ABSTRACT
Background:Many previous studies have focused on the acetaldehyde produced from ethanol by oral bacteria as a risk factor for oral cancer. Most of these studies involved low ethanol concentrations (ca. 10 mM), but oral bacteria are exposed to a wide range of ethanol concentrations (100–10,000 mM) when alcoholic beverages are consumed. In contrast, ethanol is widely used at high concentrations (> 5,000 mM) as an antiseptic/disinfectant, suggesting that ethanol has bifacial biological effects; i.e. it acts as both a metabolic substrate for bacterial acetaldehyde production and an antimicrobial agent.
Materials and methods:We examined the acetaldehyde production from ethanol by oral streptococci and the effects of ethanol exposure on the growth and viability of these bacteria at a wide range of ethanol concentrations (10–10,000 mM).
Results:Acetaldehyde production was the highest at an ethanol concentration of 2,000 mM (2.1–48-fold higher than that seen at an ethanol concentration of 10 mM). Bacterial growth was inhibited by > 1,000 mM of ethanol, and the bacteria did not seem viable in the presence of > 5,000 mM of ethanol, although they still produced acetaldehyde.
Conclusion:Ethanol has bifacial biological effects, and the concentration ranges of these effects overlap.
Introduction
Alcohol consumption, smoking, chronic mucosal irritation/trauma, and poor oral hygiene are considered risk factors for oral cancer [Citation1–5]. Regarding the carcinogenicity of alcohol, the International Agency for Research on Cancer (IARC) classified alcoholic beverages into group 1 (carcinogenic to humans), since ethanol can be metabolized to acetaldehyde, a strong carcinogen [Citation6–11]. The acetaldehyde present in the oral cavity is derived from alcoholic beverages themselves and their metabolism by the human body or oral bacteria [Citation6]. Recently, acetaldehyde production by oral bacteria has been focused on as a risk factor for oral cancer since the oral mucosa is directly exposed to the acetaldehyde produced in oral biofilms [Citation12–17].
It was reported that indigenous oral bacteria and yeasts, such as Streptococcus, Neisseria, and Candida species, produce acetaldehyde from ethanol [Citation18–23]. Previously, we examined the acetaldehyde production by various oral streptococci at an oral ethanol concentration of 11 mM derived from food and drink and obtained the following findings: (1) the production of acetaldehyde was increased at a neutral to weak alkaline pH under aerobic conditions, (2) there were differences in acetaldehyde production among the streptococcal species, and (3) the production of acetaldehyde from ethanol involves the coupling of the ethanol oxidation reaction of alcohol dehydrogenase (ADH) with the oxidation reaction of NADH by NADH oxidase [Citation24].
The concentration of ethanol in the oral cavity increases immediately after an alcoholic beverage is consumed and then decreases. It was reported after intake of an alcoholic beverage that the concentration of ethanol remaining in the oral cavity decreases gradually, as ethanol flows back into saliva from the blood for a few hours after it is taken into the body [Citation25,Citation26]. Most previous studies of acetaldehyde production by oral bacteria, including our study [Citation24], used ethanol concentrations as low as 11–22 mM (approximately 0.05–0.1%), which corresponds to the ethanol concentrations seen in saliva a few hours after alcohol consumption [Citation20,Citation22,Citation26,Citation27]. However, during real-life alcohol consumption high concentrations of ethanol (5–10% = 1,000–2,000 mM) pass through the oral cavity, and hence, actual oral ethanol concentrations will often be higher than those used in the abovementioned studies. Therefore, it is necessary to assess the ability of oral bacteria to produce acetaldehyde at a range of ethanol concentrations.
On the other hand, ethanol is widely used as a disinfectant, as it is known to have bactericidal effects; i.e. it affects bacterial proliferation and viability [Citation28–31]. However, it is unclear whether acetaldehyde is produced from ethanol by bacteria in the presence of the high concentrations of ethanol (≥5,000 mM) used in mouthwash and disinfectants [Citation32–35].
In the present study, we investigated the production of acetaldehyde from ethanol by indigenous oral streptococci at a wide range of ethanol concentrations, from moderate ethanol concentrations similar to those seen after the consumption of alcohol beverages to high ethanol concentrations similar to those used in mouthwash or disinfectants. The effects of these concentrations of ethanol on the proliferation and survival of oral streptococci were also examined. Through these experiments, we attempted to clarify the details of acetaldehyde production from ethanol by oral streptococci and to show the bifacial biological effects of ethanol; i.e. that it has antibacterial effects but also acts as a substrate for bacterial acetaldehyde production.
Materials and methods
Bacterial strains and growth conditions
In this study, Streptococcus mitis (JCM 12971), Streptococcus mutans (NCTC 10449), Streptococcus salivarius (JCM 5707), Streptococcus gordonii (JCM 12995), and Streptococcus sanguinis (ATCC 10556) were used. The strains were grown and maintained on blood agar plates (CDC anaerobe 5% sheep blood agar, BD Japan, Japan) and were stored at 4°C in air. The strains were cultured in TYG medium containing 1.7% tryptone, 0.3% yeast extract, 0.5% NaCl, 50 mM potassium phosphate buffer (PPB) [pH 7.0], and 0.5% glucose at 37°C, and then 400 μL of the culture medium was transferred to new TYG medium (40 mL) and the strains were incubated further at 37°C.
Assessment of bacterial population doubling times to estimate inhibitory effects of ethanol on bacterial growth
The bacterial stains were grown as described above until the logarithmic growth phase, and then each bacterial culture was divided into six cultures, and ethanol was added to each culture at a final ethanol concentration of 0 mM [0%], 10 mM [0.0465%], 100 mM [0.465%], 500 mM [2.33%], 1,000 mM [4.65%], or 2,000 mM [9.3%]. The cultures were continuously incubated at 37°C until the growth stopped. Bacterial growth was estimated based on the optical density (OD) of each culture at a wavelength of 660 nm, and population doubling times were calculated from the OD values. When the doubling time was ≥ 3.5 h, it was considered that no growth was occurring.
Bacterial acetaldehyde production
In the logarithmic growth phase, the bacterial cells were harvested by centrifugation (10,000 rpm for 7 min. at 4°C) and washed three times with washing buffer (2 mM PPB [pH 7] containing 75 mM KCl, 5 mM MgCl2, and 75 mM NaCl), before being suspended in the same buffer. The bacterial cell concentrations of the suspensions were adjusted based on their OD at a wavelength of 660 nm (to an OD of 10).
The reaction mixture used for the assessment of bacterial acetaldehyde production (1,000 μL) contained 200 μL of the bacterial cell suspension, 0–486 μL of ethanol as a substrate (final concentration: 0 mM [0%], 10 mM [0.0465%], 100 mM [0.465%], 1,000 mM [4.65%], 2,000 mM [9.3%], 5,000 mM [23.3%], or 10,000 mM [46.5%]), and 50 μL of PPB (pH 7.0) in washing buffer. Each reaction mixture was kept in a tube with a silicone cap, and the tube was completely closed off. The reaction was started by adding ethanol, and the reaction mixture was incubated at 37°C for 30 min. The reaction was stopped by injecting 100 μL of 7 M phosphoric acid using a sterile needle (27 G × 3/4 Terumo injection needle, Terumo Corporation, Japan) and syringe (1 mL Terumo syringe for tuberculin, Terumo Corporation, Japan) through the silicone cap, and the mixture was shaken vigorously. The headspace gas of the tube was collected using a gas-tight syringe (10 mL Norm-Ject; Henke-Sass, Wolf; Tuttlingen, Germany). Then, the concentration of acetaldehyde was measured using a sensor gas chromatograph (SGEA-P2, FIS Inc., Japan). Since this gas chromatograph can precisely measure acetaldehyde concentrations ranging from 5 to 10,000 ppb, the samples were diluted with pure nitrogen gas, if necessary [Citation24]. No acetaldehyde production was detected in control samples containing ethanol without bacterial cells.
Bacterial viability after incubation with ethanol
The reaction mixture used to examine bacterial viability was prepared in the same way as that used to assess acetaldehyde production. After the reaction mixture had been incubated with ethanol at 37 °C for 5, 10, or 20 min. (5,000 mM or 10,000 mM of ethanol) or 30 min. (all ethanol concentrations), it was spread on TYG agar medium (containing 1.7% tryptone, 0.3% yeast extract, 0.5% NaCl, 50 mM PPB [pH 7.0], and 0.5% glucose) and incubated at 37 °C for three days. Then for the reaction mixtures with 5,000 mM and 10,000 mM of ethanol, the number of colony-forming units (CFU) that developed on each culture was determined.
Statistical analysis
In the statistical analysis, the significance of differences among groups was analyzed using Tukey’s test, and p-values of < 0.05 were considered statistically significant (StatFlex Ver. 6).
Results
The effects of ethanol exposure on bacterial growth
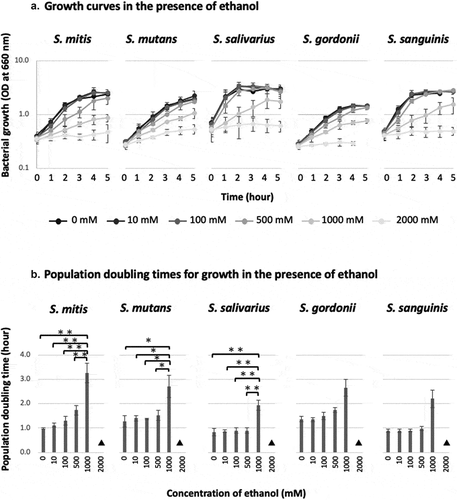
In all five strains, ethanol inhibited the growth of the bacteria and increased the population doubling time. The bacteria failed to grow at ethanol concentrations of ≥ 2,000 mM. However, the inhibitory effect of ethanol on bacterial growth varied with the ethanol concentration and the bacterial strain. In S. mutans, S. salivarius, and S. sanguinis, the addition of 0–500 mM of ethanol did not inhibit bacterial growth, whereas the addition of 1,000 mM of ethanol caused the population doubling time to increase by more than two-fold; i.e. it markedly inhibited bacterial growth. On the other hand, in S. mitis and S. gordonii, the population doubling time gradually increased at ethanol concentrations of 0–500 mM and was markedly increased at an ethanol concentration of 1,000 mM. In S. mitis, S. mutans, and S. salivarius, significant differences in the population doubling time were seen between ethanol concentrations of 1,000 mM and 0–500 mM ().
Figure 1. Population doubling times and growth curves of oral Streptococcus species in the presence of ethanol
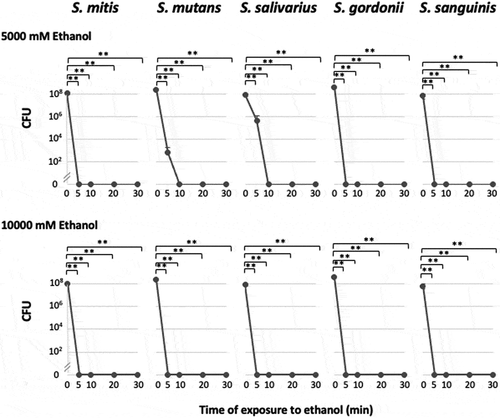
Bacterial viability after incubation with ethanol
The viability of the bacteria after they had been incubated for 30 min. in the presence of ethanol (0, 10, 100, 1,000, or 2,000 mM) was confirmed by observing bacterial colonies on TYG agar medium. All the tested bacterial strains survived in the presence of 0–2,000 mM of ethanol. When the bacteria were incubated with 5,000 mM of ethanol for 5 min., only S. mutans and S. salivarius continued to grow; however, the numbers of CFU decreased significantly to < 1% of the control value. When the other strains were incubated with 5,000 or 10,000 mM of ethanol, they lost the ability to grow or exhibited significantly reduced survival ().
Acetaldehyde production from various concentrations of ethanol
In all strains, acetaldehyde production tended to increase with the ethanol concentration at ethanol concentrations of 0–2,000 mM and peaked at an ethanol concentration of 2,000 mM. Furthermore, when the ethanol concentration was increased to 5,000 or 10,000 mM, the acetaldehyde production tended to decrease (). S. mitis and S. salivarius exhibited high maximum aldehyde production (Vmax) values, while S. mutans, S. gordonii, and S. sanguinis demonstrated low Vmax values.
Figure 3. Acetaldehyde production from ethanol by oral Streptococcus species
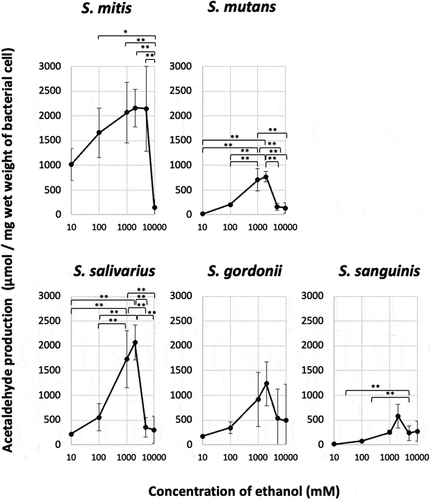
The ethanol concentration-dependent changes in acetaldehyde production were assessed by calculating the ethanol concentration that resulted in an acetaldehyde production value of half of the maximum acetaldehyde production value (C1/2). C1/2 varied among the strains. The obtained C1/2 values were 38.8 mM for S. mitis; 478.5–605.0 mM for S. mutans, S. salivarius, and S. gordonii; and 964.5 mM for S. sanguinis (). The C1/2 values of S. mitis and S. sanguinis were significantly different. S. mitis produced large amounts of acetaldehyde, even at low ethanol concentrations; and, unlike the other strains, S. mitis exhibited almost maximal levels of acetaldehyde production in the presence of 5,000 mM of ethanol ().
Table 1. Maximum acetaldehyde production (Vmax) and the concentration of ethanol at which the production was half of the maximum acetaldehyde production (C1/2)
Discussion
The presence of 1,000 mM of ethanol inhibited the growth of oral streptococci (), and incubating these bacteria with > 5,000 mM of ethanol for 10 min. seemed to abolish their viability (), indicating that concentrated ethanol is bacteriostatic and bactericidal, as is well known [Citation29,Citation30,Citation32] ().
Figure 4. Bifacial biological effects of ethanol
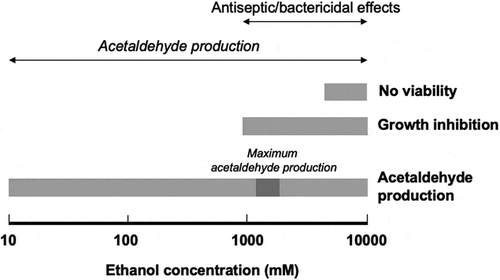
However, this study revealed that oral streptococci produce acetaldehyde at a wide range of ethanol concentrations, with maximal acetaldehyde production occurring at an ethanol concentration of 2,000 mM and some acetaldehyde production being seen at ethanol concentrations of 5,000–10,000 mM (); i.e. at concentrations that have bacteriostatic () and bactericidal () effects on oral streptococci. These observations demonstrated that ethanol has antiseptic/bactericidal effects and acts as a metabolic substrate for acetaldehyde (a carcinogen) production by oral streptococci and that the concentration ranges for these effects overlap (). These phenomena might have been due to the fact that ethanol increases the permeability of cell membranes. Flores et al. [Citation36] showed that when 10% (2,150 mM) ethanol was added to the reaction mixture, intracellular β-galactosidase activity could be detected in intact yeast (Kluyveromyces lactis) cells, indicating that the ethanol had permeabilized the cell membrane, allowing enzyme substrates to cross the cell membrane and intracellular enzyme reactions to occur in situ. Similar phenomena may occur with oral streptococci [Citation37].
The ethanol concentrations in alcoholic beverages vary from approximately 1,000 mM (4.65%) to 10,000 mM (46.5%), and it is known that immediately after the consumption of such beverages the concentration of ethanol in the oral cavity decreases to ≤ 100 mM (0.465%) due to dilution and rinsing with saliva, and it falls to approximately 10 mM (0.047%) after 2 or 3 hours [Citation25]. Therefore, bacteriostatic and bactericidal effects cannot be expected at the ethanol concentrations produced by alcohol drinking, while it is considered that carcinogenic acetaldehyde is produced from ethanol by bacteria at these concentrations. Lachenmeier [Citation38] suggested that the topical application of high concentrations of ethanol to the skin and oral cavity may induce acetaldehyde production by oral bacteria. Our findings support this possibility. Furthermore, Lachenmeier argued that the oral mucosa may be another source of acetaldehyde production because mucosal cells also exhibit ADH activity [Citation38]. This may represent as a ‘trifacial function’ of ethanol in the oral cavity, and further studies of this are needed.
In this study, acetaldehyde production was measured at low to high concentrations of ethanol (0–10,000 mM), and the maximum acetaldehyde production (Vmax) and C1/2 were determined (). The strains used in this study were divided into those with low C1/2 values (S. mitis: < 40 mM), which produced relatively large amounts of acetaldehyde from low concentrations of ethanol; those with moderate C1/2 values (S. salivarius, S. gordonii, and S. mutans: 400–600 mM); and those with high C1/2 values (S. sanguinis: > 900 mM). A previous study found that in the presence of 11 mM (approximately 0.05%) of ethanol S. mitis produced the greatest amount of acetaldehyde, and S. mutans and S. sanguinis produced extremely small amounts of acetaldehyde; however, these findings may have been largely due to differences among the C1/2 values of each strain. In other words, while S. mitis can produce a large amount of acetaldehyde from a low concentration of ethanol because of its low C1/2 value, S. mutans and S. sanguinis can only produce acetaldehyde when the ethanol concentration is high because of their high C1/2 values. The Vmax values of S. mutans and S. sanguinis were about 1/3 to 1/4 of that of S. mitis (), and the contributions of these strains to acetaldehyde production at high ethanol concentrations are estimated to be high. Thus, it is suggested that at the low concentrations of ethanol (10 mM) seen a few hours after alcohol consumption, acetaldehyde production in the oral cavity is mainly due to S. mitis, with some contribution from S. salivarius and S. gordonii. On the other hand, in addition to S. mitis, S. salivarius, and S. gordonii, S. mutans and S. sanguinis seem to be involved in acetaldehyde production in the oral cavity right after an alcoholic drink with a moderate ethanol concentration (1,000–2,000 mM) is consumed. These results suggest that an ethanol environment is maintained in the oral cavity when alcohol is consumed over a long period of time, resulting in the production of more acetaldehyde by more bacterial species, thereby increasing the risk of cancer.
As mentioned above, ADH is involved in the production of acetaldehyde from ethanol [Citation20,Citation24,Citation39,Citation40]. Kurkivuori et al. [Citation20] reported that the Km value for ADH in S. mitis is 758.3 mM, while the Km value for ADH in S. salivarius is 1.2 mM, which are not consistent with the C1/2 values calculated for these bacteria in the present study. Thus, the production of acetaldehyde by oral streptococci during ethanol metabolism may also be related to factors other than ADH activity, such as the efficiency of ethanol uptake into cells and NADH oxidase activity.
Conclusion
We demonstrated that oral streptococcal species produced acetaldehyde from ethanol at a wide range of ethanol concentrations. S. mitis can produce large amounts of acetaldehyde at low ethanol concentrations, while S. mutans and S. salivarius, which produced relatively low amounts of acetaldehyde in a previous study, can produce acetaldehyde only when the ethanol concentration is high. Since ethanol concentrations of 1,000–2,000 mM, which can be achieved by drinking alcohol, had bacteriostatic effects, and ethanol concentrations of 5,000–10,000 mM, which can be found in disinfectants, had bactericidal effects, and these concentrations of ethanol resulted in significant amounts of acetaldehyde being produced, it was concluded that ethanol has bifacial biological effects, and that the concentration ranges of these effects overlap.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–8.
- Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control. 2007 Nov;18(9):919–929.
- Mathur R, Singhavi HR, Malik A, et al. Role of poor oral hygiene in causation of oral cancer—a review of literature. Indian J Surg Oncol. 2019 Mar;10(1):184–195.
- Singhvi HR. Malik A and Chaturvedi P. The role of chronic mucosal trauma in oral cancer: a review of literature. Indian J Med Paediatr Oncol. 2017 Jan-Mar;38(1):44–50.
- Gupta B, Bray F, Kumar N, et al. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case–control study from India. Cancer Epidemiol. 2017 Dec;51:7–14.
- Stornetta A, Guidolin V, Alcohol-Derived Acetaldehyde BS. Exposure in the oral cavity. Cancers (Basel). 2018 Jan;10(1):20.
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to human. IARC. 2014. Internal Report 14/002.
- Mizumoto A, Ohashi S, Hirohashi K, et al. Molecular mechanisms of acetaldehyde-mediated carcinogenesis in squamous epithelium. Int J Mol Sci. 2017 Sep;18(9):1943.
- Kayani MA, Parry JM. The in vitro genotoxicity of ethanol and acetaldehyde. Toxicol In Vitro. 2010 Feb;24(1):56–60.
- Soffritti M, Belpoggi F, Lambertin L, et al. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in rats. Ann N Y Acad Sci. 2002 Dec;982(1):87–105.
- Garaycoechea JI, Crossan GP, Langevin F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. nature. 2018 Jan;553(7687):171–177.
- Homann N, Tillonen J, Rintamäki H, et al. Poor dental status increases acetaldehyde production from ethanol in saliva: a possible link to increased oral cancer risk among heavy drinkers. Oral Oncol. 2001 Feb;37(2):153–158.
- Chang JS, Lo H, Wong T, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013 Oct;48(10):1010–1017.
- Linderborg K, Salaspuro M, Väkeväinen S, et al. A single sip of a strong alcoholic beverage causes exposure to carcinogenic concentrations of acetaldehyde in the oral cavity. Food Chem Toxicol. 2011 Sep;49(9):2103–2106.
- Lachenmeier DW, Monakhova YB. Short-term salivary acetaldehyde increases due to direct exposure to alcoholic beverages as an additional cancer risk factor beyond ethanol metabolism. J Exp Clin Cancer Res. 2011 Jan;30(1):3.
- Perera M, Al-Hebshi NN, Perera I, et al. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J Dent Res. 2018 Jun;97(6):725–732.
- O’Grady I, Anderson A, O’Sullivan J, et al. The interplay of the oral microbiome and alcohol consumption in oral squamous cell carcinomas. Oral Oncol. 2020Nov;110:105011.
- Moritani K, Takeshita T, Shibata Y, et al. Acetaldehyde production by major oral microbes. Oral Dis. 2015 Sep;21(6):748–754.
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005 Nov;43(11):5721–5732.
- Kukivuori J, Salaspuro V, Kaihovaara P, et al. Acetaldehyde production from ethanol by oral streptococci. Oral Oncol. 2007 Feb;43(2):181–186.
- Tillonen J, Homann N, Rautio M, et al. Role of yeasts in the salivary acetaldehyde production from ethanol among risk groups for ethanol-associated oral cavity cancer. Alcohol Clin Exp Res. 1999 Aug;23(8):1409–1415.
- Gainza-Cirauqui ML, Nieminen MT, Frazer LN, et al. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J Oral Pathol Med. 2013 Mar;42(3):243–249.
- Alnuaimi AD, Wiesenfeld D, O’Brien-Simpson NM, et al. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oral Oncol. 2015 Feb;51(2):139–145.
- Tagaino R, Washio J, Abiko Y, et al. Metabolic property of acetaldehyde production from ethanol and glucose by oral Streptococcus and Neisseria. Sci Rep. 2019 Jul;9(1):10446.
- Yokoyama A, Tsutsumi E, Imazeki H, et al. Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydrogenase-2 genotype. Alcohol Clin Exp Res. 2008 Sep;32(9):1607–1614.
- Mackus M, Loo AJV, Garssen J, et al. The role of alcohol metabolism in the pathology of alcohol hangover. J Clin Med. 2020 Oct;9(11):3421.
- Alnuaimi AD, Ramdzan AN, Wiesenfeld D, et al. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016 Nov;22(8):805–814.
- Moorer WR. Antiviral activity of alcohol for surface disinfection. Int J Dent Hyg. 2003 Aug;1(3):138–142.
- Park HS, Ham Y, Shin K, et al. Sanitizing effect of ethanol against biofilms formed by three gram-negative pathogenic bacteria. Curr Microbiol. 2015 Jul;71(1):70–75.
- Duarte PHM, Da Silva PB, Rosa RAD, et al. Effect of ethanol on the antimicrobial properties of chlorhexidine over oral biofilm. Microsc Res Tech. 2018 Apr;81(4):408–412.
- Gold NA, Mirza TM and Avva U. Alcohol Sanitizer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan.
- Reidy J. McHugh E and Stassen LFA. A review of the relationship between alcohol and oral cancer. Surgeon. 2011 Oct;9(5):278–283.
- La Vecchia C. Mouthwash and oral cancer risk: An update. Oral Oncol. 2009 Mar;45(3):198–200.
- Ustrell-Borràs M, Traboulsi-Garet B, Gay-Escoda C. Alcohol-based mouthwash as a risk factor of oral cancer: a systematic review. Med Oral Patol Oral Cir Bucal. 2020 Jan;25(1):e1–e12.
- Gandini S, Negri E, Boffetta P, et al. Mouthwash and oral cancer risk - Quantitative meta-analysis of epidemiologic studies. Ann Agric Environ Med. 2012;19(2):173–180.
- Flores MV. Voget CE and Ertola RJ. Permeabilization of yeast cells (Kluyveromyces lactis) with organic solvents. Enzyme Microb Technol. 1994;16:340–346.
- Iwami Y, Yamada T. Regulation of glycolytic rate in streptococcus sanguis grown under glucose-limited and glucose-excess conditions in a chemostat. Infect Immun. 1985;50(2):378–381.
- Lachenmeier DW. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol. 2008;3:26.
- Pavlova SI, Jin L, Gasparovich, et al. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology (Reading). 2013 Jul;159(Pt 7):1437–1446.
- Fukui K, Kato K, Kodama T, et al. Kinetic study of a change in intracellular ATP level associated with aerobic catabolism of ethanol by streptococcus mutans. J Bacteriol. 1988 Oct;170(10):4589–4593.