 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction: Candida albicans is an opportunistic pathogen that causes oral candidiasis. A previous study showed that Bgl2p and Ecm33p may mediate the interaction between the yeast and saliva-coated hydroxyapatite (SHA; a model for the tooth surface). This study investigated the roles of these cell wall proteins in the adherence of C. albicans to SHA beads.
Methods: C. albicans BGL2 and ECM33 null mutants were generated from wild-type strain SC5314 by using the SAT1-flipper gene disruption method. A novel method based on labelling the yeast with Nile red, was used to investigate the adherence.
Results: Adhesion of bgl2Δ and ecm33Δ null mutants to SHA beads was 76.4% and 64.8% of the wild-type strain, respectively. Interestingly, the adhesion of the bgl2Δ, ecm33Δ double mutant (87.7%) was higher than that of both single mutants. qRT-PCR analysis indicated that the ALS1 gene was over-expressed in the bgl2Δ, ecm33Δ strain. The triple null mutant showed a significantly reduced adherence to the beads, (37.6%), compared to the wild-type strain.
Conclusion: Bgl2p and Ecm33p contributed to the interaction between C. albicans and SHA beads. Deletion of these genes triggered overexpression of the ALS1 gene in the bgl2Δ/ecm33Δ mutant strain, and deletion of all three genes caused a significant decrease in adhesion.
Introduction
Candida spp. are opportunistic pathogens and some of the most common causes of fungal infections of humans [Citation1]. Although they are considered harmless commensal organisms in healthy people, in immunocompromised individuals such as those with cancer, or who have undergone repeated surgery or the introduction of intravenous catheters, these fungi can become serious pathogens. They can cause a variety of mucosal and systemic infections, including pseudomembranous candidiasis, erythematous candidiasis, hyperplastic candidiasis, and also candidemia, which can be fatal [Citation2,Citation3]. Candida albicans is the most frequent cause of oral candidiasis [Citation4] and possesses several factors, and specific abilities, that contribute to its pathogenicity. These include polymorphism, the yeast-to-hypha morphological transition and biofilm formation. Although C. albicans frequently causes mucosal infections, it is also commonly present in the dental plaque on teeth [Citation5,Citation6]. C. albicans is acidogenic [Citation7] and thus when present in biofilms on tooth surfaces will contribute to dental caries. C. albicans biofilms form in a sequential process including adhesion to host surfaces, induction of hyphal growth, production of extracellular matrix material, and finally dispersion of yeast cells from the biofilm complex [Citation8,Citation9]. Candida cells in a biofilm matrix exhibit high resistance to antimicrobial drugs, and are hidden from the host immune system, and this contributes to the virulence of this fungus. The presence of C. albicans in dental plaque may act as a reservoir for colonization of mucosal surfaces that leads to oral candidiasis.
In the oral cavity, saliva has a number of functions. It contains antimicrobial factors such as lysozyme and histatins, and amylase to begin the breakdown of starch, but it also contains several nutrients that microorganisms can metabolize. Thus, saliva enables microbial colonization of the mouth and it has been reported that C. albicans can utilize salivary constituents for the growth and the colonization of tooth surfaces [Citation10,Citation11]. Salivary proteins adsorb to oral surfaces to form the acquired pellicle. The main component of tooth enamel is hydroxyapatite and the adsorption of salivary proteins to hydroxyapatite can promote microbial adhesion. Salivary basic proline-rich proteins (bPRPs) within the pellicle are thought to act as main receptors for the adherence of C. albicans [Citation12,Citation13].
C. albicans possesses many surface proteins involved in adhesion that bind to host receptors. These include the ALS (agglutinin-like sequence) protein family, the Sap (secreted aspartic proteinase) protein family, Hwp1p, Ywp1p, Pra1p, and Csh1p which have been well studied [Citation14–19]. In addition, surface mannoproteins, which constitute the outer layer of the cell wall, are thought to be major candidates for putative adhesins. Therefore, it is important to investigate the functions of cell wall mannoproteins in order to understand the adhesion mechanisms, biofilm formation and virulence of C. albicans. [Citation2,Citation7,Citation9,Citation10]
In previous work we discovered that two cell surface mannoproteins, Bgl2p (35 kDa) and another highly glycosylated mannoprotein (97.4 kDa) competitively inhibited the attachment of C. albicans cells to SHA beads (a model for the tooth surface), and to membrane-immobilized salivary bPRPs [Citation12]. The importance of cell wall proteins in biofilm formation is supported by studies showing that mutations in these C. albicans proteins affect cell morphology, cell integrity, adherence to different surfaces, and compromise biofilm development [Citation9,Citation20,Citation21]. Bgl2p, for example, is required for C. albicans cell wall maintenance, and disruption of the C. albicans BGL2 gene interferes with cell wall integrity [Citation22] and the dimorphic transition. In addition, the null-mutant showed attenuated in virulence for mice when compared to its parent strain [Citation22]. Our N-terminal sequencing of the 97.4 kDa putative C. albicans adhesin gave the amino acid sequence ANXXXLXXAXP which is consistent with the ANNSTLTTATP sequence in Ecm33p (residues 19–29), a glycosylphosphatidylinositol (GPI) – anchored cell wall protein. C. albicans Ecm33p is required for normal cell wall integrity and the yeast-to-hyphal transition [Citation23]. Deletion of ECM33 in C. albicans leads to delayed hypha formation and severely attenuated virulence in a mouse infection model [Citation23]. Therefore, both Bgl2p and Ecm33p are required for normal cell wall architecture as well as normal function and expression of cell surface proteins in C. albicans [Citation9,Citation22,Citation23]. In this study, we hypothesized that the two mannoproteins Bgl2p and Ecm33p are C. albicans adhesins that bind to receptors in saliva-coated surfaces.
In order to detect and quantify microbial adhesion to tooth surfaces, several assays have been employed including radiolabeling of the yeast cells [Citation4] and microscopy using crystal violet (CV) staining [Citation10,Citation19]. In general, radiolabel-based assays have high sensitivity and reproducibility compared to quantifying using microscopy or viable cell counts [Citation10]. However, radiolabeling has safety concerns, requires specialized handling and equipment, is time consuming and costly. Nile red (NR, 9-diethylamino-5-benzo-α-phenoxazine-5-one) is a lipophilic fluorescent dye that binds to intracellular neutral lipid [Citation24]. Although NR has been widely used to label various microorganisms, to the best of our knowledge, there is no study reporting the use of NR to quantify yeast adhesion.
In this research, we investigated the roles of Bgl2p and Ecm33p in the adhesion of C. albicans to SHA by generating null mutants of these genes and using a novel NR staining assay. We found that the two cell wall proteins Bgl2p and Ecm33p contribute to interactions between C. albicans and saliva-coated surfaces. Interestingly, we observed that the deletion of both genes triggered compensatory responses in C. albicans cells. In particular, the ALS1 gene, encoding a major GPI-anchored adhesin, was overexpressed in the bgl2Δ/ecm33Δ double mutant. Deletion of all three genes caused a significant decrease in the adhesion of C. albicans to SHA beads.
Material and methods
Strains and growth conditions
C. albicans strains used in this study are listed in . Yeast cells were stored in YPD medium (1% [w/v] yeast extract, 2% [w/v] peptone, 2% [w/v] glucose) containing 50% [v/v] glycerol at −80°C. They were routinely grown in YPD or glucose/salts/biotin medium (GSB) consisting of 10 [g/L] glucose, 1.0 [g/L] (NH4)2SO4, 2.0 [g/L] KH2PO4, 0.05 [g/L] MgSO4 · 7H2O, 0.05 [g/L] CaCl2 · 2H2O; and 0.05 [mg/L] biotin [Citation25].
Table 1. C. albicans strains used in this study
Gene disruption
C. albicans gene sequences were obtained from the Candida Genome Database web site (http://www.candidagenome.org/cgi-bin/seqTools). The bgl2Δ null mutant (BGL2M4) and the BGL2-restored strain bgl2Δ/BGL2 (BGL2MK2) had been constructed previously by our group using the SAT1 flipper method [Citation9]. In the present study, the same method was used to construct the ecm33Δ null mutant (ECM33M4). In brief, plasmid pSFS2 (; [Citation26]) was used which contains SAT1 (a nourseothricin resistance gene) and the FLP recombinase between direct FLP recombination target (FRT) repeats. For the ECM33 deletion, the KpnI-XhoI fragment of the ECM33 upstream region (564 bp) was PCR amplified from C. albicans SC5314 genomic DNA with ECM33 upstream forward and reverse primers (). The NotI-SacI fragment of the ECM33 downstream region (555 bp) was also amplified, using ECM33 downstream forward and reverse primers (). These two fragments were inserted on either side of the SAT1-flipper in pSFS2 (Supplementary Figure S1A). The resulting plasmid was digested with KpnI and SacI to excise the ECM33 deletion cassette which was used to transform C. albicans SC5314 with selection for nourseothricin resistance.
Table 2. Plasmids used in this study
Table 3. Primers used for BGL2, ECM33, and ALS1 deletion and reintegration
A recombinant PCR method (Supplementary Figure S1B) was used to construct the revertant of the ecm33∆/∆ strain, ecm33Δ/ECM33 (ECM33MK2 containing a functional ECM33 gene), and revertant of the bgl2∆/∆,ecm33∆/∆ strain, bgl2Δ/BGL2,ecm33Δ/ECM33 (BEMK2). Firstly, by using SC5314 genomic DNA as a template, a fragment containing ECM33 and its upstream region was PCR amplified applying the ECM33 upstream forward primer and the ECM33-FRT-Frg1-reverse primer (, Supplementary Figure S1B), which contained an overlapping region (15 nucleotides) with the ECM33-FRT-Frg2-forward primer. The other DNA fragment, which contained the nourseothricin resistance marker SAT1, the FLP recombinase, and the ECM33 downstream region, was PCR amplified from the ECM33 deletion cassette (Supplementary Figure S1A) using the ECM33-FRT-Frg2-forward primer and ECM33 downstream reverse primer. These two DNA fragments were gel extracted and purified and used as templates for recombinant PCR to generate an ECM33 reinsertion cassette using the ECM33 upstream forward primer and ECM33 downstream reverse primer (Supplementary Figure S1B). The resultant ECM33 re-insertion cassette contained the ECM33 upstream region, ECM33 coding region and SAT1-flipper (flanked by FRT sequences) followed by the ECM33 downstream region.
The same strategy was used to generate the ALS1 deletion cassette. Firstly, the KpnI-XhoI fragment of the ALS1 upstream region (544 bp) was PCR amplified from C. albicans SC5314 genomic DNA with ALS1 upstream forward and reverse primers (). Secondly, a NotI-SacI fragment of the ALS1 downstream region (515 bp) was amplified with ALS1 downstream forward and reverse primers. These two fragments were inserted on either side of the SAT1-flipper in pSFS2. The resulting plasmid was digested with KpnI and SacI to excise the ALS1 deletion cassette.
Transformation of C. albicans
C. albicans SC5314 cells were transformed with DNA fragments using the lithium acetate method [Citation27] with slight modifications. C. albicans SC5314 cells were diluted to 107 cells/ml in 50 mL fresh YPD medium and grown at 30°C for two generation times (~4 h). The cells were collected by centrifuging at 2,200 g for 5 min and washed with distilled water. Washed cells were resuspended in 600 μL distilled water, and 100 μL of cell suspension was mixed with transformation mixture (72 μL 1 M lithium acetate, 100 μL of 2.0 mg/mL single-stranded carrier DNA, and 68 μL transforming DNA solution). The transformation mixture was incubated at 30°C with rotatory mixing at 20 rpm for 2 h before heat shocking in a 42°C water bath for 45 min. Cells were harvested by centrifuging at 2,200 g for 5 min and resuspended in 1 mL fresh YPD medium before being incubated for 4 h at 30°C with shaking at 200 rpm for recovery. Finally, the cells were collected by centrifuging at 2,200 g and resuspended in 200 µl YPD. Then the cell suspension was spread on YPD plates containing 200 μg/mL nourseothricin and incubated at 30°C for 1–2 days.
qRT-PCR
C. albicans cells were diluted to an OD600 of 0.2 in 50 ml fresh YPD medium, and incubated at 30°C until the OD600 reached 1–2. The cells were harvested by centrifugation (2,200 g, 5 min) and the total RNA was extracted using the glass beads lysis method [Citation27]. The RNA samples were used as templates to synthesize first strand cDNA using the ReverTra Ace qPCR RT Master Mix and the gDNA Remover kit (Toyobo, Osaka, Japan). Real time PCR was performed using the THUNDERBIRD SYBR qPCR Mix (Toyobo) with the following conditions: 95°C for 10 min, followed by 40 cycles of 15 sec at 95°C, 30 sec at 56°C, 1 min at 72°C. All RT-PCR primers used are listed in . ACT1 mRNA was used for signal normalization, and gene expression levels were determined using Applied Biosystems software and the ΔΔCt method.
Table 4. Primers used for qRT-PCR
Preparation of SHA beads
SHA beads were prepared as previously described [Citation4] with modifications. Unstimulated whole saliva collected from eight donors was stored on ice, and an equal volume of saliva from each donor was pooled. The pooled saliva was clarified by centrifugation at 3,000 g for 1 h and the supernatant was diluted with an equal volume of KCl buffer (2 mM KH2PO4, 2 mM K2HPO4 · 3H2O, 5 mM KCl, 1 mM CaCl2, pH 6.5). Proteinase inhibitor (Protease Inhibitor Cocktail, EDTA free, Nacalai Tesque, Japan) was added to the diluted saliva at a final concentration of 1 x protease inhibitor solution to prevent proteolysis of saliva proteins. Prepared saliva solution was aliquoted in glass tubes and stored at −80°C. These stored samples were used in multiple experiments and replicate assays ensuring consistency in the coating of hydroxyapatite beads. Pooled saliva solutions were collected twice, and the protein concentrations of the saliva solutions were measured in each experiment by the Lowry method using a Bio-Rad DC Protein Assay kit (cat no. 500–0116). The saliva protein concentrations were consistently 1.3 mg/mL (Supplementary Figure S2).
Hydroxyapatite beads (Micro-Prep Ceramic Hydroxyapatite Type I 80 µm diameter, Bio-Rad) were suspended in KCl buffer, washed with distilled water 10 times, and dried in an oven at 60°C for 48 h. Adhesion assays were performed as described previously [Citation4]. In brief, exactly 12 mg beads (~4.8 x 103 beads) were placed in a 1.5 mL microfuge tube and hydrated by incubation in 1 mL KCl buffer at 4°C for 16 h prior to use in an adhesion assay. The KCl buffer was aspirated from the hydrated beads and 1 mL saliva solution (50% [v/v] in KCl buffer) was added. The tube was mixed with end-over-end rotation at 15 rpm at room temperature for 1 h to allow adsorption of saliva proteins to the hydroxyapatite beads. The supernatant was aspirated, and the beads were washed twice with 1 mL KCl buffer. As a negative control, hydroxyapatite beads were incubated in KCl buffer (non saliva-coated beads [NSHA beads]).
In some experiments, hydroxyapatite beads were coated with either skim milk (SM; Nacalai Tesque), bovine serum albumin (BSA; Nacalai Tesque), or fetal bovine serum (FBS; Gibco). These proteins were dissolved and diluted in KCl to give the same protein concentration (determined by the Lowry method using the Bio-Rad DC Protein Assay kit) as 50% whole saliva in KCl buffer, (1.3 mg/mL; Supplementary Figure S2).
Assay of C. albicans adhesion to SHA beads
Single colonies of SC5314 (wild-type), BGL2M4 (bgl2∆/∆), ECM33M4 (ecm33∆/∆), and BEM4 (bgl2∆/∆, ecm33∆/∆) were pre-cultured in 5 mL GSB medium at 30°C for 16 h. GSB medium is a defined minimal medium that more closely resembles growth conditions in the mouth than rich growth media such as YPD. Cells were harvested by centrifugation (2,200 g for 5 min) and then diluted in KCl buffer to an OD600 of 1.
To determine the ability of C. albicans cells to adhere to the saliva-coated beads, cells (1 mL) at OD600nm ranging from 0.05 to 1 were incubated with SHA or NSHA beads at room temperature with end-over-end mixing for 1.5 h. After incubation, loosely adhered C. albicans cells were removed by washing the beads twice with 1 mL KCl buffer and the washings were put in two new 1.5 mL microfuge tubes (unattached cells). Input cells and the unattached cells were stained with NR (final concentration 1 µg/mL in 10% DMSO) for 1 h, and fluorescence intensity associated with the cells was measured using a Varioskan Lux LL154104 spectrophotometer plate reader (Thermo Fisher) at 530 nm and 570 nm, excitation and emission wavelengths, respectively. The fluorescence intensity was determined using a fluorescence-intensity standard curve generated by staining various numbers of yeast cells with NR (Supplementary Figure S3). The extent of adhesion was calculated by the following formula:
In some experiments the adhesion of wild-type strain SC5314 was taken to be 100%.
The susceptibility of C. albicans strains to Calcofluor white (CFW)
SC5314 (wild-type), bgl2∆/∆ (BGL2M4), ecm33∆/∆ (ECM33M4), and bgl2∆/∆, ecm33∆/∆(BEM4) cells were cultured in 5 mL YPD medium at 30°C overnight and diluted in YPD to an OD600 of 1. The cells were then serially diluted (10-fold) in YPD medium. Three microlitres of each dilution were spotted onto YPD plates containing CFW (12.5 μg/mL), and incubated at 30°C for 24 h. Susceptibility of cells to CFW was indicated by their inability to grow on this medium.
Confocal images of C. albicans cells adhered to SHA beads
The adhesion assay was performed as described above. After loosely adhered C. albicans cells were removed from the SHA beads by washing the beads twice with 1 mL KCl buffer, the cells that were still attached to beads were stained with NR (1 mL KCl buffer containing NR in 10% DMSO at a final concentration of 1 µg/mL). Ten microlitres of the bead suspension were transferred to a glass slide and mounted with a cover slip. The C. albicans cells adhering to SHA beads were observed with a Zeiss laser scanning confocal microscope (LSCM780). Z-sections were collected over 83.3 µm. A total of 246 sections were obtained and reconstructed into 3D and 4D images using IMARIS software (Bitplane, Inc. Saint Paul, MN USA).
Ethical approval
Approval to conduct the study was obtained from the Research Ethics Committee of the Tokyo Institute of Technology in accordance with the Ethical Guidelines for Clinical Research and Ethical Guidelines for Epidemiological Research (issued by the Ministry of Health, Labor and Welfare and Ministry of Education, Culture, Sports, Science and Technology – Japan).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8 software. Results were analysed with the parametric T-test. The cut-off for statistical significance was set at p < 0.05. All data are represented as mean and standard error (SD).
Results
Construction of strains disrupted in BGL2, ECM33 and ALS1, and restoration of these genes in C. albicans disruptants
In order to investigate the role of Bgl2p and Ecm33p in the adherence of C. albicans to SHA beads we constructed several gene deletion mutants of C. albicans. The bgl2 null mutant (BGL2M4) and the BGL2-restored strain (BGL2MK2) had been constructed previously [Citation9]. We generated the ecm33 null mutant (ECM33M4) of C. albicans SC5314 as described in Material and methods. The ECM33 gene was also deleted in the bgl2 null mutant (BGL2M4), and as a result, we obtained a bgl2∆/∆ and ecm33∆/∆ double null mutant (BEM4). The doubling times of the bgl2∆/∆, ecm33∆/∆, and bgl2∆/∆/ecm33∆/∆ mutants were only slightly higher than that for wild-type C. albicans SC5314 (Supplementary Figure S4). We re-inserted the BGL2 and ECM33 genes in the null mutants, as described in Material and methods, to construct the restored strain bgl2Δ/BGL2 (BGL2MK2) and ecm33Δ/ECM33 (ECM33MK2). Further investigation of those cells identified the need to delete ALS1. Therefore, we constructed the als1 null mutant (ALS1M4), als1∆/∆,bgl2∆/∆ double null mutant (ABM4), als1∆/∆,ecm33∆/∆ double null mutant (AEM4), and als1∆/∆,bgl2∆/∆,ecm33∆/∆ triple null mutant (ABEM4), using the same protocols. All genotypes were verified by PCR amplification of the genomic loci in transformants and DNA sequencing. A list of the C. albicans strains generated is given in .
Optimizing and calibrating the C. albicans adhesion assay based on NR staining
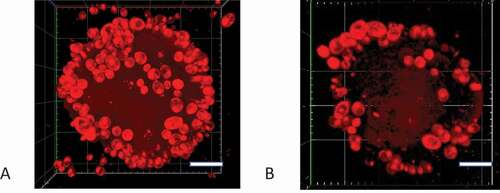
Nile red (NR) is a lipophilic fluorescent dye that has been used to stain mammalian cells [Citation28], bacteria [Citation29], microalgae [Citation30–32], and yeast cells [Citation33]. We developed a novel adhesion assay based on NR staining of C. albicans cells to overcome the limitations of radiolabeling and microscopy techniques. First, we optimized and validated the assay. We measured NR fluorescence over a range of excitation and emission wavelengths and found that the optimum excitation and emission wavelengths were 530 nm and 570 nm, respectively, (Supplementary Figure S5), and there was no autofluorescence of yeast cells at these wavelengths (Supplementary Figure S6). We also investigated the best solvent for the NR. NR gave the highest fluorescence signal in KCl buffer containing 10% DMSO (Supplementary Figure S6), the signal remained stable for at least 5 h after staining (Supplementary Figure S7), and cells stained at the stationary phase gave stronger fluorescence signals (Supplementary Figure S3). Next, we found that there was a strong relationship between the NR fluorescence and the C. albicans cell concentration over an OD600 nm range of 0.125 to 1.0 (, Supplementary Figure S8). The NR fluorescence plateaued when the OD600 nm value exceeded 2.0. There was also a linear relationship between the cell concentration and OD600 nm value with OD600 = 0.15 corresponding to a cell concentration of 1 × 106 cells/mL. This indicated that the NR fluorescence, up to values of 15 AU (arbitrary units), could be used to quantify the number of C. albicans cells adhering to hydroxyapatite beads. We confirmed that C. albicans cells adhered to the SHA beads by performing an adhesion assay, staining the C. albicans cells associated with the beads with NR, and visualizing the cells with confocal microscopy. We found that when beads were incubated with cells at OD600 = 0.15, approximately 102 cells adhered to each bead and this number was considerably higher when beads were incubated with cells at OD600 = 1.0 (, Supplementary movie 1 and 2). The number of C. albicans cells adhering to each bead corresponded to the numbers measured in a previous study using radiolabelled cells (260 cells/bead [Citation4]).
Figure 1. Correlation between NR fluorescence intensity and C. albicans cell concentration. Cell concentrations were determined by measuring the optical density at 600 nm using an ASV11D spectrophotometer. The fluorescence intensity of NR-stained cells at 530 nm excitation and 570 nm emission wavelengths was measured using a Varioskan Lux LL154104 spectrophotometer plate reader (Thermo Fisher) for cell suspensions at various OD600 nm values. Results are means (± SD) of three independent experiments
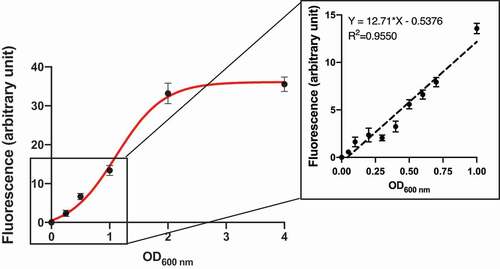
Figure 2. Confocal microscopy Z-stack imaging to visualize C. albicans adhering to saliva-coated beads. The C. albicans SC5314 cells that attached to hydroxyapatite beads were stained with NR. Confocal images were captured with a Zeiss LSCM780 laser scanning microscope. Scale-bar: 20 µm. (A) Bead that had been incubated with C. albicans SC5314 cells at OD600nm = 1, (B) Bead that had been incubated with C. albicans SC5314 cells at OD600nm = 0.1
The susceptibility of C. albicans null mutants to CFW
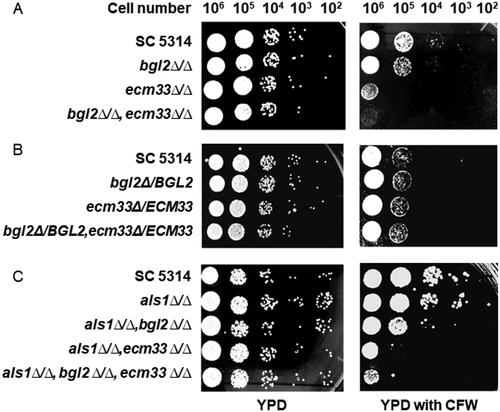
To investigate the impact of gene disruptions on C. albicans cell wall integrity, we tested the susceptibility of null mutants to CFW, a chemical which interferers with cell wall construction [Citation34]. C. albicans SC5314 (wild-type), bgl2∆/∆ (BGL2M4), ecm33∆/∆ (ECM33M4), and bgl2∆/∆, ecm33∆/∆ (BEM4) cells were serially diluted and inoculated on YPD agar and on YPD agar containing CFW (12.5 μg/mL), and incubated at 30°C for 24 h. All of the null mutants were more susceptible to CFW than the wild-type parental strain (). The ecm33∆/∆ strain was more sensitive to CFW than the bgl2∆/∆ strain and the double mutant bgl2∆/∆, ecm33∆/∆ showed the highest susceptibility to CFW (). This result indicated that when these cell surface mannoproteins are not expressed there is a decrease in the integrity of the C. albicans cell wall. Restoring the BGL2 and ECM33 genes to the respective null mutants reduced their susceptibility to CFW (). CFW binds to chitin in fungal cell walls. Although the blg2∆/∆ and ecm33∆/∆ null mutants, and especially the triple mutant bgl2∆/∆,ecm33∆/∆,als1∆/∆, were more susceptible to CFW than the wild-type SC5314 strain (), we did not determine whether this was due to a different chitin content in the cell walls.
Figure 3. Susceptibility of C. albicans strains deleted in BGL2 and ECM33 to Calcofluor white (CFW). (A) C. albicans SC5314 (wild-type), bgl2∆/∆ (BGL2M4), ecm33∆/∆ (ECM33M4), and bgl2∆/∆,ecm33∆/∆ (BEM4) were serially diluted and spotted onto YPD agar plates and YPD agar plates containing 12.5 μg/mL CFW and incubated at 30°C for 24 h; (B) C. albicans SC5314 (wild-type), and restored strains bgl2Δ/BGL2 (BGL2MK2), ecm33Δ/ECM33 (ECM33MK2), and bgl2Δ/BGL2,ecm33Δ/ECM33 (BEMK2) were serially diluted and spotted onto YPD agar plates and YPD agar plates containing 12.5 μg/mL CFW and incubated at 30°C for 24 h: (C) C. albicans SC5314 (wild-type), als1∆/∆ (ALS1M4), als1∆/∆,bgl2∆/∆ (ABM4), als1∆/∆,ecm33∆/∆ (AEM4), and als1∆/∆, bgl2∆/∆, ecm33∆/∆ (ABEM4) were serially diluted and spotted onto YPD agar plates and YPD agar plates containing 12.5 μg/mL CFW and incubated at 30°C for 24 h
Saliva promoted C. albicans cell adherence to hydroxyapatite beads
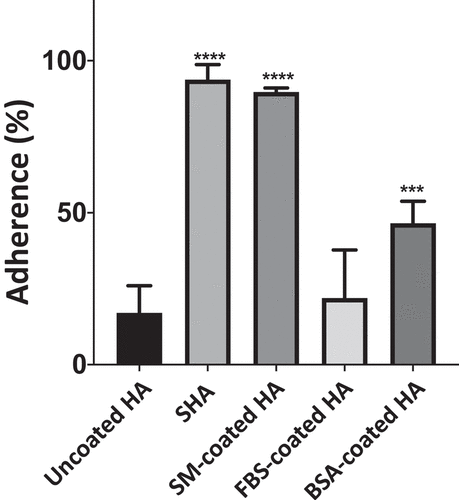
All oral surfaces are coated with a salivary protein pellicle. Therefore, it is important to include saliva in oral adhesion studies. In this study, we used hydroxyapatite (HA) beads as a model for the tooth surface and so investigated the influence of human saliva on adherence of C. albicans cells to HA beads. HA beads were incubated in human saliva for 1 h (to make SHA beads), washed and then C. albicans cells (SC5314) were added. It was found that 93.8 4.9% of the input yeast cells bound to the SHA beads, whereas only 17.0
9.0% of SC5314 cells bound to uncoated HA beads (). This result demonstrated that saliva promoted C. albicans adherence to HA beads significantly (p < 0.0001, increased adherence 5.4-fold), and was consistent with our previous results [Citation4,Citation10] and when we repeated the radiolabel assay (Supplementary Figure S9 and Figure S10) using the previous protocol. [Citation10] To determine whether this promotion of adherence was specific to salivary proteins, HA beads were coated with other proteins: bovine serum albumin (BSA), fetal bovine serum (FBS), and skim milk (SM). It was found that FBS and BSA, unlike saliva, did not promote adhesion greatly (by 1.2-fold and 2.7-fold, respectively). Interestingly, SM promoted the adhesion of C. albicans to HA beads to almost the same extent as saliva (5.2-fold) (). From these results, we inferred that saliva, and skim milk, contain components which, when bound to HA beads, act as receptors for C. albicans adherence.
Figure 4. Adherence of C. albicans SC5314 cells to HA beads coated with various proteins. C. albicans cells (1 x 107) were incubated with uncoated, saliva-, skim milk (SM)-, fetal bovine serum (FBS)-, or bovine serum albumin (BSA)-coated HA beads (12 mg) at 28°C for 1 h. The percentage of the input C. albicans cells attached to the HA beads was calculated. Results are the means (± SD) of at least three independent experiments. Asterisks indicate significant differences to the adherence to uncoated HA beads (*** p < 0.001, **** p < 0.0001)
The BGL2 and ECM33 null mutants, but not the double BGL2 and ECM33 mutant, showed significant defects in adherence to SHA beads
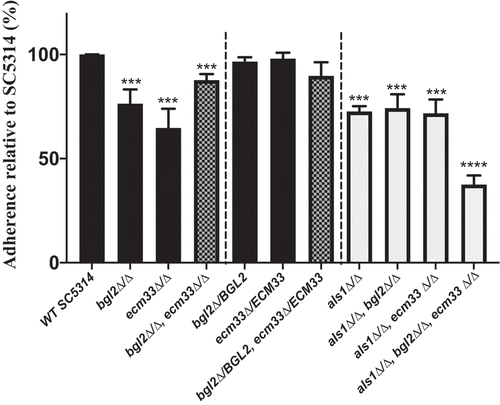
To determine whether Bgl2p or Ecm33p might be adhesins involved in adhesion to saliva-coated tooth enamel, the adherence of C. albicans null mutant strains to SHA beads was compared to that of the parental strain SC5314. The null mutants with the genes restored, bgl2Δ/BGL2 (BGL2MK2), ecm33Δ/ECM33 (ECM33MK2), and bgl2Δ/BGL2, ecm33Δ/ECM33 (BEMK2) were used in adherence assays as controls. Deletion of both alleles of either the BGL2 or the ECM33 gene reduced C. albicans adherence by more than 20% compared to the adherence of the wild-type strain (; p < 0.001). Returning the BGL2 or the ECM33 gene to the respective null mutants restored their ability to bind to SHA beads (bgl2Δ/BGL2,ecm33Δ/ECM33; ). These results suggested that both Bgl2p and Ecm33p confer some ability of C. albicans to adhere to SHA beads. Surprisingly, we found that disruption of the ECM33 gene in the bgl2∆/∆ null mutant did not reduce adhesion further but, in fact, increased adhesion of this mutant (bgl2∆/∆,ecm33∆/∆; ). Returning the BGL2 and ECM33 genes to the double disruptant did not alter adhesion to SHA beads significantly (bgl2Δ/BGL2,ecm33Δ/ECM33;). Similar results were obtained using assays measuring the adherence of radiolabeled C. albicans cells (Supplementary Figure 10). The increase in adherence when two potential adhesins had been deleted led us to hypothesize that there was a compensatory induction of expression of another adhesin in the bgl2∆/∆,ecm33∆/∆ double mutant.
Figure 5. Adherence of wild-type C. albicans SC5314 cells and homozygous BGL2, ECM33, and ALS1 single, double, and triple mutants to SHA beads. Wild-type or mutant C. albicans cells (1 x 107) were incubated with SHA beads (12 mg) at 28°C for 1 h. The results are presented as percentage adherence relative to SC5314, which is set to 100%. Results are the means of at least three independent experiments (± SD). Asterisks indicate significant differences to the adherence of SC5314 cells (*** p < 0.001, **** p < 0.0001)
The expression of adhesion-related genes in C. albicans null mutants
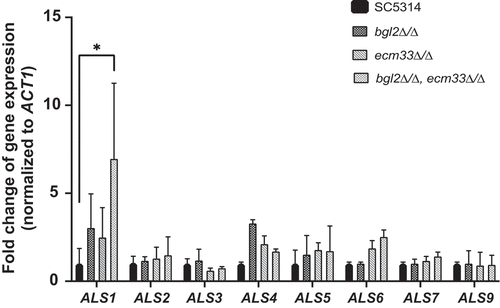
Since the bgl2∆/∆,ecm33∆/∆ strain (BEM4) showed greater adhesion to SHA beads than bgl2∆/∆ (BGL2M4) or ecm33∆/∆ (ECM33M4), we examined the expression of a family of important C. albicans adhesin genes in these strains. The ALS (Agglutinin-Like Sequence) genes encode a family of GPI-anchored cell surface glycoproteins, eight of which (Als1-Als7 and Als9) have been reported to be involved in C. albicans adhesion [Citation35–37]. Interestingly, qRT-PCR indicated that ALS1 mRNA was 3-fold higher than in SC5314 when BGL2 was deleted, 2.5 fold higher when ECM33 was deleted and more than 6 times higher in the bgl2∆/∆, ecm33∆/∆ double mutant (). The expression of ALS1 in the bgl2∆/∆, ecm33∆/∆, and the double mutant returned to 1.39, 1.12 and 1.1 times the expression level in SC5314, respectively, when the BGL2 and ECM33 genes were returned to the mutants. In contrast, there was no significant increase in the expression of ALS2, ALS5, ALS7, and ALS9 when the BGL2 and/or the ECM33 gene(s) were deleted, and indeed a reduction of expression of ALS3 when ECM33, but not BGL2 was deleted and in the bgl2∆/∆, ecm33∆/∆ double mutant (BEM4) (). This reduction in ALS3 expression in ecm33∆/∆ (39.1%) was relatively minor compared to the overexpression of the adhesin gene ALS1 (695.9%) and is unlikely to be the cause of the reduced adherence of ecm33∆/∆ cells. These results indicate that the deletion of both the BGL2 and ECM33 genes induces expression of ALS1 which may be responsible for the increase in adherence of the C. albicans double mutant to SHA beads. There were slight increases in the expression level of ALS4 in BGL2M4 and ALS6 in BEM4 (). This result suggested that in addition to changes in the ALS1 expression level, other proteins such as Als4p and Als6p might play a small role when BGL2 and ECM33 genes were deleted.
Figure 6. Expression of C. albicans ALS genes in wild-type and mutant strains. C. albicans strains were cultured in YPD medium and then total RNA was isolated and subjected to qRT-PCR. Gene expression was normalized against the C. albicans ACT1 gene and is reported for each ALS gene relative to expression in wild-type strain SC5314. All experiments consisted of triplicate measurements (technical replicates) and experiments were carried out three times (biological replicates) (± SD). (*p < 0.05)
ALS1 gene disruptions in mutant strains and investigation of the effect on adherence to SHA beads
The als1∆/∆ (ALS1M4), als1∆/∆,bgl2∆/∆ (ABM4), als1∆/∆,ecm33∆/∆ (AEM4), and als1∆/∆,bgl2∆/∆,ecm33∆/∆ (ABEM4) mutants were constructed from SC5314, bgl2∆/∆ (BGL2M4), ecm33∆/∆ (ECM33M4) and bgl2∆/∆,ecm33∆/∆ (BEM4) strains, respectively, by the gene disruption method described in the Material and Methods section. Deletion of ALS1 in SC5324 did not affect the susceptibility to CFW (), and deletion of ALS1 in the bgl2∆/∆, ecm33∆/∆, and bgl2∆/∆,ecm33∆/∆ mutants did not affect the susceptibility of those mutants to CFW further (). The adherence of the ALS1 disrupted strains to SHA beads was measured. Deletion of ALS1 in SC5314 (to form als1∆/∆) reduced the adherence ability to a similar extent (72.6 ± 2.5% of SC5314) as deletion of BGL2 (76.4 ± 13.0%) (). Deletion of ALS1 in either a bgl2∆/∆ or an ecm33∆/∆ background did not reduce adhesion to SHA further (). As predicted, however, deletion of ALS1 in the bgl2∆/∆,ecm33∆/∆ double disruptant (to form als1∆/∆,bgl2∆/∆,ecm33∆/∆) resulted in a significant reduction in adherence to SHA beads (to 37.6 ± 4.3% of the SC5414 adherence; ). These results indicated that reliance on Als1p for adherence to SHA beads is triggered when both Bgl2p and Ecm33p are inactivated, and highlight the importance of all three proteins for the attachment of C. albicans cells to tooth surfaces via saliva in the oral cavity.
Discussion
C. albicans is the Candida species most commonly isolated from the mouth and is responsible for most superficial oral fungal infections [Citation7]. The oral cavity provides many saliva-coated surfaces and niches that can be colonized by C. albicans and it is important to investigate how this opportunistic yeast interacts with tooth surfaces when covered by saliva components. The yeast cell wall is the vital point of contact between C. albicans cells and the saliva-coated oral surfaces [Citation14]. Because of this important role, and interactions with host immune systems, the C. albicans cell wall has attracted a lot of attention over the last few decades.
A method used previously for measuring C. albicans adherence to saliva-coated surfaces involved labeling the yeast with [35S]-methionine [Citation4], and GSB medium was used to mimic growth conditions in the mouth. This method is reliable, reproducible and sensitive, however, it is time-consuming and expensive and poses a risk to human health. Recently, several alternative fluorescent staining strategies have been developed to provide fast detection and quantification of yeast cells. In our research, important criteria for choosing the optimum fluorescent dye to measure yeast adhesion were chemical stability under the experiment conditions, low-cost, safety of use, and no interference with other substances in saliva. We investigated using carboxyfluorescein diacetate succinimidyl ester (CFDA) to stain C. albicans cells as this compound is cleaved by intracellular esterase enzymes to form a fluorescent amine-reactive product. We found that CFDA stained C. albicans cells and gave a strong fluorescence signal but there were also many esterases in saliva resulting in a high background signal in our SHA bead adhesion assay. We then focused on NR, a commonly used fluorescent lipophilic dye that binds to intracellular neutral lipids. NR has low fluorescence in water and other polar solvents, but gives strong fluorescence signals in nonpolar environments[Citation24]. We found, using spectroscopy and confocal microscopy, that once NR was absorbed by C. albicans cells, the signal was stable, even after 2–5 h of staining. Also, once yeast cells were stained with this dye, no washing steps were needed. Therefore, we used NR to stain C. albicans cells for the adhesion assays.
While uncoated HA beads showed low non-specific binding of 17% of the input SC5314 cells, we found that 93.8% of input yeast cells adhered to SHA beads. This is consistent with previous reports which have shown that bPRPs (basic proline-rich proteins) and statherin promote attachment of C. albicans cells to hydroxyapatite beads [Citation4,Citation10]. Control proteins FBS and BSA did not promote much adherence of C. albicans cells to hydroxyapatite beads but, surprisingly, skim milk did. It is interesting to note that milk [Citation38–40], like saliva [Citation13], contains proline-rich proteins and it might be these molecules in milk that promote C. albicans adherence to HA beads. Future studies could determine precisely which components of skim milk promote this adhesion.
The proteins Bgl2p (β-1,3-glucosyltransferase) and Ecm33p (a GPI–anchored protein) are cell wall proteins contributing to cell wall integrity [Citation9,Citation41]. In this study, it was confirmed that BGL2M4 (bgl2∆/∆) and ECM33M4 (ecm33∆/∆) had higher susceptibility to CFW than SC5314 (wild-type), and the double disruptant (bgl2∆/∆, ecm33∆/∆; BEM4) showed even higher susceptibility. As well as having compromised cell wall integrity, BGL2M4 and ECM33M4 showed lower adherence to SHA beads than SC5314. Surprisingly, the double disruptant BEM4 demonstrated increased adhesion to SHA beads. An analysis of the expression of the ALS adhesin gene family in BEM4 revealed increased expression of ALS1. The protein Als1 plays a major role in C. albicans adhesion to human epithelial cells, endothelial cells and even abiotic surfaces such as silicone [Citation42,Citation43]. It is also a key component needed for biofilm formation and the hyphal development of C. albicans [Citation43–45]. Deletion of ALS1 reduced adherence of C. albicans cells to SHA beads by 27.4%. This is similar to the reduction in adherence of C. albicans to endothelial cells caused by deletion of ALS1 reported by Fu et al [Citation35]. (35%). Deletion of either BGL2 or ECM33 reduced adherence of C. albicans cells to SHA beads by 23.6% or 35.3%, respectively. This indicates that individually, Bgl2p and Ecm33p contribute approximately as much to saliva-mediated adherence as Als1p. Our results suggested that the double deletion of BGL2 and ECM33, however, induced a compensatory overexpression of the ALS1 gene.
Deletion of ALS1 in either the bgl2∆/∆ or the ecm33∆/∆ background did not result in a strain with increased adherence to SHA beads (as was seen with the bgl2∆/∆, ecm33∆/∆ strain). These results demonstrated that ALS1 expression is only induced when both BGL2 and ECM33 are deleted, which was substantiated by the significant reduction in adherence of the bgl2∆/∆, ecm33∆/∆ strain when ALS1 was also disrupted. Als1p along with other Als proteins are well recognized adhesins, but their expression has been predominantly studied under hypha inducing conditions [Citation35,Citation46]. Much is known about ALS1 expression during morphogenesis and biofilm formation where adhesin gene expression is under the control of transcriptional regulators such as Efg1p, Bcr1p, Tup1p and Nrg1p [Citation47,Citation48]. However, there have been very few studies on the expression of adhesins in yeast cells. We were interested in adhesin expression by yeast cells as they are responsible for the colonization of saliva-coated surfaces. ALS1 is expressed by yeast cells when released from the stationary phase, but expression decreases during yeast growth [Citation49]. In contrast, expression of ALS1 is induced greatly during hyphal formation [Citation49]. The present study showed that the expression of ALS1 in yeast cells is increased in cells deleted in BGL2 and ECM33. We have previously reported that disruption of BGL2 resulted in decreased expression of transcriptional regulator CPH2 during yeast growth [Citation9] and this may be responsible, in part, for changes in the ALS1 expression. Further experiments are needed to elucidate the transcriptional network controlling the expression of Bgl2p, Ecm33p and Als1p under yeast and mycelia growth conditions.
It must be acknowledged that we only looked for compensatory expression of ALS genes in the disrupted strains. While there is strong evidence that there is induction of ALS1 when BGL2 and ECM33 are deleted, we cannot exclude the possibility that expression of other adhesins is also induced. Further research, including complete RNA sequencing of mutant strains, may provide a more complete mechanistic insight into the role of the cell wall protein network in the adhesion ability of C. albicans. This study does, however, provide strong evidence that Bgl2p, Ecm33p and Als1p contribute to C. albicans adherence to saliva-coated surfaces.
Supplemental Material
Download Zip (3.4 MB)Acknowledgments
The authors thank Suzukakedai Technical Department, Tokyo Institute of Technology, for DNA sequencing analyses.
Disclosure statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ten Cate JM, Klis FM, Pereira-Cenci T, et al. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res. 2009;88(2):105–13.
- Patil S, Rao RS, Majumdar B, et al. Clinical appearance of oral Candida infection and therapeutic strategies. Front Microbiol. 2015. DOI:https://doi.org/10.3389/fmicb.2015.01391
- Dühring S, Germerodt S, Skerka C, et al. Host-pathogen interactions between the human innate immune system and Candida albicans-understanding and modeling defense and evasion strategies. Front Microbiol. 2015. DOI:https://doi.org/10.3389/fmicb.2015.00625
- Cannon RD, Nand AK, Jenkinson HF. Adherence of Candida albicans to human salivary components adsorbed to hydroxylapatite. Microbiology. 1995. DOI:https://doi.org/10.1099/00221287-141-1-213
- de Carvalho FG, Silva DS, Hebling J, et al. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 2006. DOI:https://doi.org/10.1016/j.archoralbio.2006.06.001
- Ghasempour M, Sefidgar SAA, Eyzadian H, et al. Prevalence of Candida albicans in dental plaque and caries lesion of early childhood caries (ECC) according to sampling site. Casp J Intern Med. 2011;2(4):304–308.
- Pereira D, Seneviratne CJ, Koga-Ito CY. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2017. DOI:https://doi.org/10.1111/odi.12691
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013. DOI:https://doi.org/10.4161/viru.22913
- Chen X, Zhang R, Takada A, et al. The role of Bgl2p in the transition to filamentous cells during biofilm formation by Candida albicans. Mycoses. 2017;60(2):96–103.
- Cannon RD, Lyons KM, Chong K, et al. Adhesion of yeast and bacteria to oral surfaces. Methods Mol Bio. 2017. doi:https://doi.org/10.1007/978-1-4939-6685-1_10.
- Valentijn-Benz M, Nazmi K, Brand HS, et al. Growth of Candida albicans in human saliva is supported by low-molecular-mass compounds. FEMS Yeast Res. 2015;15(8). DOI:https://doi.org/10.1093/femsyr/fov088
- Jeng HW, Holmes AR, Cannon RD. Characterization of two Candida albicans surface mannoprotein adhesins that bind immobilized saliva components. Med Mycol. 2005. DOI:https://doi.org/10.1080/13693780410001731637
- O’Sullivan JM, Cannon RD, Sullivan PA, et al. Identification of salivary basic proline-rich proteins as receptors for Candida albicans adhesion. Microbiology. 1997;143(Pt 2):341–348.
- Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008. DOI:https://doi.org/10.1128/mmbr.00032-07
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003. DOI:https://doi.org/10.1128/mmbr.67.3.400-428.2003
- Staab JF, Datta K, Rhee P. Niche-specific requirement for hyphal wall protein 1 in virulence of Candida albicans. PLoS One. 2013. DOI:https://doi.org/10.1371/journal.pone.0080842
- Citiulo F, Jacobsen ID, Miramón P, et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012. DOI:https://doi.org/10.1371/journal.ppat.1002777.
- Alves R, Barata-Antunes C, Casal M, et al. Adapting to survive: how Candida overcomes host-imposed constraints during human colonization. PLoS Pathog. 2020. DOI:https://doi.org/10.1371/journal.ppat.1008478
- Singleton DR, Fidel PL, Wozniak KL, et al. Contribution of cell surface hydrophobicity protein 1 (Csh1p) to virulence of hydrophobic Candida albicans serotype A cells. FEMS Microbiol Lett. 2005. DOI:https://doi.org/10.1016/j.femsle.2005.02.010
- Desai JV, Mitchell AP. Candida albicans biofilm development and its genetic control. Microbiol Spectr. 2015. DOI:https://doi.org/10.1128/microbiolspec.mb-0005-2014
- Lin CH, Kabrawala S, Fox EP, et al. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. PLoS Pathog. 2013. DOI:https://doi.org/10.1371/journal.ppat.1003305
- Sarthy AV, McGonigal T, Coen M, et al. Phenotype in Candida albicans of a disruption of the BGL2 gene encoding a 1,3-β-glucosyltransferase. Microbiology. 1997. DOI:https://doi.org/10.1099/00221287-143-2-367
- Martinez-Lopez R, Park H, Myers CL, et al. Candida albicans Ecm33p Is Important for normal cell wall architecture and interactions with host cells. Eukaryot Cell. 2006;5(1):140–147.
- Rostron KA, Lawrence CL. Nile red staining of neutral lipids in yeast. Methods Mol Biol. 2017;1560:219–229.
- Holmes AR, Cannon RD, Shepherd MG. Effect of calcium ion uptake on Candida albicans morphology. FEMS Microbiol Lett. 1991. DOI:https://doi.org/10.1111/j.1574-6968.1991.tb04345.x
- Reuß O, Vik A, Kolter R, et al. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004. DOI:https://doi.org/10.1016/j.gene.2004.06.021
- Burke DJ, Dawson D, Stearns T. Methods in yeast genetics: a cold spring harbor laboratory course manual. In A cold spring harb lab course man. 2000
- Genicot G, Leroy JLMR, Van Soom A, et al. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology. 2005. DOI:https://doi.org/10.1016/j.theriogenology.2004.06.006
- Izard J, Limberger RJ. Rapid screening method for quantitation of bacterial cell lipids from whole cells. J Microbiol Methods. 2003. DOI:https://doi.org/10.1016/S0167-7012(03)00193-3
- Aleman-Nava GS, Cuellar-Bermudez SP, Cuaresma M, et al. How to use Nile Red, a selective fluorescent stain for microalgal neutral lipids. J Microbiol Methods. 2016;128:74–79.
- Rostron KA, Rolph CE, Lawrence CL. Nile red fluorescence screening facilitating neutral lipid phenotype determination in budding yeast, Saccharomyces cerevisiae, and the fission yeast Schizosaccharomyces pombe. Antonie Van Leeuwenhoek. 2015;108(1):97–106.
- Chen W, Zhang C, Song L, et al. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods. 2009;77(1):41–47.
- Ivnitski-Steele I, Holmes AR, Lamping E, et al. Identification of Nile Red as a fluorescent substrate of the Candida albicans ABC transporters Cdr1p and Cdr2p and the MFS transporter Mdr1p. Anal Biochem. 2009;394(1):87–91.
- Ram AFJ, Klis FM. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat Protoc. 2006. DOI:https://doi.org/10.1038/nprot.2006.397
- Fu Y, Ibrahim AS, Sheppard DC, et al. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002. DOI:https://doi.org/10.1046/j.1365-2958.2002.02873.x.
- Sundstrom P. Adhesion in Candida spp. Cell Microbiol. 2002. DOI:https://doi.org/10.1046/j.1462-5822.2002.00206.x
- Ho V, Herman-Bausier P, Shaw C, et al. An amyloid core sequence in the major Candida albicans adhesin Als1p mediates cell-cell adhesion. MBio. 2019. DOI:https://doi.org/10.1128/mBio.01766-19.
- Artym J, Zimecki M. Milk-derived proteins and peptides in clinical trials. Postepy Hig Med Dosw. 2013. doi:https://doi.org/10.5604/17322693.1061635.
- Zimecki M, Kruzel ML. Milk-derived proteins and peptides of potential therapeutic and nutritive value. J Exp Ther Oncol. 2007;6(2):89-106.
- Selby-Pham SN, Howell K, Jegasothy H, et al. Regulation of milk protein solubility by a whey-derived proline-rich peptide product. J Dairy Res. 2013. DOI:https://doi.org/10.1017/S0022029913000186.
- Martinez-Lopez R, Monteoliva L, Diez-Orejas R, et al. The GPI-anchored protein CaEcm33p is required for cell wall integrity, morphogenesis and virulence in Candida albicansss. Microbiology. 2004. DOI:https://doi.org/10.1099/mic.0.27320-0
- Rieg N, Fonzi WA, Belanger PH, et al. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect Immun. 1998;66(4):1783-1786.
- Nailis H, Vandenbroucke R, Tilleman K, et al. Monitoring ALS1 and ALS3 gene expression during in vitro Candida albicans biofilm formation under continuous flow conditions. Mycopathologia. 2009. DOI:https://doi.org/10.1007/s11046-008-9148-6
- Chandra J, Kuhn DM, Mukherjee PK, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001. DOI:https://doi.org/10.1128/JB.183.18.5385-5394.2001
- McCall AD, Pathirana RU, Prabhakar A, et al. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. Npj Biofilms Microbiomes. 2019. DOI:https://doi.org/10.1038/s41522-019-0094-5
- Zhao X, Oh SH, Cheng G, et al. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004. DOI:https://doi.org/10.1099/mic.0.26943-0.
- Argimón S, Wishart JA, Leng R, et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot Cell. 2007. DOI:https://doi.org/10.1128/EC.00340-06.
- Chen HF, Lan CY. Role of SFP1 in the regulation of Candida albicans biofilm formation. PLoS One. 2015. DOI:https://doi.org/10.1371/journal.pone.0129903
- Green CB, Zhao X, Yeater KM, et al. Construction and real-time RT-PCR validation of Candida albicans PALS-GFP reporter strains and their use in flow cytometry analysis of ALS gene expression in budding and filamenting cells. Microbiology. 2005. DOI:https://doi.org/10.1099/mic.0.27696-0
