 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Evidence suggest periodontal bacterial infection can contribute to oral cancer initiation and progression.
Aim: To investigate the effects of periodontal bacteria on oral cancer cell behavior using a cell-based system and a mouse carcinogenesis model.
Methods: Oral cancer cell lines were polyinfected with four periodontal bacteria. Cytokine levels and relative changes in oncogene mRNA expression were determined post-infection. Oral tumours in mice induced by 4-nitroquinoline-1-oxide (4NQO) were compared with and without administrating periodontal bacteria.
Results: Polyinfected oral cancer cells had upregulated MMP1, MMP9, and IL-8. The expression of cell survival markers MYC, JAK1, and STAT3 and epithelial-mesenchymal transition markers ZEB1 and TGF-β were also significantly elevated. Monoinfections showed F. nucleatum alone had comparable or greater effects than the four bacteria together. Fusobacterial culture supernatant, primarily LPS, was sufficient to induce IL-8 secretion, demonstrating that direct contact of live Fusobacteria with cancer cells might not be required to exert changes in cancer cell behaviour. In the 4NQO-induced oral tumour model, mice infected with bacteria developed significantly larger and more numerous lesions compared to those not infected.
Conclusion: This study demonstrated that Fusobacteria could potentially enhance cancer cell invasiveness, survival, and EMT when presented in the oral tumour microenvironment.
Abbreviations: 4NQO, 4-nitroquinoline-1-oxide; ELISA, enzyme-linked immunosorbent assay; EMT, epithelial–mesenchymal transition; IL-8, interleukin-8; JAK1, Janus kinase 1; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; OSCCs, oral squamous cell carcinomas; PK, proteinase K; PMB, Polymyxin B; qRT-PCR, quantitative real-time polymerase chain reaction; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor beta; ZEB1, zinc finger E-Box binding homeobox 1
Introduction
Oral cancer is one of the most common cancers that affect public health [Citation1]. In the USA alone, 53,260 patients will be diagnosed with oral cavity and pharyngeal cancers and more than 10,000 people will die from oral cancer in 2020 [Citation2]. Oral squamous cell carcinomas (OSCCs) are the most common oral cancers that affect the oral epithelium. The survival rate and the quality of life of patients with OSCCs are highly dependent on early diagnosis and management [Citation3]. Data in the literature support the link between inflammation and cancer progression. For example, there is increased risk of gastric cancer and colon cancer in patients with gastritis and ulcerative colitis, respectively [Citation4]. In addition, about 15% of cancers may be triggered by infections, with recent evidence suggesting that microorganisms can promote cancer progression by inducing inflammation [Citation5]. Since periodontal disease is associated with chronic inflammation [Citation6], oral pathogens may have an important role in oral cancer initiation and progression [Citation7]. Several studies have suggested an association between chronic periodontitis and increased risk of oral cancer [Citation8–10]. A recent meta-analysis showed a 2-fold increase in the risk of oral cancer in patients with periodontal disease [Citation11]. Additionally, some cancer patients suffer from depression along with the side effects of chemotherapy/radiotherapy, all of which can hinder them from maintaining proper oral hygiene. This can result in increased gingival bleeding and incidence of periodontal infection in these patients [Citation12].
Biofilms on OSCCs are also reported to be rich with anaerobic bacteria associated with periodontal disease [Citation13,Citation14]. Adding to that, there is an association between poor oral hygiene and oral cancer [Citation15]. In fact, many studies have reported the deleterious effect of periodontal pathogens on different types of cancer including oral cancer [Citation7]. For example, periodontal pathogen F. nucleatum was reported to induce chemoresistance in colorectal cancer cells by upregulating autophagy [Citation16]. Moreover, recent study on colorectal cancer have shown that the FAD-A protein from F. nucleatum upregulate Annexin A1 on cancer cells through E‐cadherin and this promotes carcinogenesis [Citation17]. Also, another periodontal pathogen P. gingivalis has been reported to induce oral cancer cells invasiveness by inducing IL-8 secretion and MMPs upregulation [Citation18]. However, there is a gap in the understanding of the biological effects of periodontal bacteria on oral cancer cell behaviour. Hence, this study was designed to investigate the effects of periodontal pathogens on cultured oral cancer cells in vitro and follow up with testing whether selected oral bacteria could enhance the formation of precancerous lesion in a mouse oral carcinogenesis model.
Materials and methods
Cell culture
HN and BHY cells were head and neck cancer cells purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen, GmbH (DSMZ, Braunschweig, Germany). OQ01 was a primary cultured head and neck cancer cell line provided by Dr. Lung-Ji Chang, University of Florida [Citation19]. These cancer cell lines were maintained in DMEM/F12 supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin, and 100 units/ml penicillin in a humidified chamber (37°C, 5% CO2) as previously described [Citation20,Citation21].
Bacterial culture
P. gingivalis strain ATCC 53,977, F. nucleatum vincentii ATCC 49,256, F. nucleatum polymorphum ATCC 10,953, F. periodonticum ATCC 33,693, Treponema denticola ATCC 35,404 and Tannerella forsythia ATCC 43,037 were grown as previously described at 37°C in an anaerobic chamber [Citation22]. Bacteria were grown in their respective culture media until their logarithmic growth phase, with growth rate measured by optical density at 550 nm. Culture characteristics and gram stains were assessed for all cultures prepared in this study. Culture supernatants were collected after centrifugation at 12,000 × g for 10 min and used for experiments described below.
Infection of cancer cells
Cancer cells were seeded at 5 x 105/ml in wells of 6-well plates at 37°C overnight. A combination of four live bacteria (P. gingivalis, F. nucleatum vincentii, T. denticola, and T. forsythia at 1:1:1:1 ratio) or individual bacteria were added at a multiplicity of infection (MOI) of 100 for each bacterium. As controls, oral cancer cells were also cultured in medium alone. Plates were incubated at 37°C with 5% CO2 for 6 or 24 h. Supernatants and cell lysates were collected for cytokine analyses and RNA isolation.
Enzyme-linked immunosorbent assay (ELISA)
Secreted cytokines and matrix metalloproteinases (MMPs) in culture supernatants were measured by ELISA using an Opt EIA ELISA kit as recommended by ELISA kit manufacturers (BD Biosciences and Affymetrix eBioscience). Absorbance was measured using a microplate reader (Model 680, Bio-Rad) and converted into concentration using standard curves of respective purified recombinant human proteins.
mRNA expression
Total RNA from harvested cells was isolated using the mirVana miRNA Isolation Kit (Thermo Fisher) and RNA concentrations were determined using a NanoDrop ND-1000 spectrophotometer. For mRNA (STAT3, JAK1, MYC, MMP1, MMP9, and ZEB1) qRT-PCR analysis, fold changes in expression levels were calculated after normalization to 18S RNA as previously described [Citation19,Citation20,Citation23].
Cell invasion assay
Cell invasion assays were carried out using Transwell chambers (8 µm pore size; Corning) [Citation20]. Cancer cells (5 x 104) infected with bacteria for 4 h or mock-infected control cells were suspended in 200 μl serum-free DMEM medium in 1:10-diluted matrigel-coated upper chamber (Matrigel, BD Biosciences, San Jose, CA). DMEM containing 10% FBS was added in the lower chamber. After 24 h, cells migrated to the bottom of the filter were fixed, stained with 0.05% crystal violet in 20% methanol for 5 min, and counted in 5 fields/filter at 200x magnification [Citation20].
Characterization of the active component in F. periodonticum supernatant
The following treatments were performed on the F. periodonticum bacterial culture supernatants to examine their ability to induce IL-8 in OQ01 cells:
Proteinase K treatment. F. periodonticum bacterial culture supernatant 100 µl was incubated with Proteinase K (Thermo Fisher) at 0, 2, or 20 ug/ml for 5 min at 55°C. Next, to deactivate Proteinase K, the mixture was heated at 95°C for 10 min. The supernatant with deactivated enzyme was then incubated with cancer cells for 24 h. Supernatant treated similarly but without the addition of Proteinase K and uninfected cells were used as additional controls. OQ01 cell supernatants were analyzed for IL-8 production by ELISA.
Nucleic acid depletion. F. periodonticum bacterial supernatant 100 µl was incubated with 10 µl of 200 K units/ml micrococcal nuclease (Cell Signaling) for 20 min at 37°C. To deactivate the nuclease, the mixture was heated at 65°C for 10 min. The supernatant with deactivated enzyme was incubated with cancer cells for 24 h. The efficiency of nucleic acid depletion using micrococcal nuclease was confirmed by agarose gel electrophoresis. OQ01 cell supernatants were analyzed for IL-8 production by ELISA.
LPS depletion. To determine whether polymyxin B (PMB) could effectively deplete LPS, Salmonella enterica LPS (Sigma-Aldrich) was used as a control to stimulate OQ01 cells to induce IL-8 production. Next, 100 units/ml PMB was mixed with Salmonella enterica LPS to determine its efficiency in depletion and inhibition of IL-8 production. F. periodonticum bacterial supernatant 100 µl was mixed similarly with PMB and the PMB/supernatant mixture was added to OQ01 cells for 24 h. Untreated F. periodonticum bacterial supernatants were used as positive controls, while uninfected cells and polymyxin B alone were used as negative controls. OQ01 cell supernatants were analyzed for IL-8 production by ELISA.
Western blot
Oral cancer cell lysates were analyzed on 10% polyacrylamide gels and transferred to nitrocellulose membranes. The dilutions of primary antibodies were 1:1000 for rabbit anti-ZEB1 (Cell Signaling) and 1:200 for mouse anti-actin antibodies (Sigma-Aldrich). Secondary antibodies conjugated to horseradish peroxidase were used at 1:10,000 dilutions (Southern Biotech).
4-nitroquinoline-1-oxide (4NQO) carcinogenesis model
Eight-weeks-old C57BL/6 J male mice were obtained from The Jackson Laboratory and housed in microisolator cages in specific pathogen-free environment. Mice were randomly divided into three groups (6 per group). Group I was untreated and served as a sham-control. Group II and III were administered 50 µg/ml 4NQO (Sigma) for 8 weeks in their drinking water from week 4 to 12 (see ). Group III was repeatedly infected (3 times per week) with P. gingivalis (strain 53977) for the first 2 weeks, F. nucleatum (strain 49,256) from week 2 to 4, and a mixture of both from week 12 to 18. Each inoculation mixture consisted of 100 μl of 109 cfu/ml bacteria suspended in 2% carboxymethylcellulose as described [Citation22]. The sequence of infection of P. gingivalis and F. nucleatum was done to simulate the natural sequence in the colonization of oral bacteria in the oral cavity. During the period of 4NQO administration, mice were not infected with the bacteria mixture. All mice were sacrificed in week 18 and their tongues were harvested, immersion-fixed in 4% paraformaldehyde, and processed and embedded in paraffin. Lesion area was calculated from the macroscopic lesion using Axiovison software (Carl Zeiss, Germany). Sections of 5 µm thickness were stained with H&E and histopathologically scored in a blinded fashion by an expert oral pathologist (I.B.). Animal experiments were approved by the Institutional Animal Care and Use Committee, University of Florida (approval 20,170,012). The University of Florida strictly follows U.S. Public Health Services policy, the Animal Welfare Act and Animal Welfare Regulations, and the guide for the Care and Use of Laboratory Animals.
Statistical analysis
All expression results are presented as meanSEM from three independent experiments. Comparisons of treated to control were analyzed using two-tailed Student’s test and multiple independent groups were analyzed using one-way ANOVA performed using Prism (Graph Pad Software).
Results
Enhanced expression of STAT3, JAK1, MYC and EMT markers in F. nucleatum-infected oral cancer cell lines
STAT3, JAK1, and MYC are three oncogenes that have both anti-apoptotic and proliferative effects on cancer cells [Citation24]. Therefore, it is expected for these oncogenes to be upregulated in cancer cells compared to normal cells. In this study, we aim to investigate further upregulation of these oncogenes by periodontal bacteria and subsequently enhancing carcinogenesis.
Infection with P. gingivalis could activate JAK/STAT3 pathway in normal gingival epithelial cells [Citation25], While F. nucleatum could upregulate MYC in colon cancer cells through E-cadherin pathway [Citation26]. Therefore, the change in expression for these oncogenes was analyzed after bacterial infection of oral cancer cells. To ensure that the effect observed from bacterial infection was not specific to a single cell line, three different oral cancer cell lines were examined. Accordingly, we measured the expression of STAT3, JAK1, and MYC in three oral cancer cell lines (OQ01, HN, BHY) after polymicrobial infection with the combination of P. gingivalis, F. nucleatum, T. denticola, and T. forsythia by qRT-PCR. STAT3 expression was enhanced almost two-fold in infected OQ01 and BHY cells compared to untreated controls ) and slightly increased in HN cells , after both 6 and 24 h of polymicrobial infection. Enhanced JAK1 expression was also seen in OQ01 and BHY 6 and 24 h post-infection (Appendix ). The same trend was seen in HN although the differences were not statistically significant (Appendix ). In contrast, enhanced MYC expression was detected in OQ01 and HN cells ), but not in BHY , and only at 24 h post-infection. In this pilot study, the activation states of the oncogenes was not examined. However, mRNA level of STAT3 was reported consistent with protein level in gingival epithelial cells [Citation27] and MYC mRNA and protein level was found closely related in colon cancer cells after Fusobacterial infection [Citation26]. Follow up studies will be required further define whether there would be changes in protein status including post-translational modifications such as phosphorylation.
Figure 1. F. nucleatum induces the expression of STAT3, MYC, and ZEB1 in oral cancer cells in vitro. (A-C) Elevated STAT3 mRNA expression levels after infection with four periodontal bacteria combined (polymicrobial infection) in oral cancer cell lines OQ01, BHY, and HN for 6 and 24 h. (D) The effect of single bacterial infection on the expression level of STAT3 in OQ01 cells after 24 h of infection. (E-G) Significantly elevated MYC mRNA expression level in OQ01 and HN, but not BHY, after 24 h of polymicrobial infection. (H) Comparison of the effects of single bacterial infections and polymicrobial infection on MYC mRNA expression in OQ01 cells after 24 h of infection. (I-K) Increased ZEB1 mRNA expression after polymicrobial infection of three oral cancer cell lines for 6 and 24 h. (L) Increased ZEB1 expression in OQ01 cells after single bacterial infection with F. nucleatum. (M) Western blot analysis of ZEB1 protein levels 24 h after F. nucleatum infection in OQ01 cells compared to uninfected cells. Uninfected cells were used as controls in all experiments. All mRNA levels were determined using qRT-PCR. All results are presented as mean ± SEM from three independent experiments. *, P <.05; **, P <.005; ****, P <.0001
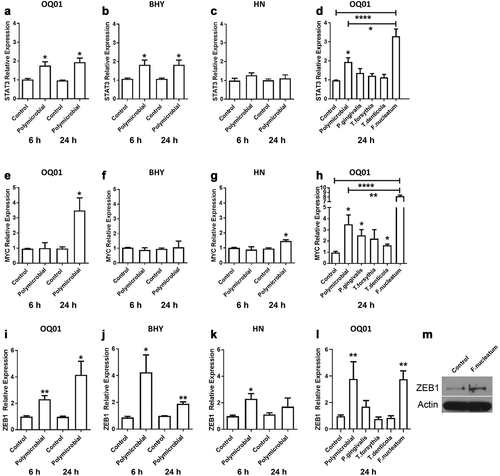
Next, we investigated whether these individual bacterial species alone could induce expression of these oncogenes. Single bacterial infections of OQ01 cells revealed that F. nucleatum alone led to a significant increase in STAT3 and MYC expression compared to control as well as to the polymicrobial infection. However, increased JAK1 expression appeared to be a synergistic effect from the four bacteria combined (Appendix ).
Cancer cells undergo epithelial-mesenchymal transition (EMT) to initiate metastasis [Citation28,Citation29]. Increased expression of ZEB1 and TGF-β is a hallmark of EMT [Citation30]. The same polymicrobial infection led to increased ZEB1 mRNA levels in all three oral cancer cell lines in both 6 and 24 h post-infection ). Simultaneously, there was an increase in TGF-β secretion in the three cell lines after polybacterial infection (Appendix ). F. nucleatum alone also significantly induced ZEB1 and TGF-β (Appendix ) expression. To confirm the effect of F. nucleatum on ZEB1 expression, Western blot analysis showed increased ZEB1 protein after 24 h F. nucleatum infection .
F. nucleatum enhanced MMP1, MMP9, and IL-8 expression and cancer cell invasiveness
Periodontal bacteria are known to induce MMPs expression in periodontal tissues and enhance tissue destruction during periodontal disease progression [Citation31]. Hence, our next step was to examine the effect of periodontal bacteria on the expression of MMPs in oral cancer cells. Enhanced MMP9 expression was observed in OQ01 and BHY ) and slightly increased in HN cells after infection . We also measured increased MMP9 protein secretion in culture supernatants of OQ01 cells after polybacterial infection by ELISA . Similarly, MMP1 mRNA expression was also enhanced in all cell lines tested 24 h after polymicrobial infection (Appendix ). Furthermore, exposure of OQ01 cells to the polymicrobial infection resulted in significantly increased IL-8 secretion compared to untreated cells .
Figure 2. F. nucleatum enhances MMP9 and IL-8 expression and cancer cell invasiveness in vitro. (A-C) Elevated MMP9 mRNA expression in OQ01 and BHY, but not HN, after polymicrobial infection for 6 and 24 h. (D) Increased MMP9 expression in OQ01 cells after single infection with P. gingivalis or F. nucleatum. (E) Increased MMP9 secretion in OQ01 cell culture supernatant after polymicrobial infection for 6 and 24 h. (F) Comparison of the effects of single bacterial infections and polymicrobial infection on MMP9 secretion in OQ01 cell supernatant. (G) Elevated IL-8 secretion in OQ01 cell supernatant after polymicrobial infection for 6 and 24 h. (H) Comparison of the effects of single bacterial infections and polymicrobial infection on IL-8 secretion in OQ01 cell supernatant. (I) elevated IL-8 levels in OQ01 cell supernatant after infection with three different Fusobacteria strains. (J) In vitro invasion assay was performed using Matrigel-coated Transwell filters. Cells infected with three Fusobacteria strains were incubated for 24 h on the filter. Cells on the upper surface were removed and cells on the lower surface were fixed and stained. Five fields at 200x magnification were counted per each filter. All mRNA levels were determined using qRT-PCR. IL-8 and MMP9 protein levels in culture supernatants were determined by ELISA. Uninfected cells were used as controls. All results are presented as mean ± SEM from three independent experiments. *, P <.05, **, P <.005, ***, P <.0005, ****, P <.0001
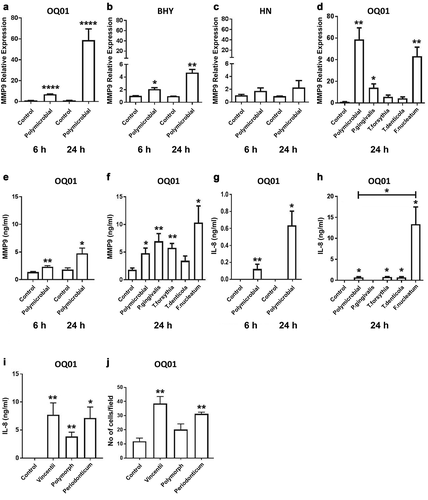
When single bacterial infections in OQ01 cells were examined, F. nucleatum appeared to have the most effect among the four bacteria in upregulating MMP1 (Appendix ) and MMP9 ). P. gingivalis alone also showed elevated MMP9 level compared to control . A dramatic increase in IL-8 secretion was also observed with F. nucleatum alone compared to control and to polymicrobial infection . There was no substantial difference between three different Fusobacteria strains (F. nucleatum vincentii, F. nucleatum polymorphum, and F. periodonticum) in enhancing IL-8 expression .
We then performed an in vitro invasion assay to measure the effects of different strains of Fusobacteria on cancer cell invasiveness. Infection with any of the three Fusobacteria strains enhanced OQ01 cell invasiveness in vitro, although no statistically significant difference was observed with F. polymorphum .
Bacterial culture supernatant alone induced expression of IL-8 and MMPs
The next experiments were designed to address the mechanism by which Fusobacteria can induce this invasive phenotype in oral cancer cells. To determine if live bacteria are needed to induce this phenotype, we performed experiments comparing live versus heat-killed bacteria and bacterial culture supernatant of F. periodonticum.
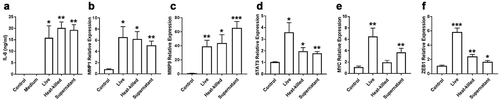
Our results showed that bacterial supernatant alone was sufficient to induce IL-8 secretion in OQ01 cells at levels comparable to cells treated with live or heat-killed bacteria . We focused on IL-8 secretion because it has been reported that IL-8 can enhance tumour invasion and elevated expression has been associated with poor patient prognosis [Citation18,Citation32]. Similarly, bacterial supernatant alone was able to enhance the expression of MMP1 and MMP9 in the same cells ). In contrast, live bacteria induced increased expression of STAT3, MYC, and ZEB1 compared to heat-killed bacteria and culture supernatant ). Comparable results were observed with other Fusobacteria strains (data not shown).
Figure 3. F. periodonticum culture supernatant alone is sufficient to induce IL-8 secretion and MMPs expression, while live bacteria give the strongest enhanced expression of STAT3, MYC, and ZEB1 in OQ01 oral cancer cells. (A) Comparison of the effects of live bacteria, heat-killed bacteria, and bacterial culture supernatant on IL-8 secretion in OQ01 cells after 24 h of incubation. Untreated cells (Control) and cells treated with bacterial culture medium alone (Medium) were negative controls. IL-8 protein level was determined by ELISA. (B-F) MMP1, MMP9, STAT3, MYC, and ZEB1 mRNA expression after treatment with live bacteria, heat-killed bacteria, or bacterial culture supernatant were determined by RT-PCR. All supernatants were obtained from F. periodonticum. Uninfected cells were used as controls. All results are presented as mean ± SEM from three independent experiments. *, P <.05, **, P <.005, ***, P <.0005
To characterize the bacterial culture supernatant component responsible for the effect exhibited in oral cancer cells, we examined whether the putative active factor(s) in the supernatant was a polypeptide, which would be highly sensitive to Proteinase K digestion. As expected, untreated supernatant showed a complex mixture of polypeptides with molecular masses ranging from >65kDa to small peptides of <15kDa (, No PK). Digestion with 2 or 20 ug Proteinase K showed substantial reduction in intensity of the majority of polypeptides. The Proteinase K-treated and boiled supernatant (Supernatant+PK) was added to OQ01 cells for 24 h and compared to control supernatant without Proteinase K treatment (Supernatant, ). Although there was ~20% decrease in IL-8 secretion, protein digestion and boiling did not have a major effect on IL-8 secretion in OQ01 cells. Similarly, there was no significant effect on MMP1 and MMP9 expression ). This indicated that polypeptides were not the main factors in the culture supernatant responsible for inducing the IL-8 secretion and MMP expression observed in OQ01 cells.
Figure 4. Effects of protein and nucleic acid digestions as well as functional LPS depletion on IL-8 secretion in OQ01 cells. (A) Polyacrylamide gel electrophoresis and Coomassie Blue stain analysis of F. periodonticum bacterial culture supernatant before and after treatment with two different concentrations of proteinase K (PK) showing almost complete degradation of proteins in the supernatant. (B) Comparing the effects of untreated (Control), supernatant, and PK-treated supernatant on IL-8 secretion from OQ01 cells after 24 h of incubation. Similarly, proteinase K treatment of supernatant did not affect the expression of MMP1 (C) and MMP9 (D) in OQ01 cells after 24 h of incubation. (E) Agarose gel and ethidium bromide staining analysis of bacterial culture supernatant before and after treatment with micrococcal nuclease (Mnuc) showing essentially complete degradation of nucleic acids in the supernatant. (F) Comparison of the effects of untreated, supernatant, and Mnuc-treated bacterial supernatant on IL-8 secretion in OQ01 cells after 24 h of infection. (G) Comparison of the effects of Salmonella enterica LPS alone and LPS+PMB treatment on IL-8 secretion in OQ01 cells after 24 h of incubation. (H) Comparison of the effects of untreated, PMB alone, supernatant, and PMB-treated bacterial supernatant on IL-8 secretion in OQ01 cells after 24 h of infection. All IL-8 protein levels were determined by ELISA. Uninfected cells were used as controls. All results are presented as mean ± SEM from three independent experiments. ns, not significant; *, P <.05; **, P <.005
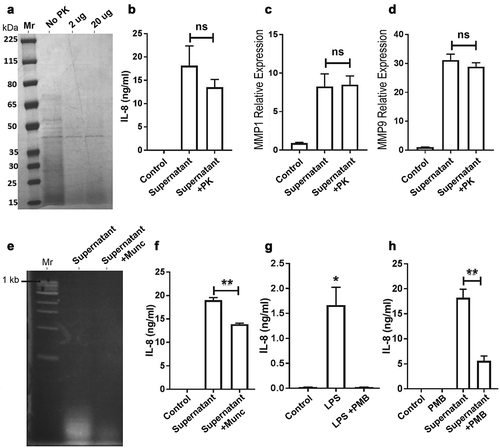
Next, we examined the effect of nucleic acid depletion of the supernatant on IL-8 secretion in OQ01 cells. Nucleic acid depletion using micrococcal nuclease (Mnuc) was effective in a control system as demonstrated by agarose gel electrophoresis and yet it had only a minor effect (20% decrease) on IL-8 secretion from OQ01 cells .
To investigate whether the active factor was LPS, polymyxin B (PMB) was employed to bind and inactivate LPS from the supernatant. First, purified LPS from Salmonella enterica added to OQ01 cells induced IL-8 secretion, which was completely abolished with the addition of PMB . Addition of PMB to the bacterial culture supernatant abolished 75% of IL-8 secretion , indicating that LPS was a major component of bacterial supernatant responsible for enhancing IL-8 secretion. Note that the same bacterial supernatant treated with PMB did not result in reduced MMPs expression (data not shown) indicating that LPS was not primarily responsible for elevated MMPs expression in OQ01 cells. Altogether, it appears that the interaction between Fusobacteria and these oral cancer cells is not solely through LPS/TLR4 pathway. Furthermore, this interaction might occur through multiple biological pathways resulting in upregulation of multiple oncogenes.
P. gingivalis and F. nucleatum promote tumour progression in vivo
Data from our in vitro experiments suggested that periodontal bacteria can enhance cancer progression. To investigate if these effects also occur in vivo, we used the 4NQO-induced oral carcinoma mouse model because it simulates human OSCC carcinogenesis progression over a reasonably short period of time in immunocompetent mice [Citation33,Citation34]. Although F. nucleatum showed most dramatic effect on oncogene upregulation and IL-8 secretion, P. gingivalis had an effect on MYC, MMP1 and MMP9. Moreover, it has been reported that combined oral infection of mice with P. gingivalis and F. nucleatum is better than single infection models of experimental periodontitis [Citation35]. Therefore, both F. nucleatum and P. gingivalis were used in the mouse model. In this pilot study, C57BL/6 J mice were randomly divided into three groups. Group I was untreated and served as sham-control . Group II and III were administered 4NQO for 8 weeks in their drinking water. Group III mice were infected with P. gingivalis and F. nucleatum orally 3 times per week as shown in . During the period of 4NQO administration, mice were not infected with bacteria. We used combined infection of P. gingivalis and F. nucleatum to simulate human infections where F. nucleatum do not present alone in periodontal infections.
Figure 5. P. gingivalis and F. nucleatum promote oral cancer progression in vivo. (A) Schematic summary of the periodontal pathogen-associated oral tumorigenesis model. Mice in Groups II and III were administered oral carcinogen 4NQO in their drinking water from week 4 to 12. In Group III, chronic periodontitis was induced by repeated oral infection with P. gingivalis alone for 2 weeks, F. nucleatum alone for another 2 weeks, and a mixture of P. gingivalis and F. nucleatum from week 12 to 18. (B) A representative image of tongue tissue showing an oral lesion (arrow) from a mouse in Group III. (C) H&E-stained section of sham-infected (Group I) mouse tongue displaying normal rete ridge formation with well-organized basal layer (sham-infected, top left). H&E-stained section of tongue from a mouse in Group II showing significant hyperkeratosis (black arrow, top right, 4NQO alone), hyperchromatism and mild hyperplasia (white arrows). H&E-stained section of tongue from a mouse in Group III showing significant hyperkeratosis (black arrows), hyperchromatism, hyperplasia, and increased nuclear/cytoplasmic ratios (white arrows, bacterial infection + 4NQO, lower panels). Higher magnification (200x) of tongue from another mouse in Group III again showing hyperkeratotic surface with foci of basilar hyperplasia nuclear hyperchromatism (white arrows, lower right). (D) The average number of macroscopic lesions differences between Group II and III. (E) The average lesion area in Group II and III. All data are presented as mean ± SEM, n = 6 mice per condition. Scale bars, 100 µm
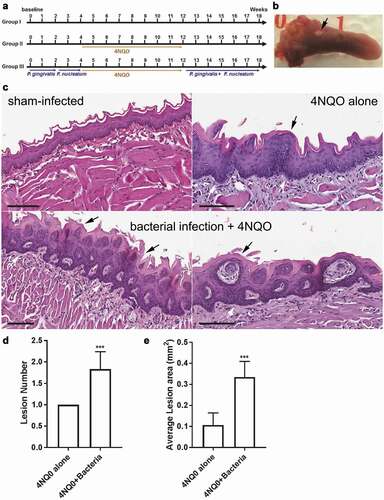
Histopathological analysis of tongue tissue sections indicated that Group III mice showed markedly enhanced hyperplasia and increased nuclear/cytoplasmic ratio compared to Group I and II ). Group III also had more oral lesions compared to Group II . Furthermore, lesions in Group III were significantly larger in size than Group II. In general, there was a significant increase in the lesion area when the bacteria were orally infected along with 4NQO . No lesions were detected in Group I.
Discussion
For many years, chronic inflammation has been linked to the initiation and progression of cancer. Chronic inflammation in periodontal disease leads to destruction of the bone surrounding the teeth [Citation36]. It has been suggested that prolonged exposure to periodontal bacteria and inflammation could increase the risk of oral cancer [Citation7]. Epidemiological studies have supported this association. Periodontitis was associated with higher risk of oral cancer in a cross-sectional study using the Third National Health and Nutrition Examination Survey [Citation9]. Another study concluded that patients with periodontitis were more likely to have poorly differentiated OSCC than those without periodontitis [Citation37]. Periodontal bacteria exist in and around cancer lesions [Citation38,Citation39]. Hence, the present study was to investigate the effect of periodontal bacteria on oral cancer cell behaviour.
The observation that Fusobacteria upregulated STAT3, JAK1, and MYC indicated that Fusobacteria infection might enhance cancer cell survival through modulation of these oncogenes, which are well implicated in pathways that promote tumorigenesis [Citation24]. Enhanced IL-8 secretion by Fusobacteria is not only associated with promoting cancer cell invasion, but also found in the saliva of oral cancer patients and implicated in disease progression [Citation32,Citation40]. The presented data together support the concept that F. nucleatum contributes to oral cancer survival and invasiveness.
F. nucleatum is a gram-negative anaerobic bacterium that was first isolated from the oral cavity and identified as a periodontal pathogen. Recent studies have reported F. nucleatum detected in different infection sites, including endocarditis, septic arthritis, and liver and brain abscesses. F. nucleatum is implicated in adverse pregnancy outcome [Citation41]. F. nucleatum has also been isolated from colon cancer lesions and proposed to have a role in cancer progression and immune system modulation [Citation42–44]. In colon cancer, F. nucleatum is linked to chemoresistance and associated with distant metastasis [Citation16,Citation45]. F. nucleatum enhanced colorectal cancer cell proliferation by activation of NF-κB and upregulation of miR-21 [Citation46]. Moreover, FadA protein on F. nucleatum can bind to E-cadherin on colorectal cancer cells, leading to activation of the potentially oncogenic E-cadherin–β catenin signalling pathway [Citation26]. F. nucleatum has also been shown to promote cell proliferation and migration in epithelial cells through up-regulation of cell cycle kinases. In oesophagal cancer, cancerous tissues contained significantly more F. nucleatum DNA than matched normal oesophagal mucosa, and increased levels were associated with poor prognosis [Citation47]. Overall, Fusobacteria have been linked to carcinogenesis in multiple cancer types and appear to impact cancer progression and treatment response. However, there are still many gaps to be filled in understanding the biological role of Fusobacteria in enhancing oral cancer progression.
To investigate the mechanism by which Fusobacteria could have such an effect on cancer cells, we infected oral cancer cells either with live bacteria, heat-killed bacteria, or bacterial supernatant. In addition to increased secretion of IL-8 and MMPs, OQ01 cells infected by live bacteria clearly induced substantially higher expression of STAT3, MYC, and ZEB1 than those treated with heat-killed bacteria or bacterial supernatant alone. The reduction in IL-8 secretion by PMB treatment indicated that LPS was the bacterial supernatant component largely responsible for enhancing IL-8 secretion. Interestingly, Fusobacteria culture supernatant alone was sufficient to induce IL-8 and MMP expression, suggesting that Fusobacteria secretes an active compound(s) that could promote carcinogenesis without direct contact of the bacteria with tumour cells. Further investigation revealed that Fusobacteria might interact with oral cancer cells through multiple pathways. One of which was the LPS/TLR4 pathway that induced IL-8 secretion in OQ01 cells. However, the same pathway did not affect MMP1 and MMP9 expression suggesting the role of a second pathway in enhancing MMPs expression. Our results are consistent with the data published on Fusobacteria/colon cancer cells interaction showing that Fusobacteria can activate multiple pathways in cancer cells, including E-cadherin and NF-KB pathways [Citation26,Citation46].
The results observed in this study showed some variability in response among the three cancer cell lines examined. This can be attributed to several factors, including the heterogeneity (different mutations) among different cancer cell lines make their response to bacterial infection variable and the potential difference in their origins in the oral cavity may account for difference in their interaction with bacteria.
The role of periodontal bacteria in oral carcinogenesis was followed up in the 4NQO carcinogenesis model. 4NQO is a carcinogen known to induce DNA damage, leading to early premalignant and malignant lesions in the oral cavity [Citation48]. Our data showed clearly that combined repeated infections with P. gingivalis and F. nucleatum induced twice as many lesions compared to exposure to 4NQO alone. The sizes of lesions were ~3 times larger in bacteria-infected versus non-infected 4NQO-treated mice. Our data is consistent with results reported by Binder Gallimidi et al. that infection with P. gingivalis and F. nucleatum promote tumorigenesis in their 4NQO carcinogenesis mouse model [Citation33]. Future studies should examine whether both bacteria are required and their relative contributions to the promotion and severity of oral lesions.
In summary, our in vitro and in vivo studies provide evidence for the role of Fusobacteria in oral cancer progression and invasion. Relevant to our observations is the recent metatranscriptomic study that reported significantly higher transcripts of Fusobacteria at oral tumour sites and adjacent sites compared to the healthy controls analyzed [Citation14]. Understanding the role of periodontal infections in cancer progression is important, as it may provide new strategies to improve treatment outcomes.
Supplemental Material
Download PDF (437.8 KB)Acknowledgements
This research was supported in part by the Andrew J. Semesco Foundation, Ocala, FL. We are also grateful for UMM ALQURA University, Saudi Arabia and Saudi Arabian Cultural Mission to the U.S. for their financial support.
Disclosure statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Messadi DV. Diagnostic aids for detection of oral precancerous conditions. Int J Oral Sci. 2013 Jun;5:59–12.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020 Jan;70:7–30.
- Bremmer JF, Brakenhoff RH, Broeckaert MA, et al. Prognostic value of DNA ploidy status in patients with oral leukoplakia. Oral Oncol. 2011 Oct;47:956–960.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006 Jan 21;12:354–362.
- Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006 Mar 28;4:14.
- D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004 Feb;83:156–160.
- Perera M, Al-Hebshi NN, Speicher DJ, et al. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiol. 2016;8:32762.
- Tezal M, Sullivan MA, Reid ME, et al. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. 2007 May;133:450–454.
- Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontol. 2005 Mar;76:406–410.
- Meyer MS, Joshipura K, Giovannucci E, et al. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008 Nov;19:895–907.
- Ye L, Jiang Y, Liu W, et al. Correlation between periodontal disease and oral cancer risk: a meta-analysis. J Cancer Res Ther. 2016 Dec;12:C237–C40.
- Holmes S. The oral complications of specific anticancer therapy. Int J Nurs Stud. 1991;28:343–360.
- Bolz J, Dosa E, Schubert J, et al. Bacterial colonization of microbial biofilms in oral squamous cell carcinoma. Clin Oral Investig. 2014 Mar;18:409–414.
- Yost S, Stashenko P, Choi Y, et al. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int J Oral Sci. 2018 Nov 12;10:32.
- Oji C, Chukwuneke F. Poor oral hygiene may be the sole cause of oral cancer. J Maxillofac Oral Surg. 2012 Dec;11:379–383.
- Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017 Jul 27;170:548–63 e16.
- Rubinstein MR, Baik JE, Lagana SM, et al. promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019 Apr;20. DOI:https://doi.org/10.15252/embr.201847638
- Ha NH, Park DG, Woo BH, et al. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine. 2016 10;86:64–72.
- Jung HM, Phillips BL, Patel RS, et al. Keratinization-associated miR-7 and miR-21 regulate tumor suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK) in oral cancer. J Biol Chem. 2012 Aug 24;287:29261–29272.
- Jung HM, Patel RS, Phillips BL, et al. Tumor suppressor miR-375 regulates MYC expression via repression of CIP2A coding sequence through multiple miRNA-mRNA interactions. Mol Biol Cell. 2013 Jun;24:1638–48, S1–7.
- Park YP, Jin L, Bennett KB, et al. CD70 as a target for chimeric antigen receptor T cells in head and neck squamous cell carcinoma. Oral Oncol. 2018 Mar;78:145–150.
- Chukkapalli SS, Velsko IM, Rivera-Kweh MF, et al. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in apoe null mice. PLoS One. 2015;10:e0143291.
- Harrandah AM, Fitzpatrick SG, Smith MH, et al. MicroRNA-375 as a biomarker for malignant transformation in oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 Dec;122:743–52 e1.
- Shirogane T, Fukada T, Muller JM, et al. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999 Dec;11:709–719.
- Whitmore SE, Lamont RJ, Goldman WE. Oral bacteria and cancer. PLoS Pathog. 2014 Mar;10:e1003933.
- Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013 Aug 14;14:195–206.
- Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009 Jun;119:1420–1428.
- Karlsson MC, Gonzalez SF, Welin J, et al. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol Oncol. 2017 Jul;11:781–791.
- Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009 Dec;1796:75–90.
- Franco C, Patricia HR, Timo S, et al. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci. 2017 Feb 17;18:440.
- Sahibzada HA, Khurshid Z, Khan RS, et al. Salivary IL-8, IL-6 and TNF-alpha as potential diagnostic biomarkers for oral cancer. Diagnostics (Basel). 2017 Apr 9;7:37-43.
- Binder Gallimidi A, Fischman S, Revach B, et al. Periodontal pathogens porphyromonas gingivalis and fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015 Sep 8;6:22613–22623.
- Rossa C Jr., D’Silva NJ. Immune-relevant aspects of murine models of head and neck cancer. Oncogene. 2019 Jan 29;38:3973-3988.
- Polak D, Wilensky A, Shapira L, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 2009 May;36:406–410.
- Di Benedetto A, Gigante I, Colucci S, et al. Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol. 2013;2013:503754.
- Tezal M, Sullivan MA, Hyland A, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009 Sep;18:2406–2412.
- Katz J, Onate MD, Pauley KM, et al. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011 Oct;3:209–215.
- Chang C, Geng F, Shi X, et al. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl Microbiol Biotechnol. 2019 Feb;103:1393–1404.
- Rao SK, Pavicevic Z, Du Z, et al. Pro-inflammatory genes as biomarkers and therapeutic targets in oral squamous cell carcinoma. J Biol Chem. 2010 Oct 15;285:32512–32521.
- Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015 Feb;23:141–147.
- Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012 Feb;22:299–306.
- Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013 Aug 14;14:207–215.
- McCoy AN, Araujo-Perez F, Azcarate-Peril A, et al. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013 Jan 15;8:e53653.
- Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017 Dec 15;358:1443–1448.
- Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology. 2017 Mar;152:851–66 e24.
- Yamamura K, Baba Y, Nakagawa S, et al. Human microbiome fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res. 2016 Nov 15;22:5574–5581.
- Hawkins BL, Heniford BW, Ackermann DM, et al. 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck. 1994 Sep-Oct;16:424–432.
