parental strain), the specific genes (and their products) could be targets for directed preventive/therapeutic approaches. Thus, if such approaches would be implemented, there was no information whether targeting these genes would interfere with S. mutans behavior in case of bacteremia (e.g., once it goes to the host bloodstream during oral hygiene procedures like flossing). There was no information whether the attenuated virulence phenotype to cause caries would also present an attenuated phenotype in a systemic infection. Therefore, a simple systemic model (larvae) was selected to verify how the deletion strains would affect S. mutans virulence.
In the caries model, the microbial population recovered from rats showed a higher proportion of S. mutans in the total microbiota for UA159 vs. deletion strains (15-fold for ΔgtfB, 3-fold for ∆lytS and 6-fold for ∆dltD). Recently, a library of UA159 transposon mutants was inoculated in mice to identify genes that are important for colonization and survival in the oral cavity; after 3 weeks, the mutant strains gtfB, dltC, dltD and lytT presented lower recovery, while dltA, dltB and lytS were recovered at a similar rate as UA159, but no caries status was reported [Citation41]. Here, the deletion strains ∆dltA and ∆dltD growth in TY+1% glucose were slower compared to the parental strain UA159 and the deletion strains ∆gtfB, ∆lytS, and ∆lytT. These two strains took 40 to 60 min more to reach mid-exponential (O.D.540nm ~ 0.5) and late exponential growth phase (O.D.540nm ~ 1.0). Thus, only ∆dltA and ∆dltD presented a slow-growth defect that may explain in part the outcome for lower colonization in the rats’ teeth and fewer and less severe carious lesions observed. Initial work with ∆lytT (∆lytR) grown in Brain Heart Infusion broth demonstrated a defect in cell division leading to longer chains because of defects in autolysin activity but without changes in planktonic growth rate or biofilm formation [Citation25]. Later, in a comprehensive study of a genomic collection of gene deletion mutants, the strains were categorized into several growth traits [Citation26]. Both ΔdltA and ΔdltD had poor acid survival in planktonic cultures and single-species biofilm formation deficiencies on polystyrene surfaces in the presence of sucrose or glucose [Citation26]. The deficiency in biofilm formation on hydroxyapatite was not observed in our in vitro study, which used TY culture media with saliva and sucrose alternated with sucrose plus starch [Citation8]. Furthermore, the pH of spent culture media did not differ between strains grown in single-species biofilms, indicating that all strains grew as the pH of culture media dropped from 7.0 to ~4.5 [Citation8]. Nevertheless, the slower growth and lower acid tolerance of ΔdltA and ΔdltD may contribute to the lower percentage of S. mutans recovered and fewer (and less severe) caries lesions.
In the current study, for smooth surfaces, UA159 yielded ≥50% more lesions than the deletion strains on enamel, while it caused the highest amount of caries lesions in dentin (≥80% more cavities). For sulcal surfaces, UA159 yielded ≅ 30% more lesions than the deletion strains on enamel, while UA159 also caused a higher amount of caries lesions in dentin (being ≥60% more severe than the deletion strains). S. mutans possesses several virulence factors associated with cariogenicity such as adhesion capacity, acidogenicity, acid tolerance, production of bacteriocins (mutacins) and biofilm formation [Citation10,Citation42]. However, the ability to synthesize water-insoluble exopolysaccharides is a trait that makes S. mutans a significant pathogen of dental caries [Citation2]. The synthesis of exopolysaccharides depends on the expression of Gtfs by this microorganism in the presence of dietary carbohydrates [Citation9]. Lack of working gtfB was related to low levels of carious lesions on the smooth surfaces of rat teeth [Citation28,Citation43]. Thus, it was expected that the ΔgtfB strain would cause less carious lesions on the surfaces of the teeth in the present study.
The infection by ΔlytS caused fewer caries lesions in enamel and dentin of rats than the parental strain UA159. Thus, the interruption of the two-component system lytST affected S. mutans cariogenicity. The lytST complex is essential for IrgAB expression in S. mutans, which participates in oxidative stress tolerance, biofilm build-up and regulation of autolysis [Citation44,Citation45]. The eDNA released by autolysis interacts with GtfB and LTA and affects the production of exopolysaccharides, colonization by S. mutans, and construction of the extracellular matrix [Citation8,Citation12,Citation17]. Previously, our group described an increased amount of eDNA in the matrix of in vitro biofilms formed by ΔlytS and ΔlytT [vs. the parental strain; Citation8]. We proposed that the loss of these genes was affecting S. mutans cell metabolism by increasing cell wall turnover, thereby increasing the secretion of microvesicles containing eDNA [Citation17], or triggering additional pathways that culminate in the augmented release of DNA into the matrix [Citation8]. The induction of additional pathways could explain the decreased cariogenicity of ΔlytS in vivo observed in this study.
In addition, the ΔdltD strain also showed a lower number of caries lesions in enamel and dentin of rats (vs. the parental strain UA159), suggesting that the product of the dltD gene could also be involved in the cariogenicity of S. mutans. This finding was expected since the dltABCD operon genes are required for the addition of D-alanine residues during LTA synthesis [Citation19]; these residues affect bacterial adhesion to the surface and formation of biofilms [Citation22,Citation23,Citation46,Citation47]. Also, a previous in vivo study induced the expression of the dltABCD operon and demonstrated a higher occurrence of caries [Citation48]. Furthermore, the S. mutans proteins encoded by the dltA, dltC and dltD genes were abundant during the early stages of cariogenic biofilm formation and were related to the amounts of intracellular polysaccharides [Citation15,Citation23]. Therefore, the loss of genes related to the synthesis of exopolysaccharides, eDNA and LTA metabolism impair cariogenicity of S. mutans.
In an earlier in vitro study, it was expected that the deletion of lytS and lytT would decrease the amount of eDNA, and deletion of dltA and dltD would reduce the quantity of LTA in the matrix (vs. the parental strain). However, the data showed more eDNA and LTA in biofilms with the respective deletion strains and that the 3D structure was distinct [Citation8]. Based on these data, it was expected that the strains yielding more eDNA and LTA in the matrix would cause more caries lesions; but this was not the outcome in the rodent model of dental caries here. An explanation is that the deletion of lytST and dltAD alters S. mutans physiology to form biofilms with distinct quantities of extracellular matrix components in vitro [Citation8]. These traits could be because of alterations in the cell wall turnover and composition (and charge), as suggested previously [Citation13,Citation44,Citation45,Citation49,Citation50]. The cell wall works as an ‘anchor’ for matrix components to connect to cells surface and mediates cell-cell binding, cell-matrix, cell-matrix-dental surface. Hence, alterations in this ‘anchor’ could lead to a weaker, smaller (with fewer cells connected to each other and to the matrix; that is, lower percentage of S. mutans recovered from rats’ dental biofilms) and more ‘porous’ 3D structure that would retain less acid inside the biofilm and at the interface biofilm/dental surface, causing fewer caries lesions. Thus, a more ‘porous’ 3D structure might be related to the distinct patterns of the distribution of bacteria and exopolysaccharides observed in the in vitro biofilms (vs. parental strain UA159), represented as percentage coverage per area from the interface substratum/biofilm (hydroxyapatite disk) to the top (outer layer) of each biofilm [Citation8].
Oral bacteria can enter the bloodstream, and usually, bacteremia can be rapidly eliminated by the immune system. However, it has been shown that if S. mutans persists in the bloodstream, it can colonize the endothelial cells of the coronary arteries [Citation36]; the cerebral arterial wall [Citation51], the renal arterial wall [Citation52], and even reach the hepatic veins in the liver [Citation53]. G. mellonella has a pathogenic defense mechanism similar to the innate immune system of mammals [Citation54], and their hemolymph is considered an analog to blood in vertebrates. This invertebrate model was used to analyze S. mutans pathogenesis in systemic diseases such as bacterial endocarditis [Citation35–Citation37]. Here, the deletion of the gtfB, lytS, lytT, dltA, and dltD genes caused lower and slower larval mortality rates. However, the ΔgtfB strain had a higher capacity to kill more larvae when compared to the other deletion strains. The larvae defense mechanisms include a plasmacyte-mediated melanocyte process [Citation38], and the production of reactive oxygen species (ROS), enzymes, and antimicrobial peptides [Citation40]. But, as G. mellonella is a limited, preliminary systemic model, further studies will need to be performed to address the contribution of these genes to S. mutans systemic virulence. In addition, the mechanism by which S. mutans reacts to the stress caused by the immune system of G. mellonella is still to be elucidated.
Nevertheless, S. mutans is susceptible to oxidative stress [Citation55]. Here, the ΔlytS, ΔlytT, ΔdltA and ΔdltD strains revealed lower tolerance to H2O2, making these genes important for providing resistance to oxidative stress. The ΔgtfB strain showed greater survival to H2O2 exposure than the other deletion strains, possibly due to the presence of lytST and dltAD that could together alleviate the deleterious effects of hydrogen peroxide. The lack of a protective matrix in ΔgtfB could render this strain more susceptible to oxidative challenge, but the data showed an opposite outcome. Thus, we hypothesize that the absence of a working gtfB gene changes the cell wall/membrane making S. mutans cells more tolerant to oxidative stress when grown in a biofilm. Further research is needed to pinpoint the exact mechanism for ∆gtfB higher tolerance to H2O2 and explain why it killed more larvae than the other four deletion strains tested. For example, transcriptomic and/or proteomic analyses of ∆gtfB challenged with H2O2 could indicate the mechanisms involved in the observed tolerance. Nevertheless, previous gene expression analysis indicated that ΔgtfB might present a slower turnover in the cell membrane in mixed-species biofilms [Citation13, used the same strain ΔgtfB used here], corroborating with earlier work using a ΔgtfBC strain [Citation56].
Moreover, bacterial adaptation to changing environmental conditions is often accomplished by TCSs that modulate gene expression in response to many stimuli [Citation57]. A previous study showed that the Cid/Lrg systems interact together to favor the colonization of S. mutans against environmental stresses [Citation58]. The expression of IrgAB is dependent on the lytST genes and confers resistance to oxidative stress [Citation44,Citation45]. LytST (lytS: sensor kinase; lytT: response regulator) is required to activate the expression of lrgAB, which is part of the S. mutans arsenal that controls biofilm formation and autolysis [Citation45]. The S. mutans IrgAB/lytST deletion strains exhibited a decreased growth rate in the presence of several ROS [Citation44,Citation45]. Before, the expression pattern was distinct for lytS and lytT. The expression of lytS was influenced by pH and abundance of carbohydrates, while lytT was expressed in very low magnitudes at distinct developmental stages for both UA159 and ΔgtfB in mixed-species biofilms, regardless of environmental pH or nutrient availability [Citation13]. The similarity between both types of biofilms indicates that lytT may interfere with multiple metabolic pathways simultaneously.
Furthermore, as the ΔgtfB biofilm aged, the expression of lytS decreased, being inversely proportional to the increase of expression recorded for lrgAB, which was not observed for UA159 biofilms. Even though LytST controls the expression of lrgAB, a ΔlrgAB mutant presented a distinct transcriptomic profile from the ΔlytS mutant [Citation45], suggesting that changes in gene expression by the mutation of lrgAB could be independent of LytST [Citation59]. Further comparison of transcriptomics and proteomics of a ΔlrgAB mutant (vs. the parental strain) subjected to distinct oxidative, heat and antimicrobial stresses indicated ‘that adaptation to a new environment may require radical proteome turnover or metabolic remodeling’ [Citation50]. Among the pathways (and resulting products) were several genes/proteins involved in cell wall biogenesis affecting cell wall turnover and stress tolerance (e.g., GtfC, AtlA, DltC) [Citation50]. In addition, alteration in the cell wall of dlt mutants may also occur.
The deletion strains ∆dltA and ∆dltD exhibited the same behaviors as the ∆lytS and ∆lytT when inoculated in G. mellonella larvae. Thus, these genes could also be contributing to the virulence of S. mutans in systemic diseases and cooperation for the tolerance to ROS products. Moreover, the expression of the dlt genes was related to the resistance of S. mutans biofilms against gentamicin, a drug used to treat endocarditis [Citation49]. The ΔdltA showed a more negatively charged surface than the parental strain, reducing the tolerance of the dltA mutant biofilms towards the positively charged gentamicin [Citation49]. Furthermore, it was shown that dltABCD affects the susceptibility of S. mutans to cationic antimicrobial peptides (innate immune factors in humans) [Citation60]. It has been shown that a D-Ala analog can block the activity of DltA and synergize with the peptidoglycan synthesis inhibitor vancomycin, leading to growth inhibition [Citation61]. Additionally, a small molecule was identified as a LtaS enzyme inhibitor [Citation62] that inhibits the growth of several medically critical Gram-positive bacteria [Citation62,Citation63]. Our preliminary work with this small molecule has shown to inhibit S. mutans growth in planktonic culture and biofilms (Castillo Pedraza et al., unpublished data). Therefore, the higher survival of larvae infected with ΔlytS, ΔlytT, ΔdltA, and ΔdltD may be related to the strains’ lower tolerance to oxidative stress after exposure to H2O2, and host antimicrobial peptides [Citation60]. Thus, it is possible that changes on cell wall/membrane in the deletion mutant tested here could explain some of the distinct and even unexpected outcomes, and thus, future research on cell wall/membrane composition and structure is warranted.
In conclusion, besides the roles that the lytST and dltAD genes play in the development and composition of in vitro biofilms [Citation8], the results from this study demonstrate that they are also factors in the virulence of S. mutans in the development of dental caries and systemic infection. Inhibition of these genes and their products with specific therapies would help decrease the virulence of S. mutans to prevent the deleterious effects of demineralization and reduce microorganism survival in systemic infections.
Supplemental Material
Download MS Word (266.9 KB)Acknowlegments
This research was supported by a research grant from the São Paulo Research Foundation (FAPESP, grant number 2014/05423-0 to M.I.K.) and scholarships (FAPESP, grant numbers 2017/00753-0 to M.C.C.P., 2014/01723-9 to A.R.F.C., and 2016/08021-5 to L.S.L.). In addition, the authors thank the National Council for Scientific and Technological Development (CNPq) for providing scholarship (grant number 141896/2017-5 to M.C.C.P for five months). M.I.K. also received funding from CNPq (grant #311707/2016-5). The authors are grateful to Eliane Franco, José Carlos, Josy G. Lazarini, and Bruno Dias Nani for their technical support. The present study is part of the PhD thesis by M.C.C.P.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50(4):353–12.
- Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92(12):1065–1073.
- Nakano K, Fujita K, Nishimura K, et al. Contribution of biofilm regulatory protein A of Streptococcus mutans, to systemic virulence. Microbes Infect. 2005;7(11–12):1246–1255.
- Nakano K, Ooshima T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 2009;4:891–902.
- Nakano K, Nemoto H, Nomura R, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. 2009;24(1):64–68.
- Branda SS, Vik S, Friedman L, et al. Biofilms: the matrix revisited. Trends Microbiol. 2005;13(1):20–26.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633.
- Castillo Pedraza MC, Novais TF, Faustoferri RC, et al. Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of cariogenic biofilms. Biofouling. 2017;33:722–740.
- Bowen WH, Koo H. Biology of Streptococcus mutans derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86.
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–303.
- Xiao J, Klein MI, Delahunty CM, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8(4):e1002623.
- Klein MI, Hwang G, Santos PHS, et al. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 2015;5:10.
- Florez Salamanca EJ, Klein MI. Extracellular matrix influence in Streptococcus mutans gene expression in a cariogenic biofilm. Mol Oral Microbiol. 2018;1–13.
- Klein MI, DeBaz L, Agidi S, et al. Dynamics of Streptococcus mutans transcriptome in response to starch and sucrose during biofilm development. PLoS One. 2010;5(10):e13478.
- Klein MI, Xiao J, Lu B, et al. Streptococcus mutans protein synthesis during mixed-species biofilm development by high-throughput quantitative proteomics. PLoS One. 2012;7(9):e45795.
- Klein MI, Duarte S, Xiao J, et al. Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilms development. Appl Environ Microbiol. 2009;75(3):837–841.
- Liao S, Klein MI, Heim KP, et al. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol. 2014;196(13):2355–2366.
- Ellwood DC, Tempest DW. Effects of environment on bacterial wall content and composition. Adv Microb Physiol. 1972;7:83–116.
- Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723.
- Kuramitsu HK, Wondrack L, McGuinness M. Interaction of Streptococcus mutans glucosyltransferases with teichoic acids. Infect Immun. 1980;29(2):376–382.
- Rölla G, Oppermann RV, Bowen WH, et al. High amounts of lipoteichoic acid in sucrose induced plaque in vivo. Caries Res. 1980;14(4):235–238.
- Gross M, Cramton SE, Gotz F, et al. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69:3423–3426.
- Spatafora GA, Sheets M, June R, et al. Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J Bacteriol. 1999;181(8):2363–2372.
- Reichmann NT, Gründling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105.
- Chatfield CH, Koo H, Quivey RG Jr. The putative autolysin regulator LytR in Streptococcus mutans plays a role in cell division and is growth-phase regulated. Microbiology. 2005;151(2):625–631.
- Quivey RG, Grayhack EJ, Faustoferri RC, et al. Functional profiling in Streptococcus mutans: construction and examination of a genomic collection of gene deletion mutants. Mol Oral Microbiol. 2015;30(6):474–495.
- Koo H, Xiao J, Klein MI, et al. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192(12):3024–3032.
- Yamashita Y, Bowen WH, Burne RA, et al. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817.
- Bowen WH, Pearson SK, Young DA. The effect of desalivation on coronal and root surface caries in rats. J Dent Res. 1988;67(1):21–23.
- Bowen WH, Schilling K, Giertsen E, et al. Role of a cell surface associated protein in adherence and dental caries. Infect Immun. 1991;59(12):4606–4609.
- Ma Y, Rutherford GC, Curran TM, et al. Membrane locus and pH sensitivity of paraben inhibition of alkali production by oral streptococci. Oral Microbiol Immunol. 1999;14(4):244–249.
- Duarte S, Rosalen PL, Hayacibara MF, et al. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Arch Oral Biol. 2006;51(1):15–22.
- König KG, Larson RH, Guggenheim B. A strain-specific eating pattern as a factor limiting the transmissibility of caries activity in rats. Arch Oral Biol. 1969;14(1):91–103.
- Larson RM. Merits and modifications of scoring rat dental caries by Keyes’ method. Symposium on Animal Models in Cariology; Washington, DC; 1981. p 203.
- Abranches J, Miller JH, Martinez AR, et al. The collagen-binding protein cnm is required for streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect Immun. 2011;79:2277–2284.
- Aviles-Reyes A, Miller JH, Simpson-Haidaris PJ, et al. Cnm is a major virulence factor of invasive Streptococcus mutans and part of a conserved threegene locus. Mol Oral Microbiol. 2014;29:11–23.
- Freires IA, Avilés-Reyes A, Kitten T, et al. Heterologous expression of Streptococcus mutans Cnm in Lactococcus lactis promotes intracellular invasion, adhesion to human cardiac tissues and virulence. Virulence. 2017;8(1):18–29.
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol. 2000;27(2):163–169.
- Loh JM, Adenwalla N, Wiles S, et al. Galleria mellonella larvae as an infection model for group A Streptococcus. Virulence. 2013;4:419–428.
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev. 2004;28:101–112.
- Shields RC, Zeng L, Culp DJ, et al. Genomewide identification of essential genes and fitness determinants of Streptococcus mutans UA159. mSphere. 2018;3(1):pii: e00031–18.
- Lemos JA, Burne RA. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology. 2008;154:3247–3255.
- Freedman ML, Tanzer JM, Coykendail AL. The use of genetic variants in the study of dental caries. Animal models in cariology. In: Tanzer JM, editors. Proceedings of the Symposium and Workshop on Animal Models in Cariology. Washington, DC: Information Retrieval Inc.; 1981. p. 247–269.
- Ahn SJ, Qu MD, Roberts E, et al. Identification of the Streptococcus mutans LytST two-component regulon reveals its contribution to oxidative stress tolerance. BMC Microbiol. 2012;12:1–12.
- Ahn SJ, Rice KC, Oleas J, et al. The Streptococcus mutans Cid and Lrg systems modulate virulence traits in response to multiple environmental signals. Microbiology. 2010;156:3136–3147.
- Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–1378.
- Qiu W, Zheng X, Wei Y, et al. d-Alanine metabolism is essential for growth and biofilm formation of Streptococcus mutans. Mol Oral Microbiol. 2016;31(5):435–444.
- Spatafora G, Rohrer K, Barnard D, et al. A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun. 1995;63(7):2556–2563.
- Nilsson M, Rybtke M, Givskov M, et al. The dlt genes play a role in antimicrobial tolerance of Streptococcus mutans biofilms. Int J Antimicrob Agents. 2016;48:298–304.
- Ahn SJ, Gu T, Koh J, et al. Remodeling of the Streptococcus mutans proteome in response to LrgAB and external stresses. Sci Rep. 2017;7(1):14063.
- Inenaga C, Hokamura K, Nakano K, et al. A potential new risk factor for stroke: streptococcus mutans with collagen-binding protein. World Neurosurg. 2018;113:e77–e81.
- Misaki T, Naka S, Kuroda K, et al. Distribution of Streptococcus mutans strains with collagen-binding proteins in the oral cavity of IgA nephropathy patients. Clin Exp Nephrol. 2015;19(5):844–850.
- Naka S, Hatakeyama R, Takashima Y, et al. Contributions of Streptococcus mutans Cnm and PA antigens to aggravation of non-alcoholic steatohepatitis in mice. Sci Rep. 2016;6:36886.
- Tsai CJY, Loh JMS, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7(3):214–229.
- Ahn SJ, Wen ZT, Burne RA. Effects of oxygen on virulence traits of Streptococcus mutans. J Bacteriol. 2007;189:8519–8527.
- Guo L, McLean JS, Lux R, et al. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep. 2015;5:18015.
- Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol. 2006;9(2):143–152.
- Ahn SJ, Rice KC. Understanding the Streptococcus mutans Cid/Lrg system through CidB function. Appl Environ Microbiol. 2016;82(20):6189–6203.
- Rice KC, Turner ME, Carney OV, Modification of the Streptococcus mutans transcriptome by LrgAB and environmental stressors. Microb Genom. 2017;3(2):e000104.
- Mazda Y, Kawada-Matsuo M, Kanbara K, et al. Association of CiaRH with resistance of Streptococcus mutans to antimicrobial peptides in biofilms. Mol Oral Microbiol. 2012;27(2):124–135.
- May JJ, Finking R, Wiegeshoff F, et al. Inhibition of the D-alanine: DAlanyl carrier protein ligase from Bacillus subtilis increases the bacterium’s susceptibility to antibiotics that target the cell wall. FEBS J. 2005;272(12):2993–3003.
- Richter SG, Elli D, Kim HK, et al. Small molecule inhibitor of lipoteichoic acid synthesis is an antibiotic for Gram-positive bacteria. Proc Natl Acad Sci U S A. 2013;110(9):3531–3536.
- Paganelli FL, van de Kamer T, Brouwer EC, et al. Lipoteichoic acid synthesis inhibition in combination with antibiotics abrogates growth of multidrug-resistant Enterococcus faecium. Int J Antimicrob Agents. 2017;49(3):355–363.