ABSTRACT
Background: Hydrogen sulfide(H2S) is a bacterial metabolite produced as a result of bacterial growth in subgingival pockets, suggested to partake in the pathogenesis of periodontitis. H2S has previously been shown to induce the secretion of the pro-inflammatory cytokines IL-1β and IL-18 via the NLRP3 inflammasome in monocytes.
Objective: To investigate the non-NLRP3 inflammasome-dependent immunological response of human peripheral blood mononuclear cells (PBMCs) of periodontitis patients and healthy controls exposed to H2S in vitro.
Methods: PBMCs of periodontitis patients(N = 31) and healthy controls(N = 32) were exposed to 1 mM sodium hydrosulfide (NaHS) at 37°C for 24 h and the secretion of cytokines was compared to resting cells. TNF-α, IFN-γ, IL-6, IL-8, IL-12p40, IL-12p70, IL-17, MCP-1, and IL-1Ra secretions were measured with Bio-Plex Pro™ Human Cytokine Assay.
Results: H2S triggered the secretion of the pro-inflammatory IFN-γ, IL-6, IL-17, TNF-α, IL-12p40, and IL-12p70, while the reverse was seen for IL-1Ra. In addition, a higher basal secretion of IFN-γ, IL-6, IL-12p70, IL-17 and MCP-1 was seen from PBMCs of periodontitis patients compared to healthy controls.
Conclusion: The bacterial metabolite H2S triggers the secretion of pro-inflammatory cytokines from PBMCs and may thus have a prominent role in the host-bacteria interplay in periodontitis.
Introduction
Periodontitis is an inflammatory disease affecting the supporting tissues of teeth. Bacteria are essential but not sufficient components in the disease etiology. The way the tissue responds to the bacterial load (host susceptibility) is comparably important for the disease development [Citation1]. According to the ecological plaque hypothesis, the disease develops due to an imbalance (dysbiosis) between the bacteria and the host, resulting in a pathological tissue response with loss of periodontal attachment, alveolar bone, and subsequently loss of teeth [Citation2]. Certain anaerobic, proteolytic, and Gram-negative bacterial species have been attributed an important role in disease development [Citation3]. These bacteria are ordinary commensals that adapt and grow in the subgingival environment, favored by the elevated access to proteins, peptides, and amino acids from the gingival crevicular fluid, and the deepening of the periodontal pocket for retention and reduction of the redox-potential. Thus, these microorganisms have, according to the current paradigm, adapted to this ecological niche created due to certain environmental changes, involving anaerobiosis and proteolytic activity, that occur in the periodontal pocket during inflammation [Citation4]. Yet, the same pathogens may also be present in healthy subjects in small numbers and the mechanisms involved in the host-bacteria interaction and pathogenesis of periodontal disease is still not fully determined [Citation1].
It has previously been claimed that it is the activity of the bacteria that is of importance for initiation and progression of periodontitis and other oral diseases, and not necessarily the biofilm composition per se [Citation5,Citation6]. Thus, it is the net effect of all bacteria collectively, usually in a biofilm, that is of significance in the host-bacteria interplay, which determines the well-being of the periodontium. The bacterial metabolites are the end products of bacterial growth and hydrogen sulfide (H2S) is such a metabolite suggested to partake in the pathogenesis of periodontitis [Citation7]. During bacterial growth in the periodontal pocket the amino acid cysteine is degraded by bacterial enzymes and H2S is, among other substances, formed [Citation8–10]. H2S is a small volatile sulfur compound that can brake disulfide bonds, and pass through cell membranes [Citation11]. It was previously known for its environmental toxicity but has in recent years been shown to, mainly depending on the concentration, have several both pro- and anti-inflammatory effects on various host cells [Citation12–14]. The cleavage of disulfide bonds has been suggested to be involved in the inhibition of antibody-mediated immune responses by reduced binding to cell surface antigens [Citation15]. It has also been shown that bacteria are more resistant to leukocyte-mediated killing in the presence of elevated H2S levels, but the detailed mechanisms of this protection are not known [Citation16]. Apart from bacterial (exogenous) production, H2S is also endogenously produced by the human body and functions as an signaling molecule, both in the brain and at other locations where it can regulate blood pressure, among many other functions [Citation14,Citation17].
We have in a previous in vitro study shown that H2S can induce the formation of the multiprotein complex NLRP3 inflammasome and the following secretion of IL-1β and IL-18 in human peripheral blood mononuclear cells (PBMCs) and in the human monocyte cell line THP1 [Citation7]. Furthermore, PBMCs of patients with periodontitis have been shown to secrete higher levels of IL-1β and IL-18 compared to healthy controls, both resting cells and cells exposed to H2S. These results have led us to hypothesize that H2S-producing microorganisms in the subgingival pocket trigger an inflammatory host response by the secretion of pro-inflammatory cytokines and that the level of this secretion may be indicative of the susceptibility of the host to periodontitis.
Aim
The aim of this study was to investigate the non-NLRP3 inflammasome-dependent immunological response to H2S by studying PBMCs of periodontitis patients and healthy controls exposed to a H2S donor in vitro.
Material and methods
Collection of blood samples
This study was approved by the Regional Ethical Review Board (Dnr 871–15) in Gothenburg, Sweden. Detailed information and clinical data of the examined subjects have previously been published [Citation18]. Briefly, the patients diagnosed with periodontitis were examined and recruited at a specialist clinic, Department of Periodontology, Södra Älvsborgs Hospital, Borås, Sweden. Of these, 66% were men with a mean age of 54 years. They had to have at least 14 teeth remaining to be included but presented a mean of 25 teeth remaining, with a mean BoP of 49%. Of the 32 subjects examined, 91% had at least one pocket with a PPD of 7 mm or more. The subjects in the healthy control group were volunteers, staff members at the dental clinics or research personnel, examined and recruited at the Institute of Odontology, University of Gothenburg, Gothenburg, Sweden. In this group there was an equal distribution regarding the gender. These subjects had almost all their teeth remaining, and they had in general a healthy gingiva and no clinical signs of attachment loss due to periodontitis with a mean BoP of 19%. In total 64 subjects were examined and 63 agreed to have peripheral blood samples taken, that were harvested at the examination visit. In the periodontitis group 28% were smokers while none of the subjects in the healthy group smoked. Snuff users were equally represented in both groups. Medical diseases such as high blood pressure and diabetes were reported in both groups but were more common in the periodontitis group [Citation18].
Isolation of cells from peripheral blood
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Paque™ Plus density gradients (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and exposed to a hydrogen sulfide generator as previously described [Citation7,Citation18]. In brief, after a washing step in PBS, the cells were resuspended in Dulbecco’s Modified Eagle Medium + GlutaMAXTM (Gibco, Life Technologies, Paisley, UK) supplemented with 5% human serum (Sigma–Aldrich Sweden AB, Stockholm, Sweden) and penicillin–streptomycin (Invitrogen, Lidingö, Sweden). The cells were seeded, 2 × 106 cells/ml, and cultured in 96-well microtiter plates at 37°C (humidified atmosphere, 5% CO2), in the presence or absence of 1 mM sodium hydrosulfide (NaHS; Fisher Scientific). This relatively high concentration was chosen since a concentration of up to 1.9 mM H2S previously has been measured from gingival crevicular fluid of deep periodontal pockets [Citation8]. The culture supernatants were collected after 24 h and stored at −80°C until further use.
Detection of cytokines
The cytokine expressions were measured using a combination of a custom-made premixed x-Plex assay for detection of TNF-α, IFN- γ, IL-6, IL-8, IL-12p70, IL-17, MCP-1, and IL-1R, and a manually added single plex set for detection of IL-12p40 (Bio-Plex Express Assay, Bio-Rad Laboratories, Hemel Hempstead, UK), using Luminex xMAP technology according to the manufacturer’s instructions.
Briefly, all samples were incubated with sets of distinctly color-coded beads conjugated with capture antibodies directed against a specific analyte. After washing, a biotinylated detection antibody was added and allowed to react with the bound proteins. After another washing step, streptavidin conjugated to the fluorescent indicator phycoerythrin was added. Then, the final washing step was followed by acquisition of data using a BioPlex 200 instrument equipped with BioManager analysis software (BioRad). The absolute concentrations of the cytokines were determined by comparing the bead color and mean fluorescence intensity from each set of beads against an automatically optimized and manually verified standard curve. The cytokine concentration was presented as pg/mL.
Statistical analyses
Statistics were performed using the GraphPad Prism version 7.0 software (GraphPad Inc., La Jolla, CA, USA). The non-parametric Mann–Whitney U-test and Wilcoxon matched-pairs signed-rank test were used for unpaired and paired samples, respectively. A p-value <0.05 was considered statistically significant; not significant (ns), p< 0.05*, p< 0.01**, p< 0.001***, p< 0.0001****.
Results
Cytokine secretion by resting PBMCs from periodontitis patients and healthy controls
The basal secretion of all cytokines tested is seen in and . Resting PBMCs from patients with periodontitis secreted more IFN- γ, IL-6, IL-12p70, IL-17 and MCP-1 compared to healthy subjects. This difference was statistically significant. Regarding IL-12p70, resting PBMCs from many of the subjects in both groups secreted undetectable levels. The secretion of IL-8 was statistically significantly lower from PBMCs of periodontitis patients compared to cells from healthy controls but also here many samples from both groups were below detection limits. There was no difference in secretion of IL-12p40, TNF-α, and IL-1Ra between the two groups. IL-12p40 was, however, not detected in any of samples from healthy subjects and in only a few samples from subjects with periodontitis.
Table 1. The median secretion (pg/mL) of the examined markers
Figure 1. Secretion of cytokines by PBMCs. The secretions of IFN- γ, IL-6, IL-17, TNF-α, IL-12p40, IL-12p70, MCP-1, IL-8, and IL-1Ra of resting PBMCs and PBMCs exposed to NaHS from patients with periodontitis and healthy controls. The median is shown as a vertical line (IFN- γ: 1.20 and 1.39 for healthy, 1.45 and 1.69 for periodontitis. IL-6: 1.73 and 2.51 for healthy, 2.18 and 2.73 for periodontitis. IL-17: 0 and 0 for healthy, 0.02 and 0.27 for periodontitis TNF-α: 1.75 and 1.98 for healthy, 1.98 and 2.15 for periodontitis. IL-12p40: 0 and 0 for healthy, 0 and 0 for periodontitis. IL-12p70: 0 and 0 for healthy, 0 and 0 for periodontitis. MCP-1: 2.28 and 2.60 for healthy, 2.78 and 2.76 for periodontitis. IL-8: 3.83 and 3.78 for healthy, 0 and 0 for periodontitis. IL-1Ra: 2.88 and 2.59 for healthy, 2.87 and 2.81 for periodontitis)
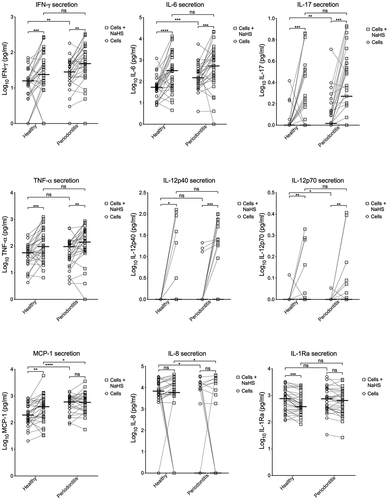
Differences in cytokine secretion by resting PBMCs and PBMCs exposed to H2S
Exposure of PBMCs to H2S triggered statistically significant higher levels of IFN-γ, IL-6, IL-17, and TNF-α secretion, both in the healthy group and in the periodontitis group. A higher secretion by PBMCs exposed to H2S was also seen for IL-12p40 and IL-12p70, but many of the subjects showed no detectable secretion, especially by resting cells. The secretion of MCP-1 was higher when the PBMCs were exposed to H2S in the healthy group while no statistical difference was seen for cells from patients with periodontitis. IL-1Ra showed a lower secretion by exposed PBMCs compared to resting cells in both groups, but the difference was statistically significant only for the healthy group. The secretion of IL-8 was not influenced by exposure to H2S in any of the two groups.
Cytokine secretion by PBMCs from periodontitis patients and healthy controls exposed to H2S
PBMCs from patients with periodontitis exposed to H2S secreted statistically significant more MCP-1 compared to healthy controls. The reverse was seen for IL-8, since many of the subjects in the periodontitis group had no detectable secretion. As with resting cells, there was no difference in secretion of IL-12p40, TNF-α, and IL-1Ra between the two groups. Additionally, no statistically significant differences were detected for IFN-γ, IL-6, IL-12p70, and IL-17 for PBMCs exposed to H2S.
Discussion
The main finding of the present study was that H2S stimulated the secretion of IFN-γ, IL-6, IL-17, TNF-α, IL-12p40, and IL-12p70, from PBMCs of both periodontitis patients and healthy controls, while the reverse was seen for IL-1Ra, that is, H2S decreased the secretion. In addition, a difference in secretion of the majority of the examined cytokines by resting (in the absence of H2S) PBMCs of periodontitis patients compared to healthy controls was seen, where higher levels were detected from cells from patients with periodontitis. These results are in line with the results of IL-1β and IL-18 secretion previously investigated in the same cohort [Citation18]. In that study, the capacity of the subgingival microbiota to produce H2S was also examined and was shown to be positively correlated to bleeding on probing scores of the patients.
Of the nine proteins measured, the ones triggered by H2S, that is, IFN- γ, IL-6, IL-17, TNF-α, IL-12p40, and IL-12p70, are all considered to be part of a pro-inflammatory panel of inflammatory mediators [Citation19]. Together with IL-1β, TNF-α has been extensively studied in relation to periodontitis as a pro-inflammatory and immunomodulatory cytokine [Citation19–21]. IL-12 is generally known for its participation in the differentiation of naïve T-cells to Th1 cells while it has, in relation to periodontitis, been reported to have both pro- and anti-inflammatory properties [Citation22]. Similarly, also IL-6 has been shown to have both pro- and anti-inflammatory properties [Citation23]. IL-6 increases the production of acute-phase proteins in the liver, and has also been linked to bone resorption in periodontitis [Citation20]. In a systematic review and meta-analysis, it was reported that the levels of IL-1β, IFN- γ, MCP-1, and IL-6 in gingival crevicular fluid of periodontitis patients were higher than in healthy individuals, while no differences were observed for IL-12 and IL-17 [Citation21]. Further, it was reported that the levels of IL-1β and IL-17 significantly decreased after periodontal treatment, while another systemic review reported reduced levels of, among others, IL-1β, IL-6, TNF-α, IFN- γ, and MCP-1, six to eight weeks after periodontal treatment [Citation24]. Thus, the inflammatory mediators measured in our study have previously been linked to periodontitis. The potential role of the bacterial metabolite H2S as a critical initiator and maintainer of this host response is, however, a novelty. Collectively, the measurement of several cytokines conducted in the present study shows a distinct pro-inflammatory pattern from PBMCs exposed to H2S, while the anti-inflammatory IL-1Ra (IL-1 receptor antagonist) which binds to the same receptor as IL-1β, is decreased [Citation25].
A higher basal secretion from PBMCs of patients with periodontitis compared to healthy controls was in the present study seen for IFN- γ, IL-6, IL-12p70, IL-17, and MCP-1, showing some similarities with the results reported from measurements in gingival crevicular fluid in the aforementioned systematic review and meta-analysis that compared periodontitis patients and healthy controls [Citation21]. Also, a statistically significantly higher secretion by resting PBMCs of the present cohort was seen for IL-1β in patients with periodontitis while there was no difference between periodontitis patients and healthy controls regarding the acute-phase protein CRP, as previously reported [Citation18]. A lack of difference regarding the CRP levels has also been reported by other studies, while the more sensitive serum amyloid A has been shown to be detected in higher levels in periodontitis patients compared to healthy controls [Citation26,Citation27]. Taken together, the data suggests that a higher basal secretion of pro-inflammatory mediators in periodontitis-susceptible individuals may be the result of a systemic inflammatory process.
In periodontitis, the host response to the colonizing bacteria is of pivotal importance for disease progression, especially considering the fact that the various bacterial species harboring the subgingival pocket are commensals [Citation1]. Thus, all individuals are colonized with various oral bacterial species that can adapt to a subgingival environment and that have the capacity to produce H2S [Citation9,Citation10,Citation28]. Nevertheless, only a fraction of approximately 10% develops severe periodontitis, while other individuals never proceed from gingivitis [Citation29]. Although, H2S can trigger the secretion of pro-inflammatory cytokines from both PBMCs of periodontitis patients and healthy controls, the differences between these two groups of patients, that is, the secretion of MCP-1 and IL-8, may be indicative of differences in host response between the periodontitis susceptible and periodontitis unaffected subjects. MCP-1 was secreted in higher levels from PBMCs of periodontitis patients, while the reverse was seen for IL-8. Both proteins are chemokines, MCP-1 is recruiting monocytes and T lymphocytes, while IL-8 activates neutrophils [Citation19,Citation30]. It should, however, be noted that the secreted IL-8 levels detected in our study were either generally high both from resting and exposed PBMCs in both groups or below the level of detection. The potential meaning of these observations needs to be addressed in future studies.
H2S produced by bacterial growth may not be the only source to its presence in the periodontal pocket. H2S is also endogenously produced and also macrophages possess the enzymes for H2S production [Citation17]. Thus, on one side the presence of H2S in the periodontal pocket may be interpreted as positive for the bacteria in the bacteria-host interplay since H2S can induce the secretion of pro-inflammatory cytokines of the host, dampen leukocyte-mediated killing of bacteria, and promote bacterial resistance to antibiotics [Citation6,Citation16,Citation31]. Consequently, the subgingival bacteria are favored by the presence of H2S. On the other hand, however, are the reports of endogenously produced H2S that in low concentrations is initiating secretion of anti-inflammatory cytokines, and is thus beneficial for the host [Citation17]. It is inarguable this fact, that H2S is recognized as an endogenous substance, that potentially can confuse the host inflammatory response. Accordingly, the in vivo situation in the periodontal pocket is complex and the role of H2S is difficult to interpret. The biphasic properties of H2S trigger the inflammatory response of the host and may simultaneously suppress the clearance of the infection, thus contributing to the never-ending bacteria-host interplay that is characteristic for periodontitis.
Conclusion
The inflammatory host response to bacteria is essential in the pathogenesis of periodontitis. In this study, we report on the effect of the bacterial metabolite H2S on the secretion of inflammatory mediators from mononuclear leukocytes in vitro. Exposure to H2S triggers the secretion of pro-inflammatory cytokines. In addition, some differences in the secretory response of cells from periodontitis patients and healthy controls are reported. Further studies are needed to assess H2S as a potential virulence factor in the pathogenesis of periodontitis.
Acknowledgments
Special thanks to Anna-Karin Östberg for help with the Bio-Plex Pro™ Human Cytokine Assay.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Van Dyke TE, Bartold PM, Reynolds EC. The nexus between periodontal inflammation and dysbiosis. Front Immunol. 2020;11:511.
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(2):279–7.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144.
- Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55(1):16–35.
- Takahashi N. Oral microbiome metabolism: from “who are they?” to “what are they doing?”. J Dent Res. 2015;94(12):1628–1637.
- Dahlen G, Basic A, Bylund J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. J Clin Med. 2019;8(9). DOI:https://doi.org/10.3390/jcm8091339
- Basic A, Alizadehgharib S, Dahlén G, et al. Hydrogen sulfide exposure induces NLRP3 inflammasome-dependent IL-1β and IL-18 secretion in human mononuclear leukocytes in vitro. Clin Exp Dent Res. 2017;3(3):115–120.
- Persson S. Hydrogen sulfide and methyl mercaptan in periodontal pockets. Oral Microbiol Immunol. 1992;7(6):378–379.
- Basic A, Blomqvist S, Carlén A, et al. Estimation of bacterial hydrogen sulfide production in vitro. J Oral Microbiol. 2015;7:1.
- Basic A, Dahlén G. Hydrogen sulfide production from subgingival plaque samples. Anaerobe. 2015;35:21–27.
- Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12(9):1111–1123.
- Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4(1):13–32.
- Zhang H, Bhatia M. Hydrogen sulfide: a novel mediator of leukocyte activation. Immunopharmacol Immunotoxicol. 2008;30(4):631–645.
- Szabo C. A timeline of hydrogen sulfide (H2S) research: from environmental toxin to biological mediator. Biochem Pharmacol. 2018;149(p):5–19.
- Zhang Z, Fang X, Yang X, et al. Hydrogen sulfide donor NaHS alters antibody structure and function via sulfhydration. Int Immunopharmacol. 2019;73:491–501.
- Toliver-Kinsky T, Cui W, Törö G, et al. H2S, a bacterial defense mechanism against the host immune response. Infect Immun. 2019;87(1). DOI:https://doi.org/10.1128/IAI.00272-18.
- Dilek N, Papapetropoulos A, Toliver-Kinsky T, et al. Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol Res. 2020;161,105119
- Basic A, Serino G, Leonhardt Å, et al. H2S mediates increased interleukin (IL)-1β and IL-18 production in leukocytes from patients with periodontitis. J Oral Microbiol. 2019;11(1). DOI:https://doi.org/10.1080/20002297.2019.1617015.
- Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010.
- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79(8 SUPPL.):1585–1591.
- Stadler AF, Angst PDM, Arce RM, et al. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. J Clin Periodontol. 2016;43(9):727–745.
- Ayuthaya IN, Everts B,V, Pavasant P. The immunopathogenic and immunomodulatory effects of interleukin-12 in periodontal disease. Eur J Oral Sci. 2018;126(2):75–83.
- Nibali L, Fedele S, D’Aiuto F, et al. Interleukin-6 in oral diseases: a review. Oral Dis. 2012;18(3):236–43.
- Koidou VP, Cavalli N, Hagi‐Pavli E, et al. Expression of inflammatory biomarkers and growth factors in gingival crevicular fluid at different healing intervals following non-surgical periodontal treatment: a systematic review. J Periodontal Res. 2020;55(6):801–809.
- Papathanasiou E, Conti P, Carinci F, et al. IL-1 superfamily members and periodontal diseases. J Dent Res. 2020;99(13):1425–1434.
- López R, Baelum V, Hedegaard CJ, et al. Serum levels of C-reactive protein in adolescents with periodontitis. J Periodontol. 2011;82(4):543–549.
- Türer ÇC, Ballı U, Güven B. Fetuin-A, serum amyloid A and tumor necrosis factor alpha levels in periodontal health and disease. Oral Dis. 2017;23(3):379–386.
- Persson S, Edlund M-B, Claesson R, et al. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5(4):195–201.
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053.
- Kurtiş B, Tüter G, Serdar M, et al. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J Periodontol. 2005;76(11):1849–1855.
- Shatalin K, Shatalina E, Mironov A, et al. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334(6058):986–990.
