ABSTRACT
Background and objectives
Porphyromonas gingivalis, a late colonizer of the periodontal biofilm, has been strongly associated with the chronic form of periodontitis. The aim of this study was to investigate the effects of a Dual Zinc plus Arginine formulation (aqueous solution and dentifrice) on the pathogenic properties of P. gingivalis and the barrier function of an in vitro gingival epithelium model.
Results
The Dual Zinc plus Arginine aqueous solution and dentifrice inhibited the hemolytic and proteolytic activities of P. gingivalis. The Dual Zinc plus Arginine aqueous solution and dentifrice enhanced the barrier function of an in vitro gingival epithelium model as determined by a time-dependent increase in transepithelial electrical resistance and decrease in paracellular permeability. This was associated with an increased immunolabeling of two important tight junction proteins: zonula occludens-1 and occludin. The deleterious effects of P. gingivalis on keratinocyte barrier function as well as the ability of the bacterium to translocate through a gingival epithelium model were attenuated in the presence of either Dual Zinc plus Arginine aqueous solution or dentifrice.
Conclusions
The Dual Zinc plus Arginine formulation may offer benefits for patients affected by periodontal disease through its ability to attenuate the pathogenic properties of P. gingivalis and promote keratinocyte barrier function.
Introduction
The oral cavity is colonized by a wide range of microbial species, mainly bacteria, which interact with each other and with host cells, contributing to physiological and pathological conditions. Dental biofilm is initially formed by Gram-positive facultative anaerobic cocci and rods, including Streptococcus and Actinomyces species. As the dental biofilm matures, colonization shifts toward strictly anaerobic Gram-negative bacterial species that contribute to the subgingival biofilm that initiates periodontal disease (gingivitis, periodontitis) [Citation1]. The progression and severity of periodontal disease is modulated by a limited group of bacteria that challenge mucosal and immune cells, leading to the establishment of a chronic inflammatory condition [Citation2]. More specifically, periodontitis is characterized by irreversible and progressive destruction of the supporting tissues surrounding the teeth, including the alveolar bone.
Although periodontal disease is considered a multifactorial polymicrobial infection, Porphyromonas gingivalis is suspected to be one of the most important causative agents of the chronic form of this disease [Citation3,Citation4]. This keystone bacterial species has been suggested to induce the transition from a symbiotic microbial community to a dysbiotic microbiota [Citation5]. P. gingivalis contributes to the pathogenesis of periodontitis through the expression of a wide range of virulence factors, including cysteine proteinases, also known as gingipains, that disturb host defense mechanisms, degrade tissue proteins, and modulate the host inflammatory response [Citation6,Citation7].
The oral epithelium creates a physical protective barrier between the underlying connective tissue and invasive periodontal pathogens and their toxic products in the oral environment, and thus plays an active role in the maintenance of periodontal health [Citation8,Citation9]. The intercellular tight junctions, which are composed of specialized transmembrane proteins that regulate transepithelial permeability, are the primary cellular determinant of epithelial barrier integrity and function [Citation10]. P. gingivalis has developed different strategies to compromise the structural and functional integrity of the oral epithelium. Using specific gingipain inhibitors and gingipain-deficient mutants of P. gingivalis, Groeger et al. [Citation11] provided evidence that these proteolytic enzymes are involved in the degradation of cell-to-cell junctions and the disruption of the epithelial barrier. Once the integrity of the oral epithelium is disrupted, P. gingivalis, along with other periodontal pathogens, can reach deeper connective tissues and trigger a marked pro-inflammatory response that modulates tissue destruction. Bacteria and their toxins can also enter the bloodstream, migrate to extra-oral sites, and cause systemic complications [Citation12,Citation13]. Taken the above into consideration, conditions or substances with the ability to attenuate the P. gingivalis-mediated destructive process or to promote the oral epithelial barrier integrity may be of high interest for maintaining or recovering oral health.
In previous human clinical studies [Citation14,Citation15], a dentifrice formulation containing zinc (zinc oxide, zinc citrate) and arginine, known as Dual Zinc plus Arginine, was reported to significantly decrease oral bacterial counts as well as plaque and gingivitis parameters compared to a regular fluoride dentifrice. The aim of the present study was to investigate the effects of the Dual Zinc plus Arginine formulation as an aqueous solution and in a fluoride dentifrice on the pathogenic properties of P. gingivalis and on the barrier function of an in vitro gingival epithelium model.
Materials and methods
Dual Zinc plus Arginine formulation
Zinc oxide and zinc citrate trihydrate were obtained from U.S. Zinc (Houston, TX, USA) and Jost Chemical (St. Louis, MO, USA), respectively. L-arginine (free form) was purchased from Ajinomoto (Tokyo, Japan). A mixture containing 0.96% zinc (zinc oxide, zinc citrate) and 1.5% arginine was freshly prepared in sterile distilled water and is referred to as the Dual Zinc plus Arginine aqueous solution. Unless indicated otherwise, the Dual Zinc plus Arginine aqueous solution was used at dilutions of 1/500, 1/1000, and 1/2000 (v/v). A dentifrice containing 0.96% zinc (zinc oxide, zinc citrate), 1.5% arginine, and 1450 ppm fluoride as sodium fluoride in a silica base marketed by Colgate-Palmolive Co. (Toronto, Canada) as Colgate Total toothpaste formula was also used. A zinc- and arginine-free control fluoride dentifrice was also tested. Unless indicated otherwise, the Dual Zinc plus Arginine dentifrice and control fluoride dentifrice were used at dilutions of 1/500, 1/1000, and 1/2000 (w/v). At the dilutions used, the amounts of zinc and arginine in the Dual Zinc plus Arginine aqueous solution and the Dual Zinc plus Arginine dentifrice were comparable. The pH of the aqueous solution was 10, while that of the dentifrice was 8.2; however, at the dilutions used, the buffer capacity of the culture tissue medium and the assay solutions brings the final pH at around 7.2. When the Dual Zinc plus Arginine aqueous solution and dentifrice were inoculated onto Todd-Hewitt agar plates (THA; Becton, Dickinson and Company, Sparks, MD, USA), no microbial contamination was observed (data not shown).
Bacteria and growth conditions
P. gingivalis ATCC 33277 was grown in an anaerobic chamber (80% N2, 10% CO2, 10% H2) for 24 h at 37°C in Todd-Hewitt broth (Becton, Dickinson and Company) supplemented with 0.001% (w/v) hemin and 0.0001% (w/v) vitamin K (THB-HK).
Hemolytic assay
Fresh sheep red blood cells (Nutri-Bact, Terrebonne, QC, Canada) were harvested from heparinized whole blood by centrifugation (600 x g for 5 min), washed three times in phosphate-buffered saline (PBS; pH 7.0), and suspended in PBS to a concentration of 2% (v/v). Equal volumes (1 ml) of red blood cells, P. gingivalis cells (optical density at 660 nm [OD660] = 1.0 in PBS), and two-fold serial dilutions of the Dual Zinc plus Arginine (aqueous solution and dentifrice) or the control dentifrice were mixed together. PBS replaced the bacteria in the negative control. Following an incubation at 37°C for 4 h, the mixtures were incubated at 4°C for 1 h and were then centrifuged (10 000 x g for 5 min) prior to recording the absorbance of the supernatants at 540 nm (A540). Assays were performed in triplicate in two independent experiments and the means ± standard deviations (SD) were calculated.
Proteolytic assay
To determine the effects of the Dual Zinc plus Arginine aqueous solution and dentifrice on the proteinase activity of P. gingivalis, a 48-h culture was centrifuged at 10 000 x g for 10 min and the supernatant was collected. Assay mixtures containing equal volumes of P. gingivalis culture supernatant, the fluorescent substrate collagen DQTM (100 µg/ml; Molecular Probes, Eugene, OR, USA), and the Dual Zinc plus Arginine aqueous solution or dentifrice were prepared and incubated for 2 h at 37°C. The fluorescence corresponding to collagen degradation was monitored at time 0, 30, 60, 90, and 120 min using a Synergy 2 microplate reader (BioTek Instruments, Winooski, VT, USA), with the excitation and emission wavelengths set at 495 nm and 525 nm, respectively. Test compounds or the fluorescent substrate alone were used as controls. Leupeptin (1 µM) was used as a positive inhibitory control. Assays were performed in triplicate in two independent experiments and the means ± SD were calculated.
Human gingival keratinocyte culture
The previously characterized B11 immortalized human gingival keratinocyte cell line [Citation16] was used to investigate the effects of the Dual Zinc plus Arginine aqueous solution and dentifrice on keratinocyte barrier integrity. Keratinocytes were cultivated in keratinocyte serum-free medium (K-SFM; Life Technologies Inc., Burlington, ON, Canada) supplemented with growth factors (50 µg/ml of bovine pituitary extract and 5 ng/ml of human epidermal growth factor) and 100 µg/ml of penicillin G-streptomycin at 37°C in a 5% CO2 atmosphere.
Transepithelial electrical resistance assay
The tight junction integrity of the B11 gingival keratinocyte barrier was assessed by determining the transepithelial electrical resistance (TER) using the procedure described by Gumbiner and Simons [Citation17]. Briefly, B11 keratinocytes were seeded onto Costar™ Transwell™ clear polyester membrane inserts (6.5-mm diameter; 0.4-µm pore size; Corning Co., Cambridge, MA, USA) at 3 × 105 cells per insert. The basolateral and apical compartments were filled with 0.6 ml and 0.1 ml of complete K-SFM, respectively, and the cultures were incubated for 3 days at 37°C in a 5% CO2 atmosphere. The conditioned medium was then replaced with fresh antibiotic-free K-SFM. Following a further incubation (16 h), the TER values were measured using an Ohm/voltmeter (EVOM2; World Precision Instruments, Sarasota, FL, USA) at 0, 2, 6, 24, and 48 h. Resistance values were calculated in Ohms (Ω)/cm2 by multiplying the resistance values by the filter surface area. Results were expressed as a percentage of the basal control values measured at time 0 h (100% values) for each condition. To investigate the effects of the Dual Zinc plus Arginine aqueous solution and dentifrice on tight junction integrity, the medium in the apical compartment was supplemented with the test compounds. The effect of these treatments on cell viability was assessed using an MTT (3-[4,5-diethylthiazol-2-yl]-2,5diphenyltetrazolim bromide) colorimetric assay, according to the manufacturer’s instructions (Roche Diagnostics, Laval, QC, Canada).
The effect of P. gingivalis on the tight junction integrity of the gingival keratinocyte barrier model was evaluated by monitoring TER at 0, 6, 24, and 48 h. P. gingivalis cells in antibiotic-free K-SFM were added to the apical compartment at a multiplicity of infection (MOI) of 104. The protective effects of adding the Dual Zinc plus Arginine aqueous solution or dentifrice were evaluated by adding them to the apical compartment at the same time as the P. gingivalis cells. All the above assays were performed in triplicate in three independent experiments and the means ± SD of a representative set of data is presented.
Paracellular permeability assay
The ability of the test compounds to enhance or protect gingival keratinocyte barrier integrity was further assessed by monitoring the paracellular transport of fluorescein isothiocyanate (FITC)-conjugated 4.4-kDa dextran (FD-4; Sigma-Aldrich Canada Co., Oakville, ON, Canada) across the keratinocyte layer. Briefly, B11 cells were cultured on Transwell™ filters, and FD-4 (1 mg/ml in culture medium) was added to the apical compartment in the presence of the test compounds. The presence of FD-4 in the basolateral compartment was determined at 0, 2, 6, 24, and 48 h by measuring the fluorescence (relative fluorescence units [RFU]; excitation wavelength 495 nm; emission wavelength 525 nm) using a Synergy 2 microplate reader. The effects of P. gingivalis (MOI = 104) on paracellular permeability and the protective effects of the Dual Zinc plus Arginine aqueous solution and dentifrice were assessed under the conditions described above. Assays were performed in triplicate in three independent experiments and the means ± SD of a representative set of data is presented.
Immunofluorescent staining of zonula occludens-1 and occludin
Gingival keratinocytes treated for 48 h as described above (test compounds ± P. gingivalis) were immunostained for two tight junction proteins (zonula occludens [zo-1] and occludin) using a previously described protocol [Citation18]. The localization of the tight junction proteins in B11 cells was visualized using an Olympus FSX100 fluorescence microscope and FSX-BSW imaging software (Olympus, Tokyo, Japan). This experiment was performed three times; a representative experiment and a representative field of this experiment is presented.
P. gingivalis translocation assay
B11 gingival keratinocytes cultured as described above were seeded at 2.25 × 105 cells per insert in high-throughput screening (HTS) 96-well Costar™ Transwell™ plates (8-µm pore size; Corning Co.), which were placed in Costar™ black receiver plates (Corning Co.). The basolateral and apical compartments were filled with 0.235 ml and 0.075 ml of K-SFM, respectively. Following a 48-h incubation, the conditioned medium was replaced with antibiotic-free K-SFM. To determine the ability of P. gingivalis to penetrate the keratinocyte layer, FITC-labeled bacteria suspended in antibiotic-free K-SFM were added to the apical compartment of the double-chamber system at an MOI of 104. Bacteria from an overnight culture were labeled with FITC as described previously [Citation19]. To evaluate the effect of the Dual Zinc plus Arginine aqueous solution and dentifrice on the invasive capacity of P. gingivalis, the keratinocyte layer was co-incubated with them and the bacteria. The translocation of FITC-labeled bacteria through the keratinocyte barrier was monitored using a Synergy 2 microplate reader by measuring the fluorescence (RFU; excitation wavelength 495 nm; emission wavelength 525 nm) in the medium recovered from the lower chamber following a 24-h incubation in an anaerobic chamber at 37°C. Assays were performed in triplicate in three independent experiments and the means ± SD of a representative set of data is presented.
Statistical analysis
Statistical analyses were performed using a one-way analysis of variance with a post hoc Bonferroni multiple comparison test (GraphPad Software Inc., La Jolla, CA, USA). All results were considered statistically significant at p< 0.05.
Results
P. gingivalis cells caused marked hemolysis of sheep red blood cells in a hemolytic assay (). Both the Dual Zinc plus Arginine aqueous solution and dentifrice significantly reduced hemolysis. At the lowest dilution tested (1/500), hemolysis was reduced by 47.5% and 37.6%, respectively. The control fluoride dentifrice did not inhibit the hemolysis caused by P. gingivalis.
Table 1. Effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice on the hemolytic activity of P. gingivalis. A value of 100% was assigned to the hemolysis induced by P. gingivalis in the absence of compounds. Results are expressed as the means ± SD of triplicate assays from two independent experiments. *, significant inhibition (p < 0.05) compared to control (no compounds).
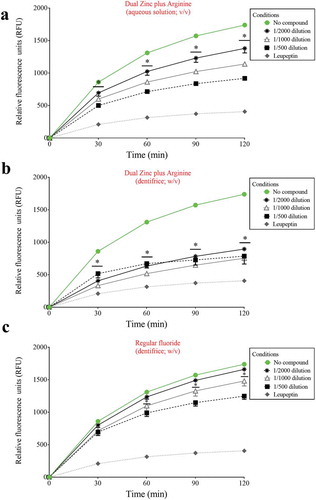
We then investigated the ability of the Dual Zinc plus Arginine formulation (aqueous solution and dentifrice) to inhibit the degradation of type I collagen by proteinases present in a culture supernatant of P. gingivalis. Significant time- and dose-dependent inhibition was observed with both the Dual Zinc plus Arginine aqueous solution and dentifrice (). More specifically, at the lowest dilution tested (1/500) and after a 2-h incubation, the Dual Zinc plus Arginine aqueous solution and the Dual Zinc plus Arginine dentifrice caused a 47.2% and 54.8% inhibition of type I collagen degradation, respectively. The control fluoride dentifrice reduced collagen degradation by 28.2%.
Figure 1. Effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice on collagen degradation by P. gingivalis. Results are expressed as the means ± SD of triplicate assays two independent experiments. *, significant decrease (p < 0.05) compared to untreated control cells.
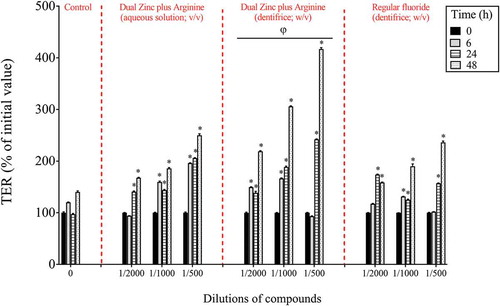
After investigating the effects of the Dual Zinc plus Arginine formulation (aqueous solution and dentifrice) on P. gingivalis, its ability to promote gingival keratinocyte barrier integrity was assessed. Preliminary assays showed that, at the concentrations used, the Dual Zinc plus Arginine aqueous solution and dentifrice did not significantly affect the viability of gingival keratinocytes as determined using a colorimetric MTT assay (data not shown). The ability of the Dual Zinc plus Arginine formulation to modulate the integrity of the gingival keratinocyte tight junction was determined by monitoring TER values over a period of 48 h. As shown in , the Dual Zinc plus Arginine aqueous solution and dentifrice induced a significant time-dependent increase in TER. A 24-h treatment of the keratinocytes with the 1/500 and 1/1000 dilutions of the Dual Zinc plus Arginine aqueous solution caused a 2.1- and 1.5-fold increase in TER, respectively, compared to untreated cells. A similar treatment with the Dual Zinc plus Arginine dentifrice caused a 2.5- and 1.9-fold increase in TER, respectively. Under the same conditions, the 1/500 and 1/1000 dilutions of the control fluoride dentifrice caused a 1.6-and 1.3-fold increase in TER, respectively.
Figure 2. Time- and dose-dependent effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice on gingival keratinocyte tight junction integrity, as determined by monitoring TER. A 100% value was assigned to the TER values at time 0. Results are expressed as the means ± SD of triplicate assays. *, significant increase (p < 0.05) compared to untreated control cells. φ, significant increase (p < 0.05) compared to the regular fluoride dentifrice.
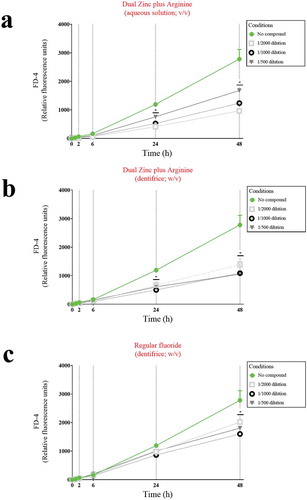
To confirm that the Dual Zinc plus Arginine aqueous solution and dentifrice enhanced the function of the keratinocyte barrier, their effect on paracellular permeability was investigated by measuring the apical-to-basolateral transport of FD-4. As shown in , the paracellular transport of FD-4 time-dependently increased in the control assay (no compounds). However, in the presence of the the Dual Zinc plus Arginine aqueous solution or dentifrice, the increase in FD-4 transport through the gingival keratinocyte barrier was significantly attenuated. More specifically, following a 24-h treatment, the aqueous solution and dentifrice at the lowest dilution tested (1/500) reduced FD-4 transport by 36.4% and 49.0%, respectively, while the control fluoride dentifrice only reduced FD-4 transport through the barrier model by 15.8%.
Figure 3. Time- and dose-dependent effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice on the paracellular permeability of gingival keratinocytes to FITC-dextran 4 (FD-4). Results are expressed as the means ± SD of triplicate assays. *, significant decrease (p < 0.05) compared to untreated control cells.
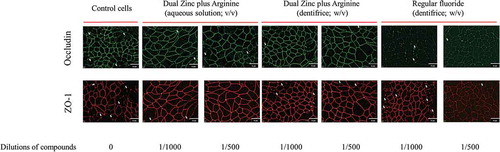
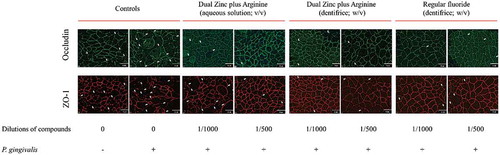
We then examined the effect of the 1/500 and 1/1000 dilutions of the Dual Zinc plus Arginine aqueous solution and dentifrice on the distribution of two junction proteins (ZO-1 and occludin) by immunofluorescence. Both the aqueous solution and dentifrice increased the immunolabeling of ZO-1 and occludin in the areas of cell-cell contact (), while the regular fluoride dentifrice had few or no effect on the immunolabeling of ZO-1 and occludin.
Figure 4. Immunofluorescence staining of the tight junction proteins occludin and zonula occludens-1 of gingival keratinocytes treated for 48 h with the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice. Arrows indicate discontinuities in protein labeling. This analysis was performed three times; a representative experiment and a representative field of this experiment is presented.
Since P. gingivalis may have a deleterious effect on keratinocyte barrier integrity, we investigated whether the Dual Zinc plus Arginine aqueous solution and dentifrice protect gingival keratinocytes from damage. Treating the keratinocytes with P. gingivalis at a MOI of 104 significantly decreased TER. After 24- and 48-h incubations, P. gingivalis decreased TER by 53.2% and 92.1%, respectively (). Despite the effect of P. gingivalis on barrier integrity, it should be noted that no significant loss of cell viability was observed using a MTT assay that determines cell metabolic activity (data not shown). We then examined the protective effect of the Dual Zinc plus Arginine aqueous solution and dentifrice on TER when the keratinocytes were challenged with P. gingivalis. As shown in , both compounds attenuated the P. gingivalis-mediated loss of keratinocyte barrier integrity. More specifically, following a 48-h incubation, a 1/500 dilution of the Dual Zinc plus Arginine aqueous solution and dentifrice reduced the ability of P. gingivalis to decrease TER 13.4-fold and 21.4-fold, respectively. A 1/500 dilution of the regular fluoride dentifrice also provided a protective effect, reducing P. gingivalis-induced damage by 11.2-fold.
Figure 5. Time- and dose-dependent protective effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice against P. gingivalis-mediated damage of gingival keratinocyte tight junction integrity as determined by monitoring TER values. A 100% value was assigned to the TER values at time 0. Results are expressed as the means ± SD of triplicate assays. *, significant increase (p < 0.001) compared to P. gingivalis-infected cells not treated with compounds. Φ, significant decrease (p < 0.05) compared to non-stimulated control cells. φ, significant increase (p < 0.05) compared to the regular fluoride dentifrice.
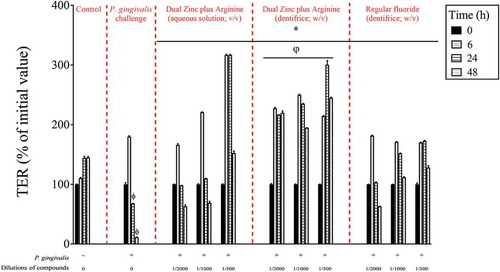
Figure 6. Protective effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice on the paracellular permeability of gingival keratinocytes to FITC-dextran 4 (FD-4) compromised by P. gingivalis. Results are expressed as the means ± SD of triplicate assays. *, significant decrease (p < 0.05) compared to P. gingivalis-stimulated cells.
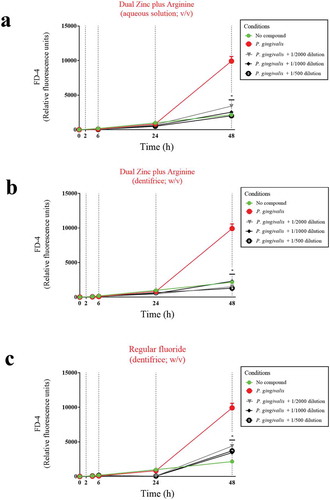
To confirm this protective effect, we investigated the effect of the Dual Zinc plus Arginine aqueous solution and dentifrice on P. gingivalis-induced paracellular flux of FD-4 through the keratinocyte barrier (). A 48-h treatment with a 1/500 dilution of the Dual Zinc plus Arginine aqueous solution and dentifrice caused a 4.9-fold and 7.6-fold decrease in FD-4 transport, respectively. The regular fluoride dentifrice caused a 2.7-fold decrease in FD-4 transport.
ZO-1 and occludin immunostaining was performed to determine whether P. gingivalis affects the keratinocyte barrier through the disruption of these two tight junction proteins. As shown in , a 48-h treatment of the keratinocytes with P. gingivalis (MOI of 104) was associated with a marked decrease in ZO-1 and occludin immunolabeling. ZO-1 and occludin immunolabeling appeared to be less intense and more discontinuous in the cell-cell contacts after a treatment with P. gingivalis compared to control cells. However, both the Dual Zinc plus Arginine aqueous solution and dentifrice prevented the discontinuous and less intense immunolabeling of ZO-1 and occludin.
Figure 7. Immunofluorescence staining of the tight junction proteins occludin and zonula occludens-1 in gingival keratinocytes following a 48-h treatment with P. gingivalis (MOI = 104) in the absence and presence of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, or the regular fluoride dentifrice. Arrows indicate discontinuities in protein labeling. This analysis was performed three times; a representative experiment and a representative field of this experiment is presented.
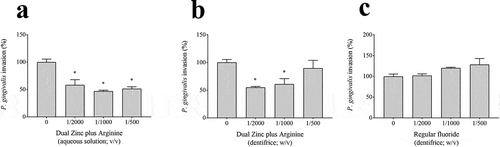
We then determined the effect of the Dual Zinc plus Arginine aqueous solution and dentifrice on the translocation of P. gingivalis through the gingival keratinocyte barrier model. The FITC-labeled P. gingivalis cells crossed the keratinocyte barrier in a double-chamber system (). A 1/1000 dilution of the Dual Zinc plus Arginine aqueous solution and dentifrice significantly reduced the migration of the FITC-labeled P. gingivalis cells through the barrier by 53.0% and 39.1%, respectively. The regular fluoride dentifrice caused no significant decrease in the migration of FITC-labeled P. gingivalis cells.
Figure 8. Effects of the Dual Zinc plus Arginine aqueous solution, the Dual Zinc plus Arginine dentifrice, and the regular fluoride dentifrice on the invasion of a gingival keratinocyte barrier by P. gingivalis. Results are expressed as the means ± SD of triplicate assays. *, significant decrease (p < 0.001) compared to P. gingivalis-infected cells not treated with the compounds.
Discussion
Two strategies can be used to promote periodontal health: (1) attenuate/neutralize the pathogenicity of periodontal pathogens, and (2) improve innate immunity by reinforcing epithelial barrier function. In the present study, we investigated the effects of a Dual Zinc plus Arginine aqueous solution and dentifrice on the pathogenic properties of P. gingivalis as well as on the barrier function of an in vitro gingival epithelium model.
We first showed that the Dual Zinc plus Arginine formulation reduced the hemolytic activity of P. gingivalis. The ability of P. gingivalis to lyse erythrocytes and release hemoglobin is considered a virulence determinant since it provides an iron source to P. gingivalis and other periodontal pathogens and thus promotes their proliferation in subgingival sites. Moreover, hemoglobin has been reported to synergize with P. gingivalis lipopolysaccharides to amplify the inflammatory response of human macrophages [Citation20]. As such, the partial inhibition of hemolysis by the Dual Zinc plus Arginine formulation may contribute to reducing the levels of pro-inflammatory mediators in periodontal sites in addition to attenuate proliferation of P. gingivalis.
Type I collagen makes up approximately 60% of the tissue volume of periodontal tissues. The collagenolytic activity of P. gingivalis has been attributed to the action of its gingipains, which are both secreted and cell-bound [Citation21,Citation22]. We showed that the Dual Zinc plus Arginine formulation dose-dependently reduces collagen degradation by P. gingivalis, suggesting that it may contribute to attenuate the tissue destructive process mediated by this periodontal pathogen. Interestingly, previous studies aimed at identifying effective inhibitors of P. gingivalis gingipains have shown that both L-arginine [Citation23] and zinc [Citation24], both of which are found in the Dual Zinc plus Arginine formulation, are highly effective in this regard.
The Dual Zinc plus Arginine formulation was found to attenuate two important virulence factors (hemolysin and gingipains) of P. gingivalis. Future studies aimed to investigate the antibacterial and anti-biofilm properties of the formulation against P. gingivalis should be carried out. Such studies are supported by the fact that Manus et al. [Citation25] recently reported that the Dual Zinc plus Arginine formulation was able to reduce bacterial viability in saliva-derived biofilm models. Evidence was brought that the antibacterial activity resulted from the action of zinc while the presence of arginine disrupts the architecture/porosity of biofilms and allows zinc to penetrate deeper into the biofilm.
The first line of host defense against both opportunistic and pathogenic microorganisms colonizing the oral cavity is the oral epithelium [Citation8,Citation9]. The physical epithelial barrier is composed of closely opposed cells that connect neighboring cells to each other by specialized intercellular tight junctions [Citation26]. These tight junctions seal the paracellular space, blocking the pathway to bacteria and toxins while allowing the flux of water and nutrients. Given the crucial protective role played by the oral epithelial barrier, compounds endowed with a capacity to enhance or protect tissue barrier function are of great interest as potential oral care products. In this regard, we previously reported that plant polyphenols, including green tea catechins [Citation18], black tea theaflavins [Citation27], and blueberry proanthocyanidins [Citation28] improve tight junction integrity in an in vitro gingival epithelium model. Although different mechanistic pathways are likely involved, in the present study, we showed that the Dual Zinc plus Arginine aqueous solution and dentifrice significantly enhance the barrier function of a gingival keratinocyte model, as determined by a time-dependent increase in transepithelial electrical resistance and decrease in paracellular permeability. Moreover, the Dual Zinc plus Arginine formulation also increased the immunolabeling of ZO-1 and occludin. The ability of the Dual Zinc plus Arginine formulation to promote gingival keratinocyte barrier function may be associated with the presence of zinc. This is supported by the results of Rybakovsky et al. [Citation29], who recently investigated the effects of various micronutrients on transepithelial electrical resistance in an in vitro oral mucosa model and showed that zinc can improve epithelial barrier function. Moreover, in preliminary assays using zinc oxide and zinc citrate, we observed a significant increase in TER in our keratinocyte model although the effects were much less important than with the Dual Zinc plus Arginine formulation (data not shown).
In addition to being a physical barrier against the invasion of the underlying connective tissue by periodontopathogenic bacteria, keratinocytes provide an immunological barrier by secreting antimicrobial β-defensin peptides active against Gram-positive and Gram-negative bacteria [Citation30]. Given that several compounds, including green tea catechins, can increase the innate immunity of oral keratinocytes by inducing human β-defensin secretion [Citation31], it may be of interest to investigate whether the Dual Zinc plus Arginine formulation can increase β-defensin secretion in oral keratinocytes. This would further support the positive impact of the formulation for improving the protective barrier function of the oral epithelium.
P. gingivalis has developed various strategies to invade the gingival epithelium and overcome its protective functions [Citation32,Citation33]. In the present study, we showed that P. gingivalis can compromise epithelial barrier function by inducing the disorganization of cell-cell interactions as shown by the decrease in TER and increase in FD-4 transport. P. gingivalis also affected the distribution of two major tight junction proteins (zonula occludens-1 and occludin). This is in agreement with the results of Katz et al. [Citation34], who used Western blotting to show that P. gingivalis (cells and supernatant) can cleave purified occludin. These effects may allow bacteria to reach and damage the underlying connective tissue. The Dual Zinc plus Arginine formulation protected the gingival keratinocyte barrier against P. gingivalis-mediated damage. This protective effect may rely on the ability of the zinc to enhance the gingival epithelium barrier function observed in the present study. It may also, at least in part, result from the ability of the Dual Zinc plus Arginine formulation to inhibit P. gingivalis gingipain activity. Groeger et al. [Citation11] provided evidence that these proteolytic enzymes are involved in the degradation of cell-to-cell junctions and the disruption of the epithelial barrier. In a recent study, Sarkar et al. [Citation35] used in vivo (mouse) and in vitro models to demonstrate that zinc can protect the intestinal epithelial barrier from damage induced by the pathogen Shigella flexneri. This protective effect was associated with a redistribution of two tight junction proteins (claudin−2 and −4) to the plasma membrane.
The intercellular spaces of the stratified oral epithelium offer a pathway for P. gingivalis to invade tissues during periodontitis [Citation36,Citation37]. We thus investigated the effect of the Dual Zinc plus Arginine formulation on the translocation of P. gingivalis through an in vitro model of the gingival epithelium. We used FITC-labeled bacteria to show that the formulation reduced the migration of P. gingivalis through the gingival keratinocyte barrier in a double-chamber system. Since gingipains contribute to the invasive capacity of P. gingivalis, their inhibition by the formulation may be responsible, at least in part, in a reduced translocation through our in vitro model of the gingival epithelium.
In conclusion, the present study provided clear in vitro evidence that the Dual Zinc plus Arginine formulation, in an aqueous solution or in a dentifrice, may offer benefits for periodontal health through its ability to attenuate the pathogenic properties of P. gingivalis and enhance epithelial barrier function. Among the limitations of the study was the fact that only one keratinocyte cell line and one P. gingivalis strain were used. Moreover, clinical trials are necessary to determine whether the beneficial effects observed with Dual Zinc plus Arginine formulation in our in vitro models can be achieved in an in vivo situation.
Acknowledgments
We thank S. Groeger (Department of periodontology, Justus-Liebeg-university Giessen) for provideing the bil cell line.
Disclosure statement
The authors report no conflicts of interest related to this study. Y. Yang, H.M. Trivedi, and J.G. Masters work as scientists for Colgate-Palmolive Co.
Additional information
Funding
References
- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–12.
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490.
- Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9.
- How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53.
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419.
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15–44.
- Mysak J, Podzimek S, Sommerova P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014:476068.
- Dale BA. Periodontal epithelium: a newly recognized role in health and disease. Periodontol 2000. 2002;30:70–78.
- Groeger SE, Meyle J. Epithelial barrier and oral bacterial infection. Periodontol 2000. 2015;69:46–67.
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70.
- Groeger S, Doman E, Chakraborty T, et al. Effects of Porphyromonas gingivalis infection on human gingival epithelial barrier function in vitro. Eur J Oral Sci. 2010;118:582–589.
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44.
- Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–491.
- Delgado E, Garcia-Godoy F, Montero-Aguilar M, et al. A clinical investigation of a Dual Zinc plus Arginine dentifrice in reducing established dental plaque and gingivitis over a six-month period of product use. J Clin Dent. 2018;29:A33–A40.
- Prasad KV, Therathil SG, Agnihotri A, et al. The effects of two new Dual Zinc plus Arginine dentifrices in reducing oral bacteria in multiple locations in the mouth: 12-hour whole mouth antibacterial protection for whole mouth health. J Clin Dent. 2018;29:A25–A32.
- Groeger S, Michel J, Meyle J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J Periodontal Res. 2008;43:604–614.
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occludin barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468.
- Ben Lagha A, Groeger S, Meyle J, et al. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog Dis. 2018;76:FTY030.
- Marquis A, Genovese S, Epifano F, et al. The plant coumarins auraptene and lacinartin as potential multifunctional therapeutic agents for treating periodontal disease. BMC Complement Altern Med. 2012;12:80.
- Bodet C, Chandad F, Grenier D. Hemoglobin and LPS act in synergy to amplify the inflammatory response. J Dent Res. 2007;86:878–882.
- Houle MA, Grenier D, Plamondon P, et al. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol Lett. 2003;221:181–185.
- Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. 2003;74:111–118.
- Nishikata M, Yoshimura F, Nodasaka Y. Possibility of Bacteroides gingivalis hemagglutinin possessing protease activity revealed by inhibition studies. Microbiol Immunol. 1989;33:75–80.
- Krauser JA, Potempa J, Travis J, et al. Inhibition of arginine gingipains (RgpB and HRgpA) with benzamidine inhibitors: zinc increases inhibitory potency. Biol Chem. 2002;383:1193–1198.
- Manus LM, Daep CA, Begum-Gafur R, et al. Enhanced in vitro zinc bioavailability through rational design of a Dual Zinc plus Arginine dentifrice. J Clin Dent. 2018;29:A10–A19.
- Meyle J, Gultig K, Rascher G, et al. Transepithelial electrical resistance and tight junctions of human gingival keratinocytes. J Periodontal Res. 1999;34:214–222.
- Ben Lagha A, Grenier D. Black tea theaflavins attenuate Porphyromonas gingivalis virulence properties, modulate gingival keratinocyte tight junction integrity and exert anti-inflammatory activity. J Periodontal Res. 2017;52:458–470.
- Ben Lagha A, LeBel G, Grenier D. Dual action of highbush blueberry proanthocyanidins on Aggregatibacter actinomycetemcomitans and the host inflammatory response. BMC Complement Altern Med. 2018;18(1):10.
- Rybakovsky E, Valenzano MC, Deis R, et al. Improvement of humanoralepithelialbarrier function and of tight junctions by micronutrients. J Agric Food Chem. 2017;65:10950–10958.
- Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2011;7:119–134.
- Lombardo Bedran TB, Feghali K, Zhao L, et al. Green tea extract and its major constituent, epigallocatechin-3-gallate, induce epithelial beta-defensin secretion and prevent beta-defensin degradation by Porphyromonas gingivalis. J Periodontal Res. 2013;49:615–623.
- Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006;85:392–403.
- Sakanaka A, Takeuchi H, Kuboniwa M, et al. Dual lifestyle of Porphyromonas gingivalis in biofilm and gingival cells. Microb Pathog. 2016;94:42–47.
- Katz J, Sambandam V, Wu JH, et al. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68:1441–1449.
- Sarkar P, Saha T, Sheikh IA, et al. Zinc ameliorates intestinal dysfunctions in shigellosis by reinstating claudin−2 and −4 on the membranes. Am J Physiol Gastrointest Liver Physiol. 2019;316:G229–G246.
- Lamont RJ, Chan A, Belton CM, et al. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885.
- Nakagawa I, Inaba H, Yamamura T, et al. Invasion of epithelial cells and proteolysis of cellular focal adhesion components by distinct types of Porphyromonas gingivalis fimbriae. Infect Immun. 2006;74:3773–3782.
