ABSTRACT
Background: Modulation of the commensal oral microbiota constitutes a promising preventive/therapeutic approach in oral healthcare. The use of prebiotics for maintaining/restoring the health-associated homeostasis of the oral microbiota has become an important research topic.
Aims: This study hypothesised that in vitro 14-species oral biofilms can be modulated by (in)direct stimulation of beneficial/commensal bacteria with new potential prebiotic substrates tested at 1 M and 1%(w/v), resulting in more host-compatible biofilms with fewer pathogens, decreased virulence and less inflammatory potential.
Methods: Established biofilms were repeatedly rinsed with N-acetyl-D-glucosamine, α-D-lactose, D-(+)-trehalose or D-(+)-raffinose at 1 M or 1%(w/v). Biofilm composition, metabolic profile, virulence and inflammatory potential were eventually determined.
Results: Repeated rinsing caused a shift towards a more health-associated microbiological composition, an altered metabolic profile, often downregulated virulence gene expression and decreased the inflammatory potential on oral keratinocytes. At 1 M, the substrates had pronounced effects on all biofilm aspects, whereas at 1%(w/v) they had a pronounced effect on virulence gene expression and a limited effect on inflammatory potential.
Conclusion: Overall, this study identified four new potential prebiotic substrates that exhibit different modulatory effects at two different concentrations that cause in vitro multi-species oral biofilms to become more host-compatible.
INTRODUCTION
The oral cavity shelters a highly organized and complex consortium of oral microbial species, characterized by the presence of a dynamic equilibrium (‘homeostasis’) between these different species [Citation1–6]. There also exists a homeostatic equilibrium between the polymicrobial microbiota, the host response, and the environment [Citation1–6]. Due to environmental changes (e.g. dietary alterations) and/or changes in the host response (e.g. immune alterations), this balance can be disrupted, resulting in oral diseases such as caries or periodontitis [Citation7–9]. These oral diseases are thus the result of a complex interplay between commensal and pathogenic bacteria, the environment and the host [Citation10–14]. Therefore, maintaining and/or restoring the homeostatic relationship between the polymicrobial oral microbiota and host tissues might create novel preventive and therapeutic options. The development of such new approaches is of high importance, considering the emerging issue of increased adaptation and resistance development of oral bacterial species to antimicrobial agents such as antibiotics or antiseptics [Citation15–19].
In an attempt to maintain and/or restore the health-associated homeostasis of the resident oral microbiota and also modulate the host response, mainly pro- and prebiotic approaches gained a lot of interest in recent years [Citation8,Citation10,Citation20,Citation21]. The microbiological and clinical benefits of probiotics have been shown and they are being used in oral healthcare for several years now [Citation22,Citation23]. However, although successfully used for gastro-intestinal diseases [Citation24], only recently an in vitro proof-of-concept for the use of prebiotics in oral healthcare was delivered [Citation25,Citation26]. The International Scientific Association for Probiotics and Prebiotics (ISAPP) recently published the most up-to-date definition and scope of prebiotics. In this statement, a prebiotic is defined as ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’[Citation27]. Therefore, prebiotics represent a whole different approach than probiotics, which are defined as ‘live microorganisms that are administered in such amounts that they eventually provide a health benefit for the host’[Citation27]. Several in vitro and in vivo studies investigated the effects of potential prebiotic substrates on oral health [Citation25,Citation26,Citation28–30]. For instance, Slomka and co-workers screened a wide range of compounds on their ability to selectively stimulate beneficial oral bacteria which in turn suppressed pathogenic oral bacteria in an in vitro dual species biofilm [Citation25]. Later on, they found that three of these potential prebiotic substrates caused a clear shift in the proportion of beneficial/commensal species (>95%) in an in vitro, 14-species oral biofilm [Citation26]. Furthermore, they also observed that the prebiotic effect was influenced by several environmental factors, such as substrate dose, oxygen concentration and nutrient availability [Citation26]. Other studies focused on the effects of one particular prebiotic substrate, namely arginine, which was found to have beneficial, health-associated effects on the composition and metabolic activity of both in vitro and in vivo microbial communities from a caries point of view [Citation28–30]. All these findings indicate promising potential for the use of prebiotics for oral health. However, further in vitro and in vivo research is still required before it can eventually be applied in humans as a widespread preventive or therapeutic strategy.
The main hypothesis of this study was that in vitro multi-species oral biofilms can be modulated by (in)direct stimulation of beneficial/commensal bacteria with certain substrates, resulting in more host-compatible biofilms that harbour lower amounts of pathogens, show decreased virulence and have less inflammatory potential. The aim was to identify new potential prebiotic substrates, which (in)directly stimulate the beneficial/commensal oral bacteria in terms of growth and/or metabolism and/or activities and by consequence inhibit pathogenic oral bacteria, decrease virulence gene expression and reduce the inflammatory response of oral keratinocytes exposed to multi-species oral biofilms treated with these substrates. The substrates were tested at two concentrations: a high concentration of 1 M and a low concentration of 1%(w/v) (with mentioning of the corresponding molar concentrations). The rationale for this was to allow for comparison with the previously mentioned proof-of-concept study of Slomka et al. [Citation26], where the effects of the potential prebiotic substrates were most pronounced at concentrations around 1 M. Although such a high concentration is acceptable for proving concepts at an in vitro level, it is difficult to apply in a real-life situation. Therefore, the lower 1%(w/v) concentration was evaluated as well, as this is a more realistic concentration for formulating compounds in commercially available mouthwashes or toothpastes.
MATERIAL AND METHODS
Bacterial strains, media and culture conditions
Bacterial species and strains used in this study as representative commensal oral bacteria, representative periodontal pathogens or representative cariogenic pathogens are listed in . Culture and incubation conditions have been described by Slomka and co-workers [Citation26].
Table 1. Overview of bacterial species and strains used in this study
Bioreactor-derived multi-species community
A 14-species community was grown in a Biostat B Twin 1 L bioreactor (Sartorius Stedim Biotech GmbH, Goettingen, Germany) with controlled environmental conditions as previously described by Slomka and co-workers [Citation26].
Substrates
N-acetyl-D-glucosamine, D-(+)-raffinose, D-(+)-trehalose (all Sigma-Aldrich Co, St. Louis, USA) and α-D-lactose (Acros Organics – Thermo Fisher Scientific, Waltham, USA) were selected from of a larger high-throughput screening for nutritional sources using Phenotype MicroArray (PM) panels and were selected because they exhibited (some) potential to function as prebiotic substrates [Citation25]. Substrates were dissolved in phosphate-buffered saline (PBS) with pH adjusted to 5.7 using citric acid, followed by filter sterilization. Substrates were tested at concentrations of 1 M and 1%(w/v), the latter, respectively, corresponding to 45 mM (NADG), 29 mM (α-D-lactose), 17 mM (D-(+)-raffinose), and 26 mM (D-(+)-trehalose).
Multi-species biofilm rinsing assays
A sample was taken from the bioreactor vessel, diluted 1:10 in fresh modified Brain Heart Infusion (BHI) medium [Citation31] and added to a 24-well plate (2 mL/well). Biofilms were grown vertically on Calcium Deficient Hydroxyapatite (CAD-HA) disks (Hitemco Medical, Old Bethpage, USA) using the Amsterdam Active Adhesion model [Citation32]. Biofilms were allowed to establish during 24 h (37°C, 170 rpm) under micro-aerophilic conditions (6% O2, 7% CO2, 7% H2, 80% N2). After this 24 h, disks were rinsed 3 times a day for 3 minutes (RT, 250 rpm), for 3 consecutive days, by transferring the lid containing the disks to a new 24-well plate containing 2 mL/well of the appropriate substrate solution. As a negative control, PBS (pH 5.7) without substrate supplementation was used. Following each rinsing step, disks were shortly dip-rinsed in a new 24-well plate containing 2 mL/well of fresh modified BHI medium to remove residual substrate traces. Next, the lid was transferred to a new 24-well plate containing 2 mL/well of fresh modified BHI medium, ultimately followed by re-incubation of the plates (37°C, 170 rpm, micro-aerophilic) until the next rinsing step. The morning after the final rinsing step on the third day, disks were dip-rinsed in PBS (pH 7.4) to remove unattached cells, followed by bacterial DNA or RNA extraction or by biofilm challenge of human cells (see lower). All experiments were repeated on three different days.
Bacterial DNA extraction and quantification
Biofilm-coated disks were dip-rinsed in PBS (pH 7.4) to remove unattached cells, after which biofilms were disrupted, bacterial cells harvested and DNA from only living bacteria was extracted, employing a propidium monoazide (PMA) treatment, as described before [Citation26]. Subsequent quantification of bacterial species was done through quantitative PCR (qPCR) assay as described previously [Citation26], species-specific primers and probes used were identical to those listed earlier by Herrero et al. [Citation33].
Organic acid analysis
Concentrations of organic acids in the filter sterilized supernatant of the multi-species biofilms were measured using a 761 Compact Ion Chromatograph (Metrohm, Switzerland) with a Metrosep Organic acids Guard/4.6 guard column. The eluent consisted of 1 mM H2SO4 at a flow rate of 0.8 mL min−1. Organic acid production/consumption was calculated as the organic acid concentrations detected in the filter sterilized biofilm supernatant, minus the concentrations of those organic acids detected in sterile modified BHI medium.
Bacterial RNA extraction and virulence gene expression analysis
Biofilm-coated disks were dip-rinsed in PBS (pH 7.4) to remove unattached cells, after which bacterial RNA was extracted through a mechanical disruption and acid phenol-chloroform extraction as described by Vandecasteele et al. [Citation34] in combination with the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. After quality and integrity assessment, a concentration-dependent normalization of all RNA samples was performed, followed by conversion of RNA to complementary DNA (cDNA) using the PrimeScript 1st strand cDNA Synthesis Kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. Expression of bacterial virulence genes was analysed through SYBR RT-qPCR and normalized for bacterial housekeeping gene (species-specific 16S rRNA or other genes) expression. Each reaction mixture consisted of 12.5 µL ROX SYBR Master Mix blue dTTP (Eurogentec, Seraing, Belgium), 5.5 µL distilled water, 1 µL of both forward and reverse species-specific primer (final primer concentration of 400 nM) and 5 µL of template cDNA. Assay conditions consisted of an initial 2 min at 50°C, followed by a denaturation step of 10 min at 95°C, 45 cycles of 15 s at 95°C and 60 s at 60°C. Specific sequences of each primer pair can be found elsewhere [Citation35]. Data were determined as a function of the threshold cycle (CT) values and relative virulence gene expression was calculated according to the ΔΔCT method (2−(ΔCTexp – ΔCTcontrol)).
Biofilm challenge of cells
Cultures of immortalized human oral keratinocytes (HOK-18A) were grown as described previously [Citation36]. Sterile silicone rings (Peleman BVBA, Wilsele, Belgium) were placed at the bottom of 24-well plates after which the HOK-18A cultures were seeded and grown until confluence. Biofilm-coated disks were dip-rinsed in PBS (pH 7.4) to remove unattached cells and set on the silicone rings with the biofilm facing the cell monolayer. The presence of the ring ensured a fixed distance of 1 mm between biofilm and cell monolayer. Following 2 h of contact (37°C, 5% CO2, 170 rpm), rings and disks were removed, cells were washed twice with PBS (pH 7.4) and fresh cell culture medium containing 0.1 mg/mL gentamycin was added, followed by incubation (37°C, 5% CO2, 170 rpm) for 2 h. Next, cells were harvested and cellular RNA was extracted with the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA was converted to cDNA and relative expression of inflammatory mediator genes was determined as described above with respect to the cellular housekeeping gene β-actin. In addition, cell culture supernatants were collected and analysed by means of enzyme-linked immunosorbent assay (ELISA) to detect CXCL8 (interleukin-8) using the Human IL-8 ELISA Kit (Thermo Fisher Scientific, Waltham, USA) according to the manufacturer’s protocol. All experiments were repeated on three different days.
Statistical analysis
GraphPad Prism version 7.04 for Windows (GraphPad Software, La Jolla, California, USA) was used for statistical analysis. For all experiments, comparisons with the control were set up and analysed through a one-way ANOVA with a confidence level of 95% and a correction for simultaneous hypothesis testing was applied according to Dunnett to detect statistically significant differences (P < 0.05). Normality of the residuals was assessed by means of a Shapiro–Wilk test and a normal quantile plot.
DATA AVAILABILITY
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files.
RESULTS
Effects of substrates on multi-species biofilm composition
N-acetyl-D-glucosamine (NADG), α-D-lactose, D-(+)-trehalose and D-(+)-raffinose were selected to determine their effects on multi-species biofilm composition. Repeated rinsing of the biofilms with the substrates at a concentration of 1 M resulted in a significant decrease in absolute numbers of all four periodontal pathogens compared to the control (, Supplementary Table S1). These decreases (expressed as logarithmic value of the amount of genome equivalents per millilitre; log(Geq/mL)) were approximately ~1–1.4 log(Geq/mL) for A. actinomycetemcomitans, ~2.3–4 log(Geq/mL) for F. nucleatum, ~2.4–2.7 log(Geq/mL) for P. gingivalis and ~2–3.2 log(Geq/mL) for P. intermedia. The numbers of the cariogenic pathogens S. mutans and S. sobrinus were usually increased with ~0.6–1.3 log(Geq/mL) compared to the control, with statistical significance being reached for S. mutans in the case of D-(+)-trehalose and D-(+)-raffinose, and for S. sobrinus in the case of α-D-lactose and D-(+)-trehalose. Interestingly, NADG resulted in a significant decrease in S. sobrinus numbers with ~3 log(Geq/mL). The numbers of the commensal species A. naeslundii (for all substrates) and A. viscosus (for D-(+)-trehalose) were often significantly decreased with ~1.9–2.5 log(Geq/mL), and V. parvula showed significant reductions of ~0.7 log(Geq/mL) for α-D-lactose and D-(+)-trehalose (, Supplementary Table S1). When considering the beneficial/commensal streptococcal species, the bacterial numbers generally increased with ~0.2–1.3 log(Geq/mL), except for S. sanguinis, with statistical significance being reached for S. gordonii (NADG), S. oralis (D-(+)-raffinose) and S. salivarius (all four substrates) (, Supplementary Table S1).
Figure 1. Effects of repeated rinsing with potential prebiotic substrates at 1 M on multi-species biofilm composition
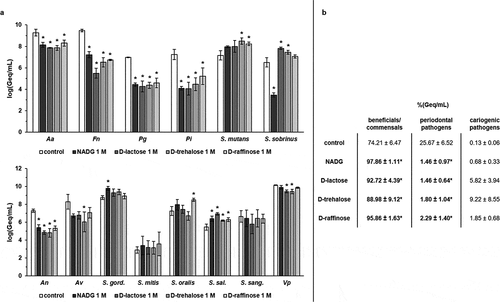
In terms of relative proportions (%(Geq/mL)) (), the control treatment resulted in a biofilm consisting of 74.21 ± 6.47% beneficial/commensal species, 25.67 ± 6.52% periodontal pathogens and 0.13 ± 0.06% cariogenic pathogens. Rinsing with the substrates resulted in significantly lower proportions of periodontal pathogens (1.46 ± 0.97%, 1.46 ± 0.64%, 1.80 ± 1.04% and 2.29 ± 1.40% for NADG, α-D-lactose, D-(+)-trehalose and D-(+)-raffinose, respectively) when compared to the control condition. All substrates also resulted in significantly higher proportions of beneficial/commensal species (97.86 ± 1.11%, 92.72 ± 4.39%, 88.98 ± 9.12% and 95.86 ± 1.63% for NADG, α-D-lactose, D-(+)-trehalose and D-(+)-raffinose, respectively). Proportions of cariogenic pathogens were elevated in all cases (0.68 ± 0.33%, 5.82 ± 3.94%, 9.22 ± 8.55% and 1.85 ± 0.68% for NADG, α-D-lactose, D-(+)-trehalose and D-(+)-raffinose, respectively), although never significantly when compared to the control.
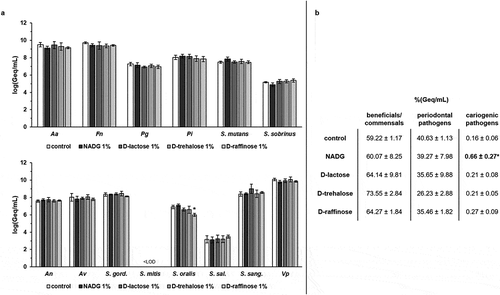
When rinsing the biofilms with the substrates at a concentration of 1%(w/v), absolute numbers of beneficial/commensal species, periodontal pathogens and cariogenic pathogens were in general only slightly affected compared to the control (changes of ~0-0.6 log(Geq/mL), without reaching statistical significance (, Supplementary Table S2). Only in the case of S. oralis, a significant decrease of ~0.9 log(Geq/mL) could be observed for D-(+)-raffinose. Tendencies for higher proportions of beneficial/commensal species and lower proportions of periodontal pathogens were observed, but significant differences compared to the control condition were never reached. The proportions of cariogenic pathogens were usually unchanged, except for those in the NADG condition, which resulted in a significantly higher proportion of cariogenic species (0.66 ± 0.27%) compared to the control (0.16 ± 0.06%) ().
Figure 2. Effects of repeated rinsing with potential prebiotic substrates at 1%(w/v) on multi-species biofilm composition
Effects of substrates on multi-species biofilm organic acid balances
The supernatants from the substrate-treated multi-species biofilms were analysed to gain more insight into the influence of the substrates on organic acid metabolism. For substrate concentrations of 1 M (, Supplementary Table S3), a significantly increased lactate production was observed in the case of α-D-lactose (3791 ± 169 mg/L), D-(+)-trehalose (4187 ± 200 mg/L) and D-(+)-raffinose (971 ± 43 mg/L) compared to the control condition (consumption of 125 ± 0 mg/L). Formate production was significantly lower for α-D-lactose and D-(+)-trehalose in comparison with the control condition (84 ± 9 mg/L and 75 ± 8 mg/L vs. 384 ± 19 mg/L). α-D-lactose (881 ± 73 mg/L), D-(+)-trehalose (1021 ± 73 mg/L) and D-(+)-raffinose (2640 ± 154 mg/L) all resulted in significantly lower acetate production compared to the control condition (3779 ± 305 mg/L). NADG, α-D-lactose, D-(+)-trehalose and D-(+)-raffinose all resulted in a significantly different production of propionate (2750 ± 40 mg/L, 1306 ± 175 mg/L, 1577 ± 46 mg/L and 4178 ± 105 mg/L, respectively) and butyrate (1255 ± 51 mg/L, 0 ± 0 mg/L, 0 ± 0 mg/L and 154 ± 26 mg/L, respectively) in comparison with the control condition (2094 ± 132 mg/L propionate and 1870 ± 93 mg/L butyrate).
Figure 3. Effects of repeated rinsing with potential prebiotic substrates on multi-species biofilm organic acid balances
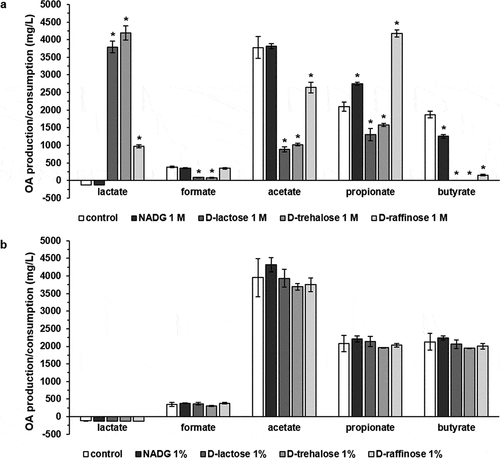
Rinsing with substrates at concentrations of 1%(w/v) did not result in significant differences in organic acid production/consumption compared to the control (, Supplementary Table S3).
Table 2. Effects of repeated rinsing of multi-species biofilms with potential prebiotic substrates on virulence gene expression from A. actinomycetemcomitans.
Table 3. Effects of repeated rinsing of multi-species biofilms with potential prebiotic substrates on virulence gene expression from P. gingivalis.
Effects of substrates on multi-species biofilm virulence gene expression
The virulence of the substrate-treated multi-species biofilms was evaluated by analysing the relative expression of 33 virulence genes from 4 periodontal pathogens. A more detailed overview of these virulence genes and the associated functions of the virulence factors they encode can be found in Supplementary Table S4. Significantly different gene expressions in the substrate-treated biofilms relative to the control biofilms were considered to be biologically relevant if their value was more than 1.5-fold upregulated or more than 2-fold downregulated. Only these results were considered.
In multi-species biofilms rinsed with the substrates at concentrations of 1 M, A. actinomycetemcomitans and P. gingivalis virulence gene expressions were significantly downregulated relative to the control condition for most substrates (). In the case of A. actinomycetemcomitans, these downregulations ranged from 2-fold up to 100-fold (NADG), 2.4-fold to 100-fold (α-D-lactose), 2.5-fold to 100-fold (D-(+)-trehalose) and 5.9-fold to 100-fold (D-(+)-raffinose). Noteworthy was the significantly upregulated expression of pgA (11.6-fold for NADG, 4.3-fold for α-D-lactose, 18.2-fold for D-(+)-trehalose and 7.3-fold for D-(+)-raffinose). For P. gingivalis, downregulations ranged from 2.6-fold to 14-fold (NADG), 17-fold to 100-fold (α-D-lactose), 7.7-fold up to undetectable (D-(+)-trehalose) and 33-fold to 100-fold (D-(+)-raffinose). In contrast, F. nucleatum virulence gene expression was in general significantly upregulated (2.5- to 250-fold) by all substrates relative to the control (). However, the upregulation induced by NADG was more limited and only significant for 2 genes, encoding the ABC transporter permease and the hemin receptor (3.3-fold in both cases). For P. intermedia, a more diverse impact of the substrates on virulence gene expression was observed (). α-D-lactose primarily significantly downregulated the expression of virulence genes (2.1- to 8.3-fold), whereas NADG (2.3- to 20.3-fold upregulation), D-(+)-trehalose (7- to 11.5-fold upregulation and 5-fold downregulation) and D-(+)-raffinose (1.9- to 10.6-fold upregulation and 3-fold downregulation) significantly up- or downregulated them.
Table 4. Effects of repeated rinsing of multi-species biofilms with potential prebiotic substrates on virulence gene expression from F. nucleatum.
Table 5. Effects of repeated rinsing of multi-species biofilms with potential prebiotic substrates on virulence gene expression from P. intermedia.
In multi-species biofilms rinsed with the substrates at concentrations of 1%(w/v), there was generally a significantly decreased A. actinomycetemcomtans virulence gene expression (2.3- to 25-fold) (). Only for the NADG condition, the expression of one gene (orf859) was significantly upregulated (2-fold). The substrates had limited impact on P. gingivalis virulence gene expression, with only D-(+)-trehalose and NADG having a significant impact on respectively kgp (3.2-fold up-regulation) and partC (2.8-fold downregulation) expression (). For F. nucleatum, and in contrast with the results obtained for substrate concentrations of 1 M, significantly decreased virulence gene expression (2- to 10-fold) was observed for most of the substrates (). For P. intermedia, D-(+)-trehalose and D-(+)-raffinose only had a limited impact on virulence gene expression, whereas NADG and α-D-lactose in general significantly downregulated virulence gene expression (2.1- to 3.8-fold) ().
Effects of substrates on multi-species biofilm inflammatory potential
The relative inflammatory potential of the substrate-treated multi-species biofilms was evaluated by analysing the expression of five inflammatory mediator genes in human oral keratinocytes (HOKs) exposed to the substrate-treated biofilms. Significantly different gene expressions in HOKs exposed the substrate-treated biofilms relative to HOKs exposed to the control biofilms were considered to be biologically relevant if their value was more than 1.5-fold upregulated or more than 2-fold downregulated. Only these results were considered. The IL-8 levels in the cellular supernatant were determined as well.
In HOKs exposed to substrate-treated (substrate concentrations of 1 M) multi-species biofilms, mostly decreases in inflammatory mediator gene expression were observed (). IL-8 gene expression was significantly downregulated for all substrates (4.5-fold to 33.3-fold). TNF-α gene expression was significantly downregulated for the α-D-lactose condition (2.5-fold) and this was also the case for IL-1β gene expression (2-fold) and IL-6 gene expression (3-fold). Absolute IL-8 levels were significantly reduced for all substrate conditions (8.4-fold to undetectable) (, Supplementary Table S5).
Table 6. Effects of repeated rinsing with potential prebiotic substrates on multi-species biofilm inflammatory potential towards human oral keratinocytes
Figure 4. Effects of repeated rinsing with potential prebiotic substrates on multi-species biofilm inflammatory potential
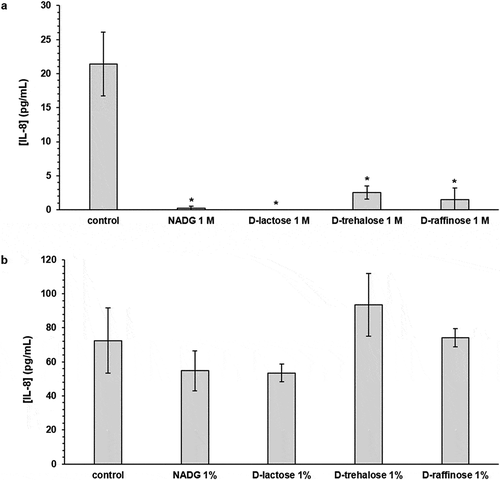
In HOKs exposed to substrate-treated (substrate concentrations of 1%(w/v)) multi-species biofilms, the relative expression of most inflammatory mediator genes was generally unaffected (). In line with the observations for IL-8 gene expression, absolute IL-8 levels were not significantly affected (, Supplementary Table S5).
DISCUSSION
Modulation of the commensal oral microbiota, by for instance prebiotic substrates, has gained a lot of interest as a promising preventive or therapeutic approach in oral healthcare. In this study, four new potential prebiotic substrates (NADG, α-D-lactose, D-(+)-trehalose and D-(+)-raffinose) were shown to modulate in vitro oral multi-species biofilms in such a way that they became more host-compatible. Repeated rinsing of established biofilms with the substrates resulted in a shift towards a more health-associated microbiological composition, an altered metabolic activity, often downregulated the expression of a selection of virulence genes and decreased the inflammatory effect on human oral keratinocytes. The higher substrate concentration (1 M) tested had pronounced effects on all four biofilm aspects, whereas the lower concentration (1%(w/v)) had a pronounced modulating effect on virulence gene expression and a limited effect on inflammatory potential. As far as we know, this study is the first one to simultaneously investigate the effects of potential prebiotic substrates on composition, metabolic activity, virulence gene expression and inflammatory potential of an in vitro, complex 14-species oral biofilm.
An important overall observation of this study was the concentration dependence of the effects the substrates had. The rationale for using two different concentrations, a high one (1 M) and a lower one (1%(w/v)), is that the 1 M concentration allows for comparison with a previous in vitro study [Citation26]. In that study, in which the prebiotic concept for oral healthcare was expanded to more complex multi-species biofilms, the beneficial effects of the potential prebiotic substrates were most pronounced for concentrations corresponding to the 1 M concentration of the current study. Although such high concentrations can be justified when working at an in vitro level to proof certain concepts, they might be more difficult to apply in a real-life situation. Therefore, a lower concentration was evaluated as well. A concentration of 1%(w/v) is a realistic concentration for formulating compounds in commercially available mouthwashes or toothpastes.
Initiation and progression of oral diseases are characterized by a dysbiosis of the oral microbiota, in which there is an increase in pathobiont species and a decrease in commensal/beneficial species abundance and activity [Citation8,Citation10,Citation11,Citation37–39]. Avoiding and/or counteracting such microbiological changes is thus of utmost importance for maintaining and/or restoring a health-associated homeostatic microbiological composition. Rinsing the biofilms with the substrates at 1 M caused pronounced increases in the proportions and amounts of beneficial/commensal species and decreases in those of the periodontal pathogens. These alterations resulted in a theoretically more health-associated biofilm composition from a periodontitis point of view [Citation1,Citation3,Citation11,Citation40–42]. On the other hand, changes were often less favourable from a caries point of view, as reflected by most of the changes in numbers of S. mutans and S. sobrinus, two of the primary organisms associated with dental caries [Citation43–46]. However, for NADG, a decrease in S. sobrinus was observed, which could originate in a competitive advantage for S. mutans based on differences in the ability to ferment NADG, as has been described previously [Citation47–50]. The overall less favourable effects on the cariogenic pathogens could be addressed by, for instance, combinations with L-arginine, a substrate that has been shown to have several beneficial effects from a caries point of view [Citation28–30,Citation51,Citation52]. Pronounced changes in multi-species oral biofilm composition following rinsing with potential prebiotic substrates similar to the ones observed in the current study were also previously reported by Slomka and co-workers [Citation26]. In that study, the most promising potential prebiotic substrate tested at a concentration of 1 M resulted for instance in a biofilm composition consisting of 97% beneficial/commensal species and reduced F. nucleatum and P. gingivalis numbers with 1.5–2 log(Geq/mL). In contrast with all the above, limited to no compositional changes were observed for substrates at 1%(w/v). Such different effects on microbiological composition for different concentrations have been reported previously for other potential prebiotic substrates both in oral and gastro-intestinal research [Citation26,Citation53,Citation54]. In addition, also the relatively limited duration of the treatment might play a role.
Microbiological composition and interactions might influence metabolic activity and vice versa [Citation55–64], which is why also the function, i.e. metabolic activity, of the microbiota should be evaluated [Citation27]. In this study, changes in levels of organic acids playing key roles in oral bacterial metabolic pathways were investigated. This was done to partially cover the complexity of our multi-species model, as different metabolic profiles can be distinguished based on bacterial species and localization in the oral cavity [Citation60,Citation64]. Streptococcus and Actinomyces species are usually found more supragingivally and have a saccharolytic metabolism resulting in the production of lactate, formate and acetate [Citation64]. The produced lactate forms an energy source for Veillonella, Actinomyces and Aggregatibacter species [Citation55,Citation56,Citation64,Citation65]. Veillonella species metabolize it into formate, acetate and propionate [Citation64], whereas Actinomyces species metabolize it into acetate [Citation64]. Subgingival sites are more dominated by asaccharolytic and/or proteolytic species like Fusobacterium, Porphyromonas and Prevotella that metabolize nitrogenous substrates into small peptides and amino acids, which are in turn degraded to propionate, butyrate, acetate and formate [Citation60,Citation64]. The amino sugar NADG and sugars α-D-lactose, D-(+)-trehalose and D-(+)-raffinose are mainly metabolized through saccharolytic pathways to provide a carbon source and also a nitrogen source in the case of NADG [Citation47,Citation66–72]. Biofilm treatment with the substrates at 1 M caused pronounced changes in organic acid levels that can often be at least partially explained by the observed compositional changes. For instance, only NADG rinsing did not increase lactate production. NADG rinsing had stimulating effects on two streptococcal numbers, but it also decreased S. sobrinus numbers and simultaneously did not decrease V. parvula numbers, in contrast with the three other substrates. On the other hand, Actinomyces spp. were decreased in the substrate-treated biofilms, although less pronounced for NADG and D-(+)-raffinose than for the two other substrates. Altogether, this can explain the observed changes in lactate and acetate levels. Similar changes have already been described in literature [Citation32,Citation55]. Fernandez y Mostajo et al. reported decreased Veillonella and Streptococcus species accompanied by increased lactate and decreased acetate levels following treatment of in vitro multi-species biofilms [Citation55]. Similarly, different magnitudes of decreases in formate and propionate levels can be attributed to the different magnitudes in decreases in, for instance, F. nucleatum, P. gingivalis, P. intermedia, V. parvula and Actinomyces spp. Increased propionate levels could be due to unaffected numbers of V. parvula and different magnitudes of decreases in periodontal pathogens. Finally, decreased butyrate levels can be attributed to pronounced decreases in anaerobic proteolytic species caused by all substrates. Given the extensive metabolic cross-feeding in oral biofilms, it is challenging to fully elucidate what the metabolic impact of the substrate treatments is. The lack of changes observed for substrates at 1%(w/v) was most likely due to the absence of compositional changes.
The virulence profiles of our multi-species biofilms were evaluated at the expression level of a selection of well-known virulence genes from the periodontal pathobionts [Citation73–78] incorporated in the biofilms. Treatment of the biofilms with the substrates at 1 M caused pronounced changes in virulence gene expression. Expression of nearly all A. actinomycetemcomitans and P. gingivalis virulence genes was strongly downregulated, which are genes encoding proteins involved in a wide range of pathology-associated processes such as cell adhesion, invasion and colonization, cytotoxicity, host tissue degradation and immune evasion (Supplementary Table S4). Remarkable was the upregulated expression of pgA obtained for all substrates. This gene is involved in the synthesis of a linear polymer of NADG residues in β(1,6)linkage that is part of an extracellular polysaccharide matrix involved in biofilm formation and colonization [Citation79,Citation80]. Upregulation of pgA as part of the extracytoplasmatic stress response has been described for other Aggregatibacter species [Citation81]. Apart from being a widespread structural component of bacterial cell walls (peptidoglycan) [Citation47], NADG is also known to function as a signalling molecule and to be involved in the regulation of virulence gene expression [Citation82,Citation83]. However, it is unclear whether the substrates themselves directly affect virulence gene expression in the biofilms or if the observed changes are the result of the complex interplay between other biofilm aspects. Noteworthy was the upregulation of F. nucleatum virulence gene expression, something which is usually seen in dysbiotic biofilms [Citation35,Citation84,Citation85]. However, for the NADG treatment the expression of most F. nucleatum virulence genes remained unaffected. The varying effects of NADG treatment on gene expression in the different periodontopathogens correspond with what has been previously described for different microbial species [Citation82]. Even though treatment with the substrates at 1%(w/v) did not result in significant compositional and metabolic changes, virulence gene expression was still affected. This is in line with what has been described for subinhibitory concentrations of some antibiotics and antiseptics, which affected bacterial virulence gene expression in certain species at concentrations too low to influence bacterial growth/survival [Citation86–88]. Highly remarkable was that gene expression patterns were sometimes completely opposed to those observed for treatment with the substrates at 1 M. However, the explanation for this remains currently unclear. To conclude, a wide range of studies on dysbiotic oral biofilms reported increased expression of virulence genes involved in the same or similar processes and functions as those evaluated in this study [Citation35,Citation84,Citation85,Citation89–91]. In the current study, effects on virulence gene expression were found to be highly dependent on the substrate, substrate concentration and the bacterial species and gene under consideration. However, the initiation and progression of oral pathologies are a consequence of the collective composition, function and virulence of the entire, synergistic polymicrobial community [Citation11,Citation92–94]. Therefore, it is important to look at the bigger picture. When considering the overall effect, often a favourable decreased virulence gene expression was observed.
Inflammation is considered an ecological driver of dysbiosis, with the initiation and progression of periodontal diseases seeming to be the product of a reciprocal, self-sustaining feedforward loop between dysbiosis and inflammation [Citation95]. Therefore, it was evaluated whether the substrate-treated biofilms showed an altered inflammatory potential. The evaluated genes encode inflammatory mediators that are all well known for their involvement in periodontitis [Citation36,Citation96–100]. Gene expression data revealed that the effects on the inflammatory potential of the biofilms were highly dependent on the substrate and substrate concentration. For substrates at 1 M, especially IL-8 gene expression levels were strongly downregulated, which was also reflected in the strongly decreased absolute IL-8 levels. In periodontitis, oral keratinocytes produce IL-8 in the presence of pro-inflammatory cytokines, such as IL-1β and TNF-α, which in turn acts as a potent activator and attractant of neutrophils [Citation98,Citation100,Citation101]. Elevated IL-8 levels have been described in patients with severe periodontitis and are also considered to be important indicators for the onset of oral diseases [Citation100,Citation102,Citation103]. Our data thus point towards a theoretically more periodontal health-associated inflammatory status. The effects on inflammatory response can also be linked to the observed changes in biofilm composition, metabolic activity and virulence gene expression. It is well known that periodontal pathogens and dysbiotic biofilms can elicit strong epithelial inflammatory responses [Citation12,Citation35,Citation36,Citation96–100]. The shifts in biofilm composition obtained for substrates at 1 M probably contributed to the lowered inflammatory response. This is also related to the observed changes in metabolic activity, as periodontal inflammation is thus associated with a dysbiotic community predominantly consisting of proteolytic and asaccharolytic species [Citation12,Citation95]. Metabolic changes associated with an improvement of periodontal disease status are, for instance, increases in lactate levels and decreases in propionate, butyrate and acetate levels [Citation55]. Especially decreased butyrate levels can be considered favourable, as butyrate production by periodontal pathogens is highly associated with the inflammation that occurs during periodontal disease [Citation104–106]. All substrates tested in this study caused at least one, and often three or four, of such changes at a concentration of 1 M. Furthermore, many of the genes for which expression was downregulated encode virulence factors involved in cell adhesion and invasion, cytotoxicity and other processes that play crucial roles in the onset and progression of inflammation. Even though the treatment of the biofilms with substrates at 1%(w/v) only had a limited effect on their inflammatory potential, they could affect the inflammatory response more indirectly, like through the observed downregulated expression of such virulence genes.
When evaluating the effects of potential therapies at an in vitro level, one needs to be aware of certain limitations and differences when compared to the in vivo situation. Complex microbial communities are characterized by inter- and intra-species interactions and metabolic cross-feeding, which has to be taken into account when evaluating potential prebiotic effects [Citation58,Citation107]. Other factors of importance are, for instance, the limited application time in the mouth and wash-out and dilution effects of the salivary and crevicular fluid flow [Citation108]. Given all of this, the experimental set-up and approach used in this study tried to consider several of these factors. Therefore, it was decided to immediately look at the effects of the substrates on the level of complex, multi-species biofilms grown on vertical hydroxyapatite disks, mimicking the tooth surface and orientation. The applied rinsing protocol simulated regular, repeated exposure to the substrates, as would also be the case in real-life when brushing the teeth or using mouthwashes. In line with that, the pH of the substrate solutions was adjusted to 5.7, a pH also found in commercially available mouthwashes. To conclude, future research should focus on certain aspects that were not addressed in the current study. Such aspects include, for instance, the exact mode of action of the substrates, the inclusion of other types of relevant immune cells, determination of actual protein levels of the evaluated virulence factors and looking into possible combinations of substrates to reduce the concentrations needed to achieve certain effects. Regarding the latter, an additional study will be performed to investigate a substrate concentration range from 1%(w/v) to 5%(w/v) and to look into the combinatory effects of different substrates at physiologically relevant concentrations. Eventually, the substrates will need to be tested under in vivo conditions to fully determine their microbiological and clinical potential.
Overall, this study identified four new potential prebiotic substrates, NADG, α-D-lactose, D-(+)-trehalose and D-(+)-raffinose, that exhibit different modulatory effects at two different concentrations, that cause in vitro multi-species oral biofilms to become more host-compatible. The observed compositional, metabolic and inflammatory changes were usually only observed for the highest concentration (1 M) tested, whereas changes in virulence gene expression were found to be highly dependent on substrate type and concentration, bacterial species and gene under consideration.
ACKNOWLEDGEDMENTS
This study was supported by grants from the KU Leuven (Belgium) (C24/17/086), from the Research Foundation Flanders (FWO, Belgium) (FWO G091218N) and from Colgate-Palmolive (NJ, USA).
We thank Ioanna Chatzigiannidou (CMET, UGent) for the help with the organic acid analysis.
AUTHOR CONTRIBUTIONS
T.V. contributed to conception, design, data acquisition and analysis, data interpretation, drafted and critically revised the manuscript; K.B. and N.B. contributed to design and data interpretation, critically revised the manuscript; W.V.H., N.Z., C.D., J.M. and M.Q. contributed to data interpretation, critically revised the manuscript; W.T. contributed to conception, design, data analysis and interpretation, critically revised the manuscript.
DISCLOSURE OF INTEREST
Authors W.T, T.V, K.B., M.Q. (in the name of Katholieke Universiteit Leuven), N.B. (in the name of Universiteit Gent), C.D and J.M. (in the name of Colgate-Palmolive Company) are listed as inventors on a patent application filed by Colgate-Palmolive Company, Katholieke Universiteit Leuven and Universiteit Gent related to specific aspects of this manuscript. Dr. Daep and Dr. Masters are employed by Colgate-Palmolive, which partially sponsored this study. All other authors report no conflicts of interest related to this study.
MATERIALS & CORRESPONDENCE
Correspondence and requests for materials should be addressed to W.T.
Supplemental Material
Download MS Word (34.7 KB)Supplementary Material
Supplemental data for this article can be accessed here.
Disclosure statement
Authors W.T, T.V, K.B., M.Q. (in the name of Katholieke Universiteit Leuven), N.B. (in the name of Universiteit Gent), C.D and J.M. (in the name of Colgate-Palmolive Company) are listed as inventors on a patent application filed by Colgate-Palmolive Company, Katholieke Universiteit Leuven and Universiteit Gent related to specific aspects of this manuscript. Dr. Daep and Dr. Masters are employed by Colgate-Palmolive, which partially sponsored this study. All other authors report no conflicts of interest related to this study.
Additional information
Funding
References
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(5721–5732). DOI:https://doi.org/10.1128/JCM.43.11.5721-5732.2005
- Burne RA, Zeng L, Ahn SJ, et al. Progress dissecting the oral microbiome in caries and health. Adv Dent Res. 2012;24(2):77–17.
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017.
- Human Microbiome PC. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214.
- Verma D, Garg PK, Dubey AK. Insights into the human oral microbiome. Arch Microbiol. 2018;200(4):525–540.
- Zaura E, Keijser BJ, Huse SM, et al. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9(1):259.
- Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol. 2017;44(Suppl 18):S23–S38.
- Rosier BT, Marsh PD, Mira A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J Dent Res. 2018;97(4):371–380.
- Silva N, Abusleme L, Bravo D, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23:329–355.
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–490.
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419.
- Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10(5):497–506.
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(2):279–294.
- Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63(4s):322–331.
- Cieplik F, Jakubovics NS, Buchalla W, et al. Resistance toward chlorhexidine in oral bacteria - is there cause for concern? Front Microbiol. 2019;10:587.
- Kitagawa H, Izutani N, Kitagawa R, et al. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J Dent. 2016;47:18–22.
- Kulik EM, Waltimo T, Weiger R, et al. Development of resistance of mutans streptococci and Porphyromonas gingivalis to chlorhexidine digluconate and amine fluoride/stannous fluoride-containing mouthrinses, in vitro. Clin Oral Investig. 2015;19(6):1547–1553.
- Roberts AP, Mullany P. Oral biofilms: a reservoir of transferable, bacterial, antimicrobial resistance. Expert Rev Anti Infect Ther. 2010;8(12):1441–1450.
- Verspecht T, Rodriguez Herrero E, Khodaparast L, et al. Development of antiseptic adaptation and cross-adaptation in selected oral pathogens in vitro. Sci Rep. 2019;9(1):8326.
- Devine DA, Marsh PD. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol. 2009;1(1). DOI:https://doi.org/10.3402/jom.v1i0.1949
- Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(Suppl 1):S11–15.
- Gruner D, Paris S, Schwendicke F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J Dent. 2016;48:16–25.
- Teughels W, Durukan A, Ozcelik A, et al. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2013;40(11):1025–1035.
- Patel R, DuPont HL. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60(Suppl suppl_2):S108–121.
- Slomka V, Hernandez-Sanabria E, Rodriguez Herrero E, et al. Nutritional stimulation of commensal oral bacteria suppresses pathogens: the prebiotic concept. J Clin Periodontol. 2017;44(4):344–352.
- Slomka V, Rodriguez Herrero E, Boon N, et al. Oral prebiotics and the influence of environmental conditions in vitro. J Periodontol. 2018;89(6):708–717.
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502.
- Koopman JE, Hoogenkamp MA, Buijs MJ, et al. Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch Oral Biol. 2017;73:79–87.
- Koopman JE, Röling WFM, Buijs M, et al. Stability and resilience of oral microcosms toward acidification and Candida outgrowth by arginine supplementation. Microb Ecol. 2015;69(2):422–433.
- Wolff M, Corby P, Klaczany G, et al. In vivo effects of a new dentifrice containing 1.5% arginine and 1450 ppm fluoride on plaque metabolism. J Clin Dent. 2013;24(Spec no A):A45–54.
- Alvarez G, Gonzalez M, Isabal S, et al. Method to quantify live and dead cells in multi-species oral biofilm by real-time PCR with propidium monoazide. AMB Express. 2013;3(1):1.
- Exterkate RA, Crielaard W, Ten Cate JM. Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 2010;44(4):372–379.
- Herrero ER, Slomka V, Boon N, et al. Dysbiosis by neutralizing commensal mediated inhibition of pathobionts. Sci Rep. 2016;6(1):38179.
- Vandecasteele SJ, Peetermans WE, Merckx R, et al. Use of gDNA as internal standard for gene expression in staphylococci in vitro and in vivo. Biochem Biophys Res Commun. 2002;291(3):528–534.
- Herrero ER, Fernandes S, Verspecht T, et al. Dysbiotic biofilms deregulate the periodontal inflammatory response. J Dent Res. 2018;97(5):547–555.
- Sliepen I, Van Damme J, Van Essche M, et al. Microbial interactions influence inflammatory host cell responses. J Dent Res. 2009;88(11):1026–1030.
- Diaz PI, Hoare A, Hong BY. Subgingival microbiome shifts and community dynamics in periodontal diseases. J Calif Dent Assoc. 2016;44(7):421–435.
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. Isme J. 2012;6(6):1176–1185.
- Wang GP. Defining functional signatures of dysbiosis in periodontitis progression. Genome Med. 2015;7(1). DOI:https://doi.org/10.1186/s13073-015-0165-z
- Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. Isme J. 2013;7(5):1016–1025.
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8(2):263–271.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144.
- Conrads G, de Soet JJ, Song L, et al. Comparing the cariogenic spe Streptococcus sobrin S. mutans on whole genome level. J Oral Microbiol. 2014;6(1):26189.
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50(4):353–380.
- Marsh PD, Featherstone A, McKee AS, et al. A microbiological study of early caries of approximal surfaces in schoolchildren. J Dent Res. 1989;68(7):1151–1154.
- Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44(2):331–384.
- Moye ZD, Burne RA, Zeng L. Uptake and Metabolism of N-Acetylglucosamine and Glucosamine by Streptococcus mutans. Appl Environ Microbiol. 2014;80(16):5053–5067.
- Beighton D, Russell RR, Whiley RA. A simple biochemical scheme for the differentiation of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1991;25(3):174–178.
- Kral TA, Daneo-Moore L. Biochemical differentiation of certain oral streptococci. J Dent Res. 1981;60(9):1713–1718.
- Homer KA, Patel R, Beighton D. Effects of N-acetylglucosamine on carbohydrate fermentation by Streptococcus mutans NCTC 10449 and Streptococcus sobrinus SL-1. Infect Immun. 1993;61(1):295–302.
- He J, Hwang G, Liu Y, et al. l-Arginine Modifies the Exopolysaccharide Matrix and Thwarts Streptococcus mutans Outgrowth within Mixed-Species Oral Biofilms. J Bacteriol. 2016;198(19):2651–2661.
- Zheng X, He J, Wang L, et al. Ecological Effect of Arginine on Oral Microbiota. Sci Rep. 2017;7(1):7206.
- Palframan RJ, Gibson GR, Rastall RA. Effect of pH and dose on the growth of gut bacteria on prebiotic carbohydrates in vitro. Anaerobe. 2002;8(5):287–292.
- Davis LM, Martinez I, Walter J, et al. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol. 2010;144(2):285–292.
- Fernandez YMM, Exterkate RAM, Buijs MJ, et al. Effect of mouthwashes on the composition and metabolic activity of oral biofilms grown in vitro. Clin Oral Investig. 2017;21(4):1221–1230.
- Periasamy S, Kolenbrander PE. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J Bacteriol. 2010;192(12):2965–2972.
- Keller L, Surette MG. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol. 2006;4(4):249–258.
- Bradshaw DJ, Homer KA, Marsh PD, et al. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology. 1994;140(12):3407–3412.
- Levy R, Borenstein E. Metabolic modeling of species interaction in the human microbiome elucidates community-level assembly rules. Proc Natl Acad Sci U S A. 2013;110(31):12804–12809.
- Takahashi N. Microbial ecosystem in the oral cavity: metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congress Series. 2005;1284:103–112.
- Nyvad B, Crielaard W, Mira A, et al. Dental caries from a molecular microbiological perspective. Caries Res. 2013;47(2):89–102.
- Takahashi N, Washio J. Metabolomic Effects of Xylitol and Fluoride on Plaque Biofilm in Vivo. J Dent Res. 2011;90(12):1463–1468.
- Takahashi N, Washio J, Mayanagi G. Metabolomics of supragingival plaque and oral bacteria. J Dent Res. 2010;89(12):1383–1388.
- Takahashi N. Oral Microbiome Metabolism: from “Who Are They?” to “What Are They Doing?”. J Dent Res. 2015;94(12):1628–1637.
- Brown SA, Whiteley M. A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans. J Bacteriol. 2007;189(17):6407–6414.
- Zeng L, Das S, Burne RA. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J Bacteriol. 2010;192(9):2434–2444.
- Chen YY, Betzenhauser MJ, Snyder JA, et al. Pathways for lactose/galactose catabolism by Streptococcus salivarius. FEMS Microbiol Lett. 2002;209(1):75–79.
- Elbein AD. The metabolism of alpha,alpha-trehalose. Adv Carbohydr Chem Biochem. 1974;30:227–256.
- Iturriaga G, Suarez R, Nova-Franco B. Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci. 2009;10(9):3793–3810.
- Baker JL, Lindsay EL, Faustoferri RC, et al. Characterization of the Trehalose Utilization Operon in Streptococcus mutans Reveals that the TreR Transcriptional Regulator Is Involved in Stress Response Pathways and Toxin Production. J Bacteriol. 2018;200(12). DOI:https://doi.org/10.1128/JB.00057-18
- Nagasawa R, Sato T, Senpuku H. Raffinose Induces Biofilm Formation by Streptococcus mutans in Low Concentrations of Sucrose by Increasing Production of Extracellular DNA and Fructan. Appl Environ Microbiol. 2017;83(15). DOI:https://doi.org/10.1128/AEM.00869-17
- Hachem MA, Fredslund F, Andersen JM, et al. Raffinose family oligosaccharide utilisation by probiotic bacteria: insight into substrate recognition, molecular architecture and diversity of GH36 α-galactosidases. Biocatalysis and Biotransformation. 2012;30(3):316–325.
- Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23(4):473–480.
- Cugini C, Klepac-Ceraj V, Rackaityte E, et al. Porphyromonas gingivalis : keeping the pathos out of the biont. J Oral Microbiol. 2013;5(1). DOI:https://doi.org/10.3402/jom.v5i0.19804
- Arimatsu K, Yamada H, Miyazawa H, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4(1):4828.
- Norskov-Lauritsen N, Claesson R, Birkeholm Jensen A, et al. Aggregatibacter Actinomycetemcomitans: clinical Significance of a Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens. 2019;8(4). DOI:https://doi.org/10.3390/pathogens8040243
- Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147.
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a Potential Community Activist for Disease. J Dent Res. 2012;91(9):816–820.
- Kaplan JB, Velliyagounder K, Ragunath C, et al. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol. 2004;186(24):8213–8220.
- Umeda JE, Longo PL, Simionato MR, et al. Differential transcription of virulence genes in Aggregatibacter actinomycetemcomitans serotypes. J Oral Microbiol. 2013;5(1). DOI:https://doi.org/10.3402/jom.v5i0.21473
- Bosse JT, Sinha S, Li MS, et al. Regulation of PGA operon expression and biofilm formation in Actinobacillus pleuropneumoniae by sigmaE and H-NS. J Bacteriol. 2010;192(9):2414–2423.
- Naseem S, Konopka JB. N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens. PLoS Pathog. 2015;11(7):e1004947.
- Konopka JB. N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica (Cairo);2012. DOI:https://doi.org/10.6064/2012/489208 2012
- Duran-Pinedo AE, Yost S, Small F-LJ. RNA Transcriptome of the Oral Microbiome during Periodontitis Progression. Appl Environ Microbiol. 2015;81(19):6688–6699.
- Frias-Lopez J, Duran-Pinedo A. Effect of periodontal pathogens on the metatranscriptome of a healthy multispecies biofilm model. J Bacteriol. 2012;194(8):2082–2095.
- Knudsen GM, Holch A, Gram L. Subinhibitory concentrations of antibiotics affect stress and virulence gene expression in Listeria monocytogenes and cause enhanced stress sensitivity but do not affect Caco-2 cell invasion. J Appl Microbiol. 2012;113(5):1273–1286.
- Liu Q, Zheng Z, Kim W, et al. Influence of subinhibitory concentrations of NH125 on biofilm formation & virulence factors of Staphylococcus aureus. Future Med Chem. 2018;10(11):1319–1331.
- Kastbjerg VG, Larsen MH, Gram L, et al. Influence of sublethal concentrations of common disinfectants on expression of virulence genes in Listeria monocytogenes. Appl Environ Microbiol. 2010;76(1):303–309.
- Szafranski SP, Deng ZL, Tomasch J, et al. Functional biomarkers for chronic periodontitis and insights into the roles of Prevotella nigrescens and Fusobacterium nucleatum; a metatranscriptome analysis. NPJ Biofilms Microbiomes. 2015;1(1):15017.
- Duran-Pinedo AE, Chen T, Teles R, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. Isme J. 2014;8(8):1659–1672.
- Yost S, Duran-Pinedo AE, Teles R, et al. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015;7(1):27.
- Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–183.
- Yost S, Duran-Pinedo AE, Krishnan K, et al. Potassium is a key signal in host-microbiome dysbiosis in periodontitis. PLoS Pathog. 2017;13(6):e1006457.
- Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nature Rev Microbiol. 2018;16(12):745–759.
- Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759.
- Mysak J, Podzimek S, Sommerova P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014:476068.
- Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–374.
- Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11:30.
- Stathopoulou PG, Benakanakere MR, Galicia JC, et al. Epithelial cell pro-inflammatory cytokine response differs across dental plaque bacterial species. J Clin Periodontol. 2010;37(1):24–29.
- Groeger S, Meyle J. Oral Mucosal Epithelial Cells. Front Immunol. 2019;10:208.
- Fukui A, Ohta K, Nishi H, et al. Interleukin-8 and CXCL10 expression in oral keratinocytes and fibroblasts via Toll-like receptors. Microbiol Immunol. 2013;57(3):198–206.
- Goutoudi P, Diza E, Arvanitidou M. Effect of periodontal therapy on crevicular fluid interleukin-6 and interleukin-8 levels in chronic periodontitis. Int J Dent. 2012;2012:362905.
- Schueller K, Riva A, Pfeiffer S, et al. Members of the Oral Microbiota Are Associated with IL-8 Release by Gingival Epithelial Cells in Healthy Individuals. Front Microbiol. 2017;8:416.
- Pollanen MT, Overman DO, Salonen JI. Bacterial metabolites sodium butyrate and propionate inhibit epithelial cell growth in vitro. J Periodontal Res. 1997;32(3):326–334.
- Tsuda H, Ochiai K, Suzuki N, et al. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J Periodontal Res. 2010;45(5):626–634.
- Qiqiang L, Huanxin M, Xuejun G. Longitudinal study of volatile fatty acids in the gingival crevicular fluid of patients with periodontitis before and after nonsurgical therapy. J Periodontal Res. 2012;47(6):740–749.
- Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009;191(22):6804–6811.
- Marsh PD. Contemporary perspective on plaque control. Br Dent J. 2012;212(12):601–606.
