ABSTRACT
Aggregatibacter actinomycetemcomitans belongs to the HACEK group of fastidious Gram-negative organisms, a recognized cause of infective endocarditis. A. actinomycetemcomitans is also implicated in periodontitis, with rapid progress in adolescents. We recently demonstrated that the major outer membrane protein, OmpA1 was critical for serum survival of the A. actinomycetemcomitans serotype a model strain, D7SS, and that the paralogue, OmpA2 could operate as a functional homologue to OmpA1 in mediating serum resistance. In the present work, an essentially serum-sensitive ompA1 ompA2 double mutant A. actinomycetemcomitans strain derivative was exploited to elucidate if A. actinomycetemcomitans OMVs can contribute to bacterial serum resistance. Indeed, supplementation of OMVs resulted in a dose-dependent increase of the survival of the serum-sensitive strain in incubations in 50% normal human serum (NHS). Whereas neither OmpA1 nor OmpA2 was required for the OMV-mediated serum protection, OMVs and LPS from an A. actinomycetemcomitans strain lacking the LPS O-antigen polysaccharide part were significantly impaired in protecting D7SS ompA1 ompA2. Our results using a complement system screen assay support a model where A. actinomycetemcomitans OMVs can act as a decoy, which can trigger complement activation in an LPS-dependent manner, and consume complement components to protect serum-susceptible bacterial cells.
Introduction
The HACEK group of fastidious Gram-negative organisms is an identified cause of infective endocarditis, responsible for 1.4 to 3% of cases [Citation1]. The genus Aggregatibacter is now the dominant etiology of HACEK endocarditis [Citation2]. Carriage of Aggregatibacter actinomycetemcomitans is strongly associated with periodontitis with rapid progress in adolescents and young adults [Citation3–Citation5]. A. actinomycetemcomitans is a genetically diverse species, with the serotypes a-g representing different lineages [Citation5]. A. actinomycetemcomitans genotypes that produce high levels of leukotoxin, i.e., JP2 and cagE, respectively (serotype b) are strongly linked to periodontal attachment loss progression in North and West African adolescents [Citation4,Citation6]. The systemic role of A. actinomycetemcomitans, further to its involvement in endocarditis, includes its association with conditions of soft tissue abscesses, and osteomyelitis [Citation7]. In addition, A. actinomycetemcomitans has been identified in atheromatous plaque [Citation8] and is a candidate bacterial trigger of anti-citrulline autoimmunity in rheumatoid arthritis [Citation9,Citation10].
Serum resistance represents an important virulence factor of bacteria that enter into the bloodstream and cause infection, allowing the bacterial cells to evade the innate immune defense mechanisms present in serum, including the complement system and antimicrobial peptides. Mechanisms of bacterial resistance against complement-mediated killing include protection via extracellular polysaccharide capsules, insertion of membrane attack complexes into nonlethal bacterial targets, and expression of factors that inhibit or interfere with the complement cascade [Citation11–Citation14]. Bacterial factors that mediate serum resistance, therefore, represent targets for vaccine and drug development [Citation15,Citation16]. Serum resistance appears to be crucial for A. actinomycetemcomitans virulence and is a prevalent feature among strains of this species [Citation17–Citation19]. The outer membrane protein, Omp100 (also referred to as ApiA) was previously shown to be required for full serum resistance of some serotype b, and d A. actinomycetemcomitans strains, and to physically interact with the alternative complement pathway negative regulator, Factor H in vitro [Citation17,Citation20]. We recently demonstrated that the major outer membrane protein, OmpA1 was critical for serum survival of the A. actinomycetemcomitans serotype a model strain, D7SS [Citation21]. Interestingly, serum resistant ompA1 mutants were fortuitously obtained, which expressed increased levels of the paralogue, OmpA2. Thus, OmpA2, which normally seems to be expressed at low levels in vitro, could apparently operate as a functional homologue to OmpA1 in A. actinomycetemcomitans serum resistance [Citation21]. As several complement activation pathways, i.e., classical, alternative, and mannose-binding lectin (MBL) complement activation were needed to completely eliminate serum-sensitive ompA1 ompA2 double mutant A. actinomycetemcomitans derivatives [Citation21], it is plausible that serum resistance in this species, similar to in Acinetobacter baumanii [Citation22], is highly complex, and depend on multiple gene products.
It has been clearly demonstrated that most Gram-negative bacteria release outer membrane vesicles (OMVs) during normal growth [Citation23,Citation24]. A. actinomycetemcomitans OMVs have been shown to deliver virulence factors, such as leukotoxin, and cytolethal distending toxin (CDT) to human cells [Citation25,Citation26], and to internalize into the host cells to act as a trigger of innate immunity [Citation27]. Proteomics, and Western blot analysis of A. actinomycetemcomitans OMVs have identified several additional vesicle-associated proteins that can contribute to evasion of the immune defense, including the IL1β-binding lipoprotein, BilRI, Omp100, OmpA1, and OmpA2, and a Factor H-binding protein homologue [Citation21,Citation28,Citation29]. A role of OMVs in contributing to bacterial serum resistance has been demonstrated in a number of bacterial species, including Moraxella catarrhalis, Neisseria gonorrhoeae, Porphyromonas gingivalis, and Vibrio cholerae [Citation30–Citation33]. Whether A. actinomycetemcomitans OMVs may also be involved in serum resistance is not known. A functional role of A. actinomycetemcomitans OMVs in complement interaction would be consistent with in vitro observations that the vesicles, in an OmpA1- and OmpA2-dependent manner, respectively, could bind to C4-binding protein [Citation21], a major inhibitor of classical and MBL complement activation [Citation34]. Lipopolysaccharide (LPS) is one of the most abundant components of OMVs, including those released by A. actinomycetemcomitans, and is displayed on the outer surface of the vesicles [Citation23–Citation25]. It was recently shown that LPS is involved in the binding of IL-8 by A. actinomycetemcomitans OMVs [Citation35], and it cannot be excluded that the LPS in OMVs also may interact with complement. The serotype-specific polysaccharide (S-PA) determinant of A. actinomycetemcomitans resides in the LPS O antigen, which is immunodominant [Citation36–Citation39]. A. actinomycetemcomitans strains are occasionally isolated, which lack the ability to produce the serotype-specific antigen [Citation40]. As speculated previously [Citation40], this may represent a mechanism to evade from antibody-based host responses, which could be advantageous in blood circulation. However, a contradiction with this idea is that the absence of S-PA expression in A. actinomycetemcomitans appears to be scarce.
The aim of the present work was to investigate if OMVs may play a role in serum resistance in A. actinomycetemcomitans.
Materials and methods
Ethics considerations
All procedures were conducted in accordance with the guidelines of the local ethics committee at the Medical Faculty of Umeå University, which are in accordance with the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, October 2013). For the assays using normal human serum (NHS), blood was sampled from healthy volunteers after informed consent.
Bacterial strains and growth conditions
A. actinomycetemcomitans strain D7SS is a naturally genetic competent, smooth-colony derivative of D7S (serotype a), which was originally isolated from a patient with rapidly progressing periodontal disease [Citation41]. An A. actinomycetemcomitans ompA1 ompA2 double mutant strain, i.e. D7SS ompA1::spe, ompA2::kan [Sper, Kmr] [Citation21] was used as a serum-sensitive test strain. SA3138 is a serotype a A. actinomycetemcomitans wild-type strain [Citation40]. SA3139, which was isolated from the same patient as SA3138, carries the serotype a-specific antigen gene cluster, however, does not express the LPS O-antigen polysaccharide, rendering this strain non-serotypeable using immunoassay [Citation40]. DNA from strain D7SS ompA1 [Citation21] was used in the present work to generate mutant derivatives of SA3138 and SA3139, i.e., SA3138 ompA1::spe [Sper], and SA3139 ompA1::spe [Sper], respectively. Escherichia coli C600 is a prototypical K-12 laboratory strain [Citation42]. Strain SE600 is an ompA mutant, generated in C600 S, which is a streptomycin-resistant derivative of C600 [Citation43]. The bacterial strains were routinely cultivated in air supplemented with 5% CO2, at 37°C, on blood agar plates (5% defibrinated horse blood, 5 mg hemin/l, 10 mg Vitamin K/l, Columbia agar base). Alternatively, for transformation assays, the strains were grown on Trypticase soy broth supplemented with 0.1% yeast extract, 5% heat-inactivated horse serum, and 1.5% agar (sTSB agar). When needed, the growth media was supplemented with 100 μg/ml (final concentration) kanamycin or spectinomycin, respectively.
Construction of ompA1 gene replacement mutants
The ompA1::spe allele from D7SS ompA1 was transferred to strains SA3138 and 3139, using natural transformation [Citation41], generating SA3138 ompA1 and SA3139 ompA1, respectively. Confirmation of the allelic replacements was done by PCR. For this, we used ompA1-specific oligonucleotide primers as described earlier [Citation21].
SDS-PAGE and Western blot analysis
The procedures used for SDS-PAGE and Western blot analysis have been described previously [Citation44,Citation45]. For Western blot, we used normal human serum samples from six periodontally healthy donors. Two sera were shown earlier to exhibit high, and one to exhibit low reactivity, respectively, towards recombinant A. actinomycetemcomitans leukotoxin [Citation46]. Moreover, one serum was sampled from an individual who was confirmed to be A. actinomycetemcomitans-negative according to cultivation from subgingival plaque [Citation47], albeit exhibited reactivity towards A. actinomycetemcomitans peptidoglycan-associated lipoprotein (PAL) [Citation28]. All serum samples tested were used at a final dilution of 1:2,000. As secondary antibody, anti-human horseradish peroxidase (HRP)-conjugate was used (Jackson ImmunoResearch, Newmarket, UK) (1:10,000). Immunoreactive bands were visualized using Clarity™ Western ECL Substrate (Bio-Rad), and the ChemiDoc™ MP imaging system (Bio-Rad).
Isolation of outer membrane vesicles
OMVs were isolated from A. actinomycetemcomitans cells harvested from an average of 10 blood agar plates, using ultracentrifugation as described earlier [Citation26,Citation27]. OMV pellets were washed twice with PBS (85,000 × g; 2 h, 4°C) using a 70 Ti rotor (Beckman Instruments Inc.), resuspended in ≈200 μl PBS, and then used as the OMV preparation. The yield of OMVs was estimated by determining protein concentrations using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific), and preparations were assessed for OMV particle concentration using Nanoparticle Tracking Analysis software (NanoSight Ltd.). OMVs were tested for the absence of bacterial contamination by cultivating small aliquots on blood agar plates in air supplemented with 5% CO2, at 37°C for 3 days.
Isolation of LPS
LPS was isolated from A. actinomycetemcomitans cells harvested from 12 blood agar plates, using a combination of procedures [Citation48,Citation49], as described [Citation35]. LPS preparations were resuspended in 100 µl H2O and the yield (typically 1.5–2.8 × 106 endotoxin units [EU]) was estimated using the ToxinSensor™ chromogenic Limulus Amebocyte Lysate (LAL) endotoxin kit (BioNordika, Sweden).
Bacterial serum sensitivity assay
To determine the sensitivity of A. actinomycetemcomitans cells to normal human serum, NHS was taken from healthy volunteers. We essentially followed procedures described previously [Citation21], using A. actinomycetemcomitans strains grown on agar. In brief, prior to being used in the assays, bacteria were harvested and suspensions were adjusted to 1.0 × 109 cells/ml in PBS buffer. Reaction mixtures contained 50% NHS, i.e. 105 μl NHS, 95 μl PBS, and 10 μl bacterial suspension, and were incubated at 37°C for 1-2 h. For the serum sensitivity assay with OMVs, the PBS was supplemented with OMVs isolated from A. actinomycetemcomitans strains. The PBS was supplemented with vesicles equivalent to 20, 100, and 200 μg protein, respectively, yielding a final concentration of 95.2, 476.2, and 952.4 μg/ml in the reaction mixtures. Alternatively, 50 ng (500 EU) LPS was added to the reaction mixtures. Bacterial serum survival was determined by viable count, and the increase in serum survival upon OMV or LPS supplementation was determined relative to incubations in vesicle-free controls (50% NHS in PBS).
Assessment of complement consumption by OMVs
To determine the consumption of complement by A. actinomycetemcomitans OMVs, vesicles (20 and 100 μg, respectively) in PBS were incubated in 50% NHS at 37°C for 1 h in a reaction volume of 200 μl, yielding final concentrations of 95.2 and 476.2 μg protein/ml. Alternatively, 50 ng (500 EU) LPS was used instead of OMVs. The remaining complement activity in the NHS samples was subsequently quantified using the Wieslab® Complement System Screen kit, COMPL300 (Svar Life Science AB, Malmö, Sweden), according to the instructions of the manufacturer. NHS incubated with PBS alone served as a negative control for complement consumption.
Statistical analysis and image processing
The statistical significance of the data was calculated using two-tailed Student’s t-test. The level of statistical significance was set to P < 0.05, based on at least three independent experiments unless otherwise stated. Images for figures were assembled using Adobe Photoshop CS6, or Microsoft PowerPoint.
Results
OMVs mediate LPS-dependent serum protection of A. actinomycetemcomitans cells
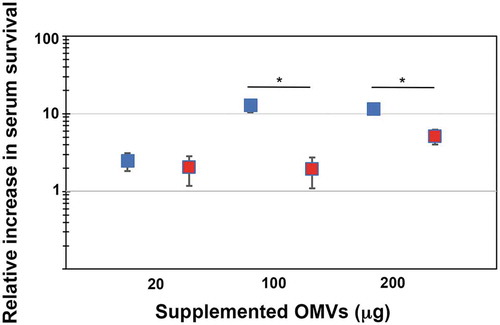
In order to test if outer membrane vesicles may be involved in serum resistance of A. actinomycetemcomitans, OMVs were isolated from the wild-type strain, D7SS, and from its ompA1 ompA2 double mutant derivative, as described in Materials and methods. Total protein concentrations of obtained vesicle preparations were in average ≈6-7 mg/ml, and which contained approximately 1 × 109 OMV particles/ml according to NanoSight analysis. A. actinomycetemcomitans strain D7SS ompA1 ompA2 was utilized in the serum protection assays as it is essentially serum-sensitive [Citation21]. According to our findings (), the survival rate of this strain was clearly increased (up to approximately 100-fold) in a dose-dependent manner upon supplementation of D7SS OMVs equivalent to 20 and 100 μg protein. In contrast, there was no further increase in serum survival when D7SS vesicles corresponding to 200 μg protein was supplemented, suggesting that the serum protection was saturated. To investigate whether the protective effect by A. actinomycetemcomitans OMVs was OmpA-dependent, the same assay was performed, but instead supplementing OMVs obtained from the ompA1 ompA2 double mutant strain (). According to our results, the ompA double mutant OMVs had a slightly, however not statistically significantly reduced, protective effect on the serum survival of D7SS ompA1 ompA2. We, therefore, concluded that A. actinomycetemcomitans OMVs could mediate serum protection, essentially independent of OmpA. To investigate the potential role of LPS in OMV-mediated serum protection, vesicles were isolated from the A. actinomycetemcomitans wild-type strain, SA3138, and from strain SA3139, which has an LPS lacking the O-antigen polysaccharide part. According to our results, OMVs obtained from the LPS O-antigen-deficient strain, SA3139, were significantly attenuated in protecting the D7SS ompA1 ompA2 double mutant in serum sensitivity assays (). We, therefore, concluded that serum protection by A. actinomycetemcomitans OMVs was LPS-dependent. A similar result was obtained in serum protection assays using purified LPS instead of OMVs. Upon supplementation of 50 ng (500 EU) LPS, there was a higher (3.4-fold ± 0.375 [SEM]) survival of D7SS ompA1 ompA2 in presence of SA3138 LPS, compared to when LPS from SA3139 was used instead. On the other hand, strains SA3138 and SA3139 were both found to be serum resistant (survival rate ≥100%), whereas, upon inactivation of the ompA1 gene in these strains, their serum resistance was essentially lost (survival rate ≤1%), consistent with OmpA1 being a major factor contributing to the intrinsic serum resistance of A. actinomycetemcomitans strains [Citation21].
Figure 1. Enhanced serum survival of A. actinomycetemcomitans strain D7SS ompA1 ompA2 upon supplementation of OMVs. A. actinomycetemcomitans cells were incubated in 50% normal human serum (NHS) at 37°C for 1 h. The assay was performed in the absence or presence of 20, 100, and 200 μg of OMVs, respectively, as indicated. Bacterial serum survival was determined by viable count, and shown is the increase (fold change) in survival relative to incubations in vesicle-free controls (50% NHS in PBS). Supplemented OMVs were obtained from the wild-type strain D7SS (blue squares), and D7SS ompA1 ompA2 (red squares), respectively. Shown are means ± SEM from four independent experiments. *P < 0.05 vs control. **P < 0.05 vs supplementation of 20 μg of OMVs. P > 0.05, D7SS OMVs vs D7SS ompA1 ompA2 OMVs
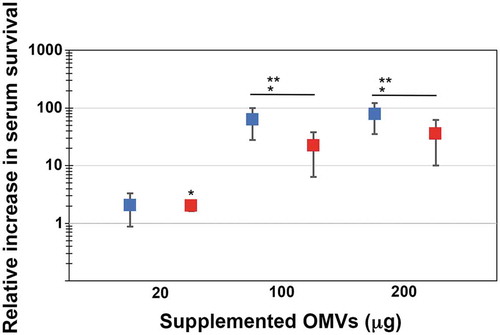
Figure 2. LPS-dependent serum protection of A. actinomycetemcomitans strain D7SS ompA1 ompA2 upon supplementation of OMVs. A. actinomycetemcomitans cells were incubated in 50% normal human serum (NHS) at 37°C for 1 h. The assay was performed in the absence or presence of 20, 100, and 200 μg of OMVs, respectively, as indicated. Bacterial serum survival was determined by viable count, and shown is the increase (fold change) in survival relative to incubations in vesicle-free controls (50% NHS in PBS). Supplemented OMVs were obtained from strain SA3138 (serotype a, wildtype; blue squares), and SA3139 (as SA3138 but LPS lacks the O-antigen polysaccharide part; red squares). Shown are means ± SEM from four independent experiments. *P < 0.05 SA3138 OMVs vs SA3139 OMVs
Complement consumption by A. actinomycetemcomitans OMVs is LPS-dependent
As supplementation of A. actinomycetemcomitans OMVs protected strain D7SS ompA1 ompA2 in serum sensitivity assays, we investigated whether the vesicles could activate, and consume serum complement components. To this end, 50% NHS was preincubated with OMVs equivalent to 20 and 100 μg protein, respectively, and then assessed with the COMPL300 assay (Materials and methods) to determine the remaining activity of the classical, alternative, and MBL pathways of complement activation, respectively. According to our results, upon addition of the 20 μg samples, OMVs from D7SS, the D7SS ompA1 ompA2 double mutant, and SA3138 all efficiently activated complement, and consumed components of the tested activation pathways (). Using 100 μg OMVs there was <1% residual activity in the three pathways, consistent with complement component titration by the vesicles. In contrast, most of the complement activity in the three pathways were remaining in the NHS preincubated with SA3139 OMVs, lacking the LPS O-antigen polysaccharide part (). We, therefore, concluded that LPS under the present conditions was a major OMV antigen responsible for complement consumption through the tested activation pathways. A similar result was obtained using LPS, purified from SA3138 and SA3139, i.e. LPS from the latter strain consumed less complement activity ().
Table 1. Remaining complement activity (%) in the indicated activation pathways after consumption of complement in NHS by A. actinomycetemcomitans OMVs (20 μg; rendering a final OMV protein concentration of 95.2 μg/ml) for 1 h at 37°C. NHS incubated with PBS served as a negative control. Complement consumption was thereafter determined using the COMPL300 kit, as described in Materials and methods. Values are given as mean percentage (range) from two independent experiments
Table 2. Remaining complement activity (%) in the indicated activation pathways after consumption of complement in NHS by A. actinomycetemcomitans LPS (50 ng; 500 EU) for 1 h at 37°C. Complement consumption was thereafter determined using the COMPL300 kit, as described in Materials and methods. Values are given as mean percentage (range) from two independent experiments. P < 0.05 SA3138 OMVs vs SA3139 OMVs for all three tested pathways
Normal human serum of periodontally healthy subjects contains antibodies recognizing A. actinomycetemcomitans OMV antigens
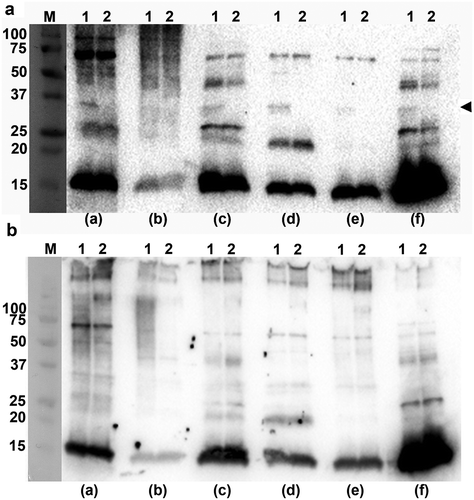
Albeit classical complement consumption by A. actinomycetemcomitans OMVs to a large extent was OmpA-independent (), we wanted to clarify whether normal human serum may contain antibodies recognizing A. actinomycetemcomitans OMV antigens, which could play a role in the classical complement activation by the vesicles. To this end, Western blot was used to assess a selection (n = 6) of NHS from periodontally healthy subjects for their ability to recognize antigens of OMVs obtained from A. actinomycetemcomitans strains. Comparing the reactivity of the sera towards OMVs isolated from D7SS, and D7SS ompA1 ompA2, respectively ()), revealed that all sera, including one sampled from a confirmed, A. actinomycetemcomitans-negative individual, recognized species-specific antigens, although at varying degrees. We concluded that A. actinomycetemcomitans OmpA1 was recognized by at least five of the six tested sera, as a reactive band specific for this protein was not detected when assessing OMVs from the D7SS ompA1 ompA2 double mutant. It cannot be excluded that such antibodies have been developed against OMPs and/or surface proteins of commensals, as E. coli OMV antigens, including OmpA, were recognized by at least four of the six tested sera (Supplementary Figure 1). A similar pattern of antigen recognition was observed comparing OMVs obtained from the serotype a wild-type strain, SA3138, and the LPS O-antigen-deficient strain SA3139 ()). In Western blots, the serotype-specific antigen of A. actinomycetemcomitans LPS is typically detected as a diffuse smear pattern [Citation40,Citation50], also when assessing OMV samples [Citation28]. This type of banding pattern was detected for SA3138 OMVs when testing serum sample (b), but not for OMVs from the O-antigen deficient SA3139. Hence, serum (b) was most likely sampled from an individual carrying a serotype a A. actinomycetemcomitans. Collectively, these observations are in accordance with the notion that there are antibodies present in NHS of periodontally healthy subjects, which can recognize A. actinomycetemcomitans OMV antigens, to trigger classical complement activation. However, our results are also consistent with an apparent antibody-independent complement activation by LPS in A. actinomycetemcomitans OMVs.
Figure 3. Immunoreactivity of normal human sera to A. actinomycetemcomitans and E. coli OMVs. Western blot analysis of reactivity of NHS from periodontally healthy individuals with (a) OMVs obtained from the A. actinomycetemcomitans strains D7SS (wild-type; lane 1), and D7SS ompA1 ompA2 (lane 2), and (b) OMVs obtained from the A. actinomycetemcomitans strains SA3138 (wild-type; lane 1), and SA3139 (LPS lacks the O-antigen polysaccharide part; lane 2). The NHS samples a-f were used for immunodetection as indicated, and include sera known to exhibit high (a, and f) and low (b) reactivity, respectively, towards recombinant A. actinomycetemcomitans leukotoxin, and one from a confirmed A. actinomycetemcomitans-negative individual (e). Samples equal to 10 μg protein were applied on the gels. The reactive band corresponding to A. actinomycetemcomitans OmpA1 is indicated with an arrowhead. LPS is detected as a diffuse, high-molecular smear pattern for serum sample (b), when assessing SA3138 OMVs. The sizes (kDa) of the proteins in the pre-stained molecular weight marker (M) are indicated along the left sides
Discussion
In this work, we have used a highly serum-sensitive ompA1 ompA2 double mutant A. actinomycetemcomitans strain to demonstrate that OMVs play a role in serum protection in this organism. Such a function of A. actinomycetemcomitans OMVs is in agreement with findings with OMVs of a number of other bacterial species, i.e. M. catarrhalis, N. gonorrhoeae, P. gingivalis, and V. cholerae [Citation30–Citation33].
Based on previous studies with A. actinomycetemcomitans [Citation17,Citation21,Citation51], we routinely determined the serum survival in 50% NHS. A. actinomycetemcomitans strains of all serotypes are ubiquitously serum resistant [Citation17,Citation19], and due to the apparent unavailability of a natural serum-susceptible strain, a strain D7SS ompA1 ompA2 double mutant was selected for the present experimental studies. This derivative exhibits an essentially abolished serum resistance and is susceptible to the classical, alternative, and MBL pathways of complement activation, whereas its survival rate is similar to the wild-type, D7SS, in heat-inactivated NHS [Citation21]. The serum sensitivity of the double mutant is likely accompanied by at least a partially impaired membrane stability, as electron microscopy revealed larger membrane-vesicle like structures occasionally projecting from the cell surface, and the strain has also a somewhat reduced growth rate in liquid cultures [Citation21].
From the present work, we could conclude that A. actinomycetemcomitans OMVs contributed to serum protection, and strongly activated, and consumed the components of all three tested complement activation pathways. Activation of the classical complement pathway by the vesicles is consistent with our current observations that naturally occurring antibodies in NHS can recognize A. actinomycetemcomitans OMV antigens, including OmpA1. However, classical complement activation has also been demonstrated to be induced by bacterial OMPs and/or surface proteins in an antibody-independent manner [Citation52–Citation54]. We observed that OmpA1 and OmpA2 were not critical for serum protection and complement consumption by the OMVs. This was somewhat unexpected as these OMPs were necessary for the serum resistance of strain D7SS and bound to recombinant C4-binding protein in in vitro incubations with A. actinomycetemcomitans OMVs [Citation21]. Thus, A. actinomycetemcomitans OMVs appeared to mediate serum protection via a different mechanism than, e.g., V. cholerae OMVs, which were found to divert naturally occurring antibodies against Gram-negative organisms away from the bacterial cells via OmpU on the released vesicles [Citation30]. Also, OMVs of M. catarrhalis and N. gonorrhoeae seemingly depended on OMPs when contributing to serum resistance [Citation31,Citation32]. It can be speculated that the requirement of OMPs for OMV-mediated serum protection might be stronger in some bacterial species, such as Neisseria meningitidis, where LPS has a weak complement-activating capability [Citation55].
In the present work, we compared the serum protection efficiency of OMVs, and LPS, derived from the A. actinomycetemcomitans serotype a strains SA3138 and SA3139, which were originally isolated from the same individual, but with the latter strain not expressing the LPS O-antigen polysaccharide part [Citation40]. Our observation that SA3139 OMVs, and LPS, only weakly activated complement through any of the tested pathways is consistent with a major role of the LPS O-antigen polysaccharide in the complement activation and consumption by A. actinomycetemcomitans OMVs. Concomitantly, and most likely as a consequence of their modest level of complement consumption, OMVs and LPS derived from SA3139, as compared to SA3138, had a clearly lower ability to protect the highly serum-sensitive strain D7SS ompA1 ompA2 double mutant in 50% NHS. Hence, our present results support a model in which A. actinomycetemcomitans OMVs contribute to serum protection as a complement target, which in an LPS-dependent manner can consume and titrate complement components to prevent them from interacting with the bacterial cells. LPS-dependent serum protection by OMVs has been demonstrated previously, for vesicles released by the periodontal pathogen P. gingivalis [Citation33]. Such function of A. actinomycetemcomitans OMVs is plausible, considering the efficient consumption of complement components by LPS of this species [Citation56]. Moreover, LPS derived from a number of Gram-negative bacteria can block the serum bactericidal activity [Citation33,Citation57], and interact with the classical complement factor C1q, in an antibody-independent fashion, to activate the classical pathway [Citation58,Citation59]. LPS is also a well-recognized alternative complement activator [Citation60], and furthermore, it can trigger the MBL pathway [Citation61].
The potential role in vivo of OMV-mediated serum protection is not known. Evidently, bacteria release OMVs during infection in vivo, and vesicles have been detected in, e.g., fluids from infected hosts, demonstrating their ability to disseminate distant from the site of infection [Citation32,Citation62,Citation63]. Notably, using a mouse model, it was recently demonstrated that A. actinomycetemcomitans OMVs can pass the blood-brain barrier [Citation64]. Bacterial production of OMVs, and their composition is influenced by various environmental factors and sources of cellular stress, which bacterial pathogens experience inside the host [Citation23,Citation24,Citation65]. Evidence from electron microscopy supports that OMV production can be increased upon exposure to host components such as serum, and tissues [Citation23,Citation66,Citation67]. Increased vesicle production appears to improve bacterial survival under stress [Citation68], and higher levels of released vesicles may be associated with virulence, e.g., in leukotoxic as compared to non-leukotoxic A. actinomycetemcomitans strains [Citation65,Citation69]. It remains, however, to be determined if vesicle production during bacterial infections on occasions can reach levels in vivo to make a significant contribution in serum protection of complement-susceptible bacteria. Complement consumption by OMVs may represent a strategy by pathogens in which they collaborate to conquer innate immunity. The idea of interbacterial serum protection by OMVs is supported by observations that M. catarrhalis vesicles can protect Haemophilus influenzae from complement-mediated killing in vitro [Citation32]. Furthermore, interestingly, P. gingivalis vesicles protected two serum-sensitive oral species in vitro, i.e., Bacteroides loeschii and Capnocytophaga ochracea [Citation33], suggesting the possibility that OMV-mediated serum protection could favor the pathogenic progress of periodontitis.
In summary, the present work has revealed a role of OMVs in contributing to A. actinomycetemcomitans serum protection, by acting as a decoy, consuming complement in an LPS-dependent fashion. Detailed comprehension of OMV-host interactions, which mediate serum protection may advance the development of tailor-made OMV-based vaccines, agents that reduce bacterial serum resistance, and/or that can target bacteria that have hitherto evaded vaccination achievements.
Acknowledgments
We are grateful to Tova Vestman for technical assistance. We thank Dr. Anders Johansson for contributing the patient sera, and Dr. Sun Nyunt Wai for providing the E. coli strains C600 and SE600, respectively.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
References
- Chambers ST, Murdoch D, Morris A, et al. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One. 2013;8:e63181.
- Norskov-Lauritsen N. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev. 2014;27:214–10.
- Fine DH, Patil AG, Velusamy SK. Aggregatibacter actinomycetemcomitans (Aa) under the radar: myths and misunderstandings of Aa and its role in aggressive periodontitis. Front Immunol. 2019;10:728.
- Haubek D, Ennibi OK, Poulsen K, et al. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–242.
- Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000. 2010;54:78–105.
- Johansson A, Claesson R, Höglund Åberg C, et al. cagE gene sequence as a diagnostic marker to identify JP2 and non-JP2 highly leukotoxic Aggregatibacter actinomycetemcomitans serotype b strains. J Periodontal Res. 2017;52:903–912.
- van Winkelhoff AJ, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol. 2000;1999(20):122–135.
- Bale BF, Doneen AL, Vigerust DJ. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis. Postgrad Med J. 2017;93:215–220.
- Gómez-Banuelos E, Mukherjee A, Darrah E, et al. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Clin Med. 2019;8:E1309.
- Konig MF, Abusleme L, Reinholdt J, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176.
- Abreu AG, Barbosa AS. How Escherichia coli circumvent complement-mediated killing. Front Immunol. 2017;8:452.
- Berends ET, Kuipers A, Ravesloot MM, et al. Bacteria under stress by complement and coagulation. FEMS Microbiol Rev. 2014;38:1146–1171.
- Hovingh ES, van den Broek B, Jongerius I. Hijacking complement regulatory proteins for bacterial immune evasion. Front Microbiol. 2016;7:2004.
- Joiner KA. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230.
- Jongerius I, Schuijt TJ, Mooi FR, et al. Complement evasion by Bordetella pertussis: implications for improving current vaccines. J Mol Med (Berl). 2015;93:395–402.
- Vila-Farres X, Parra-Millan R, Sanchez-Encinales V, et al. Combating virulence of Gram-negative bacilli by OmpA inhibition. Sci Rep. 2017;7:14683.
- Asakawa R, Komatsuzawa H, Kawai T, et al. Outer membrane protein 100, a versatile virulence factor of Actinobacillus actinomycetemcomitans. Mol Microbiol. 2003;50:1125–1139.
- Oscarsson J, Claesson R, Lindholm M, et al. Tools of Aggregatibacter actinomycetemcomitans to evade the host response. J Clin Med. 2019;8:1079.
- Sundqvist G, Johansson E. Bactericidal effect of pooled human serum on Bacteroides melaninogenicus, Bacteroides asaccharolyticus and Actinobacillus actinomycetemcomitans. Scand J Dent Res. 1982;90:29–36.
- Ramsey MM, Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci USA. 2009;106:1578–1583.
- Lindholm M, Min Aung K, Nyunt Wai S, et al. Role of OmpA1 and OmpA2 in Aggregatibacter actinomycetemcomitans and Aggregatibacter aphrophilus serum resistance. J Oral Microbiol. 2019;11:1536192.
- Sanchez-Larrayoz AF, Elhosseiny NM, Chevrette MG, et al. Complexity of complement resistance factors expressed by Acinetobacter baumannii needed for survival in human serum. J Immunol. 2017;199:2803–2814.
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94.
- Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053.
- Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1–13.
- Rompikuntal PK, Thay B, Khan MK, et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80:31–42.
- Thay B, Damm A, Kufer TA, et al. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-κB activation. Infect Immun. 2014;82:4034–4046.
- Kieselbach T, Zijnge V, Granström E, et al. Proteomics of Aggregatibacter actinomycetemcomitans outer membrane vesicles. PLoS One. 2015;10:e0138591.
- Kieselbach T, Oscarsson J. Dataset of the proteome of purified outer membrane vesicles from the human pathogen Aggregatibacter actinomycetemcomintans. Data Brief. 2017;10:426–431.
- Aung KM, Sjöström AE, von Pawel-rammingen U, et al. Naturally occurring IgG antibodies provide innate protection against Vibrio cholerae bacteremia by recognition of the outer membrane protein U. J Innate Immun. 2016;8:269–283.
- Pettit RK, Judd RC. The interaction of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae with normal human serum. Mol Microbiol. 1992;6:729–734.
- Tan TT, Mörgelin M, Forsgren A, et al. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J Infect Dis. 2007;195:1661–1670.
- Grenier D, Belanger M. Protective effect of Porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun. 1991;59:3004–3008.
- Suankratay C, Mold C, Zhang Y, et al. Mechanism of complement-dependent haemolysis via the lectin pathway: role of the complement regulatory proteins. Clin Exp Immunol. 1999;117:442–448.
- Ahlstrand T, Kovesjoki L, Maula T, et al. Aggregatibacter actinomycetemcomitans LPS binds human interleukin-8. J Oral Microbiol. 2019;11:1549931.
- Lakio L, Paju S, Alfthan G, et al. Actinobacillus actinomycetemcomitans serotype d-specific antigen contains the O antigen of lipopolysaccharide. Infect Immun. 2003;71:5005–5011.
- Page RC, Sims TJ, Engel LD, et al. The immunodominant outer membrane antigen of Actinobacillus actinomycetemcomitans is located in the serotype-specific high-molecular-mass carbohydrate moiety of lipopolysaccharide. Infect Immun. 1991;59:3451–3462.
- Wilson ME, Schifferle RE. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect Immun. 1991;59:1544–1551.
- Sims TJ, Moncla BJ, Darveau RP, et al. Antigens of Actinobacillus actinomycetemcomitans recognized by patients with juvenile periodontitis and periodontally normal subjects. Infect Immun. 1991;59:913–924.
- Kanasi E, Dogan B, Karched M, et al. Lack of serotype antigen in A. actinomycetemcomitans. J Dent Res. 2010;89:292–296.
- Wang Y, Goodman SD, Redfield RJ, et al. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002;184:3442–3449.
- Appleyard RK. Segregation of new lysogenic types during growth of a doubly lysogenic strain derived from Escherichia coli K12. Genetics. 1954;39:440–452.
- Emory SA, Belasco JG. The ompA 5ʹ untranslated RNA segment functions in Escherichia coli as a growth-rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency. J Bacteriol. 1990;172:4472–4481.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Paul-Satyaseela M, Karched M, Bian Z, et al. Immunoproteomics of Actinobacillus actinomycetemcomitans outer-membrane proteins reveal a highly immunoreactive peptidoglycan-associated lipoprotein. J Med Microbiol. 2006;55:931–942.
- Brage M, Holmlund A, Johansson A. Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J Periodontal Res. 2011;46:170–175.
- Johansson A, Hänström L, Kalfas S. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol Immunol. 2000;15:218–225.
- Al-Hendy A, Toivanen P, Skurnik M. Rapid method for isolation and staining of bacterial lipopolysaccharide. Microbiol Immunol. 1991;35:331–333.
- Paju S, Saarela M, Chen C, et al. Altered antigenicity is seen in the lipopolysaccharide profile of non-serotypeable Actinobacillus actinomycetemcomitans strains. FEMS Immunol Med Microbiol. 2000;27:171–177.
- Wilson ME, Bronson PM. Opsonization of Actinobacillus actinomycetemcomitans by immunoglobulin G antibodies to the O polysaccharide of lipopolysaccharide. Infect Immun. 1997;65:4690–4695.
- Tang-Siegel G, Bumgarner R, Ruiz T, et al. Human serum-specific activation of alternative sigma factors, the stress responders in Aggregatibacter actinomycetemcomitans. PLoS One. 2016;11:e0160018.
- Agarwal V, Ahl J, Riesbeck K, et al. An alternative role of C1q in bacterial infections: facilitating Streptococcus pneumoniae adherence and invasion of host cells. J Immunol. 2013;191:4235–4245.
- Alberti S, Marques G, Camprubi S, et al. C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect Immun. 1993;61:852–860.
- Merino S, Nogueras MM, Aguilar A, et al. Activation of the complement classical pathway (C1q binding) by mesophilic Aeromonas hydrophila outer membrane protein. Infect Immun. 1998;66:3825–3831.
- Bjerre A, Brusletto B, Mollnes TE, et al. Complement activation induced by purified Neisseria meningitidis lipopolysaccharide (LPS), outer membrane vesicles, whole bacteria, and an LPS-free mutant. J Infect Dis. 2002;185:220–228.
- Kiley P, Holt SC. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980;30:862–873.
- Allen RJ, Scott GK. The effect of purified lipopolysaccharide on the bactericidal reaction of human serum complement. J Gen Microbiol. 1980;117:65–72.
- Dumestre-Perard C, Doerr E, Colomb MG, et al. Involvement of complement pathways in patients with bacterial septicemia. Mol Immunol. 2007;44:1631–1638.
- Loos M, Euteneuer B, Clas F. Interaction of bacterial endotoxin (LPS) with fluid phase and macrophage membrane associated C1Q. The FC-recognizing component of the complement system. Adv Exp Med Biol. 1990;256:301–317.
- Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12:1074–1084.
- Man-Kupisinska A, Swierzko AS, Maciejewska A, et al. Interaction of mannose-binding lectin with lipopolysaccharide outer core region and its biological consequences. Front Immunol. 2018;9:1498.
- Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with “blebbing” meningococcus. Lancet. 2002;360:1741.
- Unal CM, Schaar V, Riesbeck K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin Immunopathol. 2011;33:395–408.
- Han EC, Choi SY, Lee Y, et al. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. Faseb J. 2019;33:13412–13422.
- Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655.
- Dutson TR, Pearson AM, Price JF, et al. Observations by electron microscopy on pig muscle inoculated and incubated with Pseudomonas fragi. Appl Microbiol. 1971;22:1152–1158.
- Yonezawa H, Osaki T, Kurata S, et al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009;9:197.
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558.
- Lai CH, Listgarten MA, Hammond BF. Comparative ultrastructure of leukotoxic and non-leukotoxic strains of Actinobacillus actinomycetemcomitans. J Periodontal Res. 1981;16:379–389.
