ABSTRACT
Oral health and declining cognition may have a bi-directional association. We characterized the subgingival microbiota composition of subjects from normal cognition to severe cognitive decline in two cohorts.
Memory and Periodontitis (MINOPAR) include 202 home-living participants (50–80 years) in Sweden. Finnish Oral Health Studies in Older Adults (FINORAL) include 174 participants (≥65 years) living in long-term care in Finland. We performed oral examination and assessed the cognitive level with Mini Mental State Examination (MMSE). We sequenced the 16S-rRNA gene (V3-V4 regions) to analyse the subgingival bacterial compositions.
The microbial diversities only tended to differ between the MMSE categories, and the strongest determinants were increased probing pocket depth (PPD) and presence of caries. However, abundances of 101 taxa were associated with the MMSE score. After adjusting for age, sex, medications, PPD, and caries, only eight taxa retained the significance in the meta-analyses of the two cohorts. Especially Lachnospiraceae [XIV] at the family, genus, and species level increased with decreasing MMSE.
Cognitive decline is associated with obvious changes in the composition of the oral microbiota. Impaired cognition is accompanied with poor oral health status and the appearance of major taxa of the gut microbiota in the oral cavity. Good oral health-care practices require special deliberations among older adults.
Introduction
Cognitive impairment is a transitional condition between healthy cognition and dementia. Even though dementia predominantly affects older people, it is not a part of normal aging. Both infection and inflammation are suggested to play a role in cognitive impairment and dementia [Citation1,Citation2]. It has been hypothesized that long-term priming of brain glia due to bacterial entry from the host’s dysbiotic microbiota elsewhere in the body provide a slow inflammatory damage in the central nervous system [Citation3–9].
Although experimental and clinical studies suggest an interconnection between oral dysbiotic microbiota related to common oral diseases, such as caries and periodontal diseases, and brain health/cognitive function, robust evidence is still lacking [Citation10,Citation11]. The oral microbiome comprises many organisms, which can induce inflammatory conditions in vulnerable individuals, such as older adults and immunocompromised people. Those inflammatory conditions can develop in several extra-oral tissues, including the brain, causing infection-induced neuroinflammation and gradual cognitive decline. In an animal study, neurodegenerative features in brain tissue, including fewer number of intact neuronal cells, were evident after repeated oral application of Porphyromonas gingivalis/gingipain, which suggests a role of oral pathogen in the development of neuropathology [Citation12]. P. gingivalis and/or its bacterial components have been identified in post-mortem brain specimens from Alzheimer disease (AD) patients suggesting the evasion of oral pathogens to the brain [Citation13,Citation14]. Also, Treponema denticola has been identified from brain samples by molecular and immunological techniques [Citation15], and serum antibody IgG levels to Fusobacterium nucleatum, Prevotella intermedia [Citation16] and Actinomyces naeslundii [Citation17] have been associated with declining cognition. Another proposed mechanism of oral-brain connection is via the communication of systemic inflammation to the brain induced by bacteraemia [Citation18].
Although older adults with declining cognition have limited capability to maintain oral hygiene and often develop oral health problems, epidemiological studies have suggested a bi-directional association between oral health and declining cognition/dementia [Citation19,Citation20]. This bidirectional relationship between some neurodegenerative diseases and periodontitis is associated with an increase in inflammatory biomarkers, in IgG related to periodontopathogenic bacteria, and in periodontitis severity [Citation21]. Mild cognitive impairment (MCI) may progress in some people to dementia, but others may remain stable or recover full function [Citation22]. It has been argued based on experimental animal studies that brain infection is an early event much before cognitive decline and diagnosis of dementia [Citation13]. It seems plausible that the oral dysbiosis promoting systemic inflammation may play a role in several of these neurodegenerative diseases [Citation23].
The Mini-Mental State Examination (MMSE) [Citation24] is the best-known and the most often used short screening tool for providing an overall measure of cognitive impairment, and it is commonly used as part of the evaluation for possible dementia [Citation25]. Because better understanding of the origin and mechanisms of the interaction between oral microbial burden and cognitive impairment is needed, we analysed in this study the periodontal microbiome in two cohorts comprising individuals categorised by MMSE scores from normal cognition to severely weakened cognition. We investigated whether the oral (periodontal) microbiome of participants divided based on the MMSE display special features associating with cognitive decline.
Materials and methods
Study design and population
This study is based on two separate cohorts, which were investigated separately. Both include oral examination data and a determination of cognition performed with the MMSE test. Periodontal microbiome samples were collected similarly and analysed using the same method, in the same laboratory.
Memory and Periodontitis (MINOPAR) study. N = 202 home living 50–80 years old participants who were enrolled from the Karolinska Memory Clinic at the Karolinska University Hospital in Huddinge, Sweden, and from the population register in Huddinge, Sweden, comprising participants with AD, MCI, subjective cognitive impairment, and those who had no signs/symptoms of memory disorder (healthy controls). The study was conducted from 2013 to 2017. The study design has been published by Holmer et al. [Citation26] including precise description of diagnostic criteria, control population criteria, and study exclusion criteria. Ethical approval for the study was obtained from the Regional Ethical Review Board in Stockholm (2012/652–31/1).
Finnish Oral Health Studies in Older Adults (FINORAL) study. N = 174 65 years or older residents in the capital area of Helsinki, Finland, who are living in long-term care (nursing homes and assisted living facilities). This cohort comprises the dentate participants of the FINORAL, which is a random subsample of participants of the nutrition study that included older residents in the capital area of Helsinki [Citation27]. Study was conducted from 2017 to 2019. The study design has been earlier published by Hiltunen et al. [Citation28] and Julkunen et al. [Citation29]. The City of Helsinki and the Ethics Committee of the Hospital District of Helsinki and Uusimaa approved the study protocol (HUS/2042/2016 and HUS/968/2017).
Both cohorts adhere to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants and/or their proxies.
Data collection
All study participants either completed a questionnaire about personal data (medical, dental, educational information etc.) (MINOPAR), or a registered nurse most familiar with the participants in each facility filled in a questionnaire concerning study participants’ demographic characteristics (age, sex, education) (FINORAL).
In both cohorts, the clinical oral examination (conducted for MINOPAR at the Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden; for FINORAL at each participant’s long-term care facility) comprised a comprehensive assessment of the oral soft and hard tissues including a periodontal examination comprising probing pocket depth (PPD), both at six sites on all existing teeth, and registration of caries lesions [Citation26,Citation29]. In MINOPAR, the MMSE examination was performed prior to the oral examination at the same visit; other diagnoses were obtained from medical records, and use of medications was self-reported (questionnaire). In FINORAL, the MMSE examination was performed in connection with the implementation of the nutrition study, and the residents’ medical diagnoses and use of medications were retrieved from medical records.
After completion of the oral examination, the deepest or the most representative periodontal pocket was selected in each quadrant for subgingival microbial sampling. In MINOPAR, the quadrant to be sampled was isolated with cotton rolls/pads and a saliva ejector. Supragingival plaque was carefully removed at the selected sampling site using a sterile curette, leaving the subgingival biofilm undisturbed. All samples were taken with a new individual sterile curette with a single pull, in a coronal direction, from the base of the periodontal pocket. The procedure was repeated in all four quadrants. For FINORAL, due to the exceptional conditions, there are some exceptions to the sampling protocol: no saliva ejector was available, cotton rolls/pads were used, if possible, not all supragingival plaque could be removed from everyone, all samples were taken with one sterile curette. The procedure was repeated in all four quadrants where teeth were present. The samples were pooled in 1.5 mL microcentrifuge tubes (Thermo Fisher Scientific®) containing PCR grade water (Roche®) and stored at−80°C until further processing.
Sample processing and microbiota profiling
The methodology of DNA extraction, PCR amplification and 16S rRNA gene amplicon sequencing is detailed elsewhere [Citation30,Citation31]. In brief, DNA was extracted from the subgingival samples using FastDNATM Spin Kit for Soil (MP Biomedicals) according to the manufacturer’s instructions. PCR amplifications were performed on ARKTIK Thermal Cyclers (Finnzymes/Thermo Scientific) and the V3–V4 hypervariable regions of the 16S rRNA gene were amplified with primers reported previously [Citation32] using Illumina MiSeq (Illumina Inc., San Diego, CA, United States; paired-end sequencing; read lengths: forward: 326 bp; reverse: 278 bp) at the DNA Sequencing and Genomics Laboratory of the Institute of Biotechnology, University of Helsinki, Finland.
Primers were trimmed from paired-end read with cutadapt (v. 2.8 with Python 3.8.10) [Citation33] and were merged together to reconstruct full-length sequences following the standard operating procedure (SOP) for MiSeq data using mothur pipeline (v. 1.45.2) [Citation34]. The following criteria were applied: (i) removal of homopolymers (>8 bp), (ii) removal of sequence reads containing ambiguous bases, and (iii) removal of assembled reads>435 bp in length. This reduced the number of assembled reads, ensuring high quality of data. Sequences with chimeric reads were removed by UCHIME algorithm incorporated in the mothur. We used Silva reference database (v. 132) [Citation35] for alignment and Human Oral Microbiome Database (HOMD v. 15.1) [Citation36] for taxonomy and identified the Operational Taxonomic Unit (OTU). Singleton sequences were removed from data during the mothur analysis. The raw data are available in the European Nucleotide Archive (accession numbers: PRJEB35923 for MINOPAR and PRJEB57333 for FINORAL).
Statistical analyses
For statistical analyses, both cohorts were stratified according to points achieved in MMSE test: normal cognition or very mild cognitive decline (hereafter referred to as normal cognition; 25–30 points), mild cognitive decline (20–24 points), moderate cognitive decline (10–19 points), and severe cognitive decline (0–9).
The categorical variables were described as numbers and percentages (%), and the continuous variables as means and standard deviations (SDs) or medians with interquartile range (IQR, shown as 25th and 75th percentiles).
We removed OTUs with fewer than six sequence reads, samples with a low number of sequence reads (<10000 reads), and a high number of sequences (> = 150000 reads) before analysis. After these steps, the final number of good-quality sequences was 17,193 729 (mean ± SD: 78870 ± 23 155 per sample) and 10,921 316 (mean ± SD: 43685 ± 9 526 per sample) for MINOPAR and FINORAL, respectively.
We compared the alpha diversity measures (observed richness and Shannon index) between the MMSE categories using Phyloseq (v.1.34.0). Kruskal–Wallis tests, Kendall rank correlation, Jonckheere-Terpstra test, and linear regression (combined model with multiple variables) were used for diversity analyses. Results were shown in two models; model 1, adjusted for age and sex, and model 2, adjusted for age, sex, education, caries, smoking, medications, and PPD.
The differences in microbial community composition in terms of beta diversity between the MMSE categories were tested using Bray-Curtis dissimilarities with the permutational multivariate analysis of variance (PERMANOVA) in R package vegan (v. 2.6–0) and visualization with non-metric multidimensional scaling (NMDS). Differentially abundant OTUs at different taxonomy levels (focusing on OTUs, genera, and species that had more than one read in more than one-fourth of the samples) were identified using general linear models with a negative binomial distribution using the R package DESeq2 (1.30.1).
The relative abundance of the 18 most common genera was compared in both cohorts. We tested the associations between the differential abundant taxa and MMSE scores in crude model. Finally, for the taxa that were significant in crude model of either cohort, we performed meta-analyses with additional adjustments for age, sex, number of medications, PPD≥4 mm and presence of caries.
The statistical analyses were performed in the R version 4.0.5, and we set the level of statistical significance to p < 0.05.
Results
The characteristics of the participants of MINOPAR (n = 202) and FINORAL (n = 174) are presented in .
Table 1. Characteristics of the participants.
The mean age (SD) of MINOPAR participants was 67.7 (7.1) years, while the FINORAL participants were older, 74.8 (10.9) years. Thus, the age range in the two cohorts was from 51 to 101 years. The number of females was 108 (53.5%) and 126 (72.4%) in MINOPAR and FINORAL, respectively. The MINOPAR participants lived at home and 78 (38.6%) had academic education, while the FINORAL participants lived in nursing home [80 (46.0%)] or assisted living facilities [94 (54.0%)] and 23 (13.2%) had university level education. 101 (50.0%) and 59 (62.8%) of MINOPAR and FINORAL participants had never smoked.
The median (IQR) number of teeth was 26 (24—28) and 13 (6—20) in MINOPAR and FINORAL, and caries was observed in 31(15.3%) and 119 (68.4%) of the participants, respectively. 189 (93.6%) in MINOPAR and 54 participants (33.8%) in FINORAL had deepened periodontal pockets (at least 4 mm deep).≥6 mm periodontal pockets were found in 87 (43.1%) and 34 (21.3%) participants in MINOPAR and FINORAL, respectively.
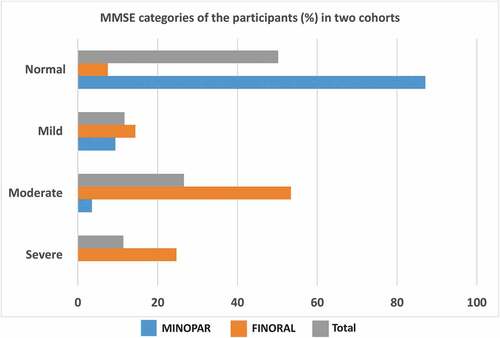
We divided the cohorts into categories based on the MMSE test results. In MINOPAR, 176 (87.1%) had normal cognition, while 19 (9.4%) and 7 (3.5%) had mild or moderate cognitive decline, respectively. In FINORAL, the corresponding frequencies were 13 (7.5%), 25 (14.4%), 93 (53.4%), respectively, and 43 (24.7%) had severe cognitive decline. Accordingly, the two cohorts comprised a continuum of MMSE results complementing each other ().
Figure 1. MMSE categories in MINOPAR and FINORAL. MINOPAR (n = 202) and FINORAL (n = 174) participants were divided into subgroups according to the MMSE score. The scores for normal cognition, and mild, moderate and severe decline of cognition were 25–30, 20–24, 10–19, and 0–9.
We amplified and sequenced 16S rRNA gene V3-V4 hypervariable regions from subgingival plaque samples resulting in 28,115,045 good-quality sequence reads. We investigated first the richness and evenness of the microbial communities, the alpha diversity, by comparing observed richness and Shannon index between the MMSE categories ( , ). We observed a significant declining trend between MMSE categories in MINOPAR (Shannon index 0.021), whereas in FINORAL the alpha diversity did not differ between the categories. Alpha diversity measures differed significantly between sexes and participants with or without caries or increased PPD and had a significant correlation with the number of teeth (). In multivariate linear models, alpha diversity did not associate with MMSE categories, but gender, number of teeth, caries and PPD were significantly associated in either of the cohorts ().
Figure 2. Alpha diversity of the oral microbiome in the MMSE categories. MINOPAR (n = 202) and FINORAL (n = 174) participants were divided into subgroups according to the MMSE score as normal, mild, moderate, and severe cognitive decline. Alpha diversity as observed richness and Shannon index were calculated for the subgingival microbiome composition in both cohorts. p-values for the significance of the difference between the MMSE groups are shown. The box plots present median (x), mean (line), IQR (box), and 95% CI (error bars).
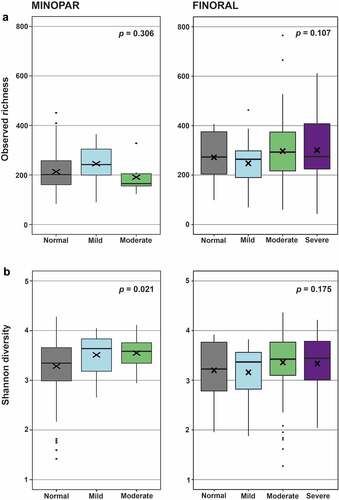
Table 2. Comparisons of alpha diversity according to the characteristics of the cohorts.
In univariate analysis, differences were observed in the beta-diversity between the MMSE categories, but they did not reach statistical significance (MINOPAR, p = 0.163; FINORAL, p = 0.077). Beta-diversity was significantly different between sexes, and between participants with or without caries or increased PPD and correlated with age and number of medications in use ( , Figure S1). In multivariate analyses, increased PPD had the strongest association with beta-diversity in MINOPAR explaining almost 4% of the variability, while the beta-diversity in FINORAL was associated with the presence of caries, which explained 1.2% of the variability.
Table 3. Comparisons of beta diversity according to the characteristics of the cohorts.
Subgingival microbiota was mainly composed of five genera: Fusobacterium, Prevotella, Streptococcus, Veillonella, and Capnocytophaga. The 18 most abundant genera are presented according to the MMSE categories in Supplemental Table S1. Streptococcus (p = 0.010) and Actinomyces (p = 0.041) exhibited a significant decreasing, and Tannerella (p = 0.013), Treponema (p = 0.003), Corynebacterium (p = 0.009), and Saccharibacteria G-5 (p = 0.003) a significant increasing trend with declining MMSE score in FINORAL cohort. In MINOPAR, no significant trends were observed.
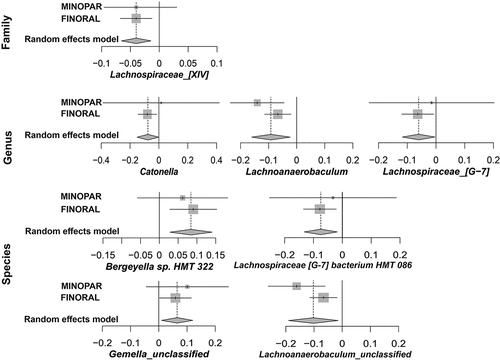
The differential abundance of several OTUs associated significantly with the MMSE score in general linear models (Supplemental Table S2). In MINOPAR, the number of associated taxa was 39, while in FINORAL 67 taxa were associated with MMSE score. The largest estimates were observed for Prevotella saccharolytica (−0.585/MMSE unit) and Saccharibacteria [G-1] bacterium (−0.437/MMSE unit) in MINOPAR, and Bacteroidetes (−0.184/MMSE unit) and Saccharibacteria (−0.177/MMSE unit) families in FINORAL. All significant taxa were further subjected to meta-analyses of both cohorts with additional adjustments for age, sex, number of medications, PPD≥6 mm and presence of caries (Supplemental Table S3). In the final random effects models with full adjustment (), Lachnospiraceae [XIV] family [beta (95% CI), p-value] [−0.04 (−0.07; −0.01), 0.002], genera Lachnospiraceae [G-7] [−0.08 (−0.15; −0.00), 0.046], Lachnoanaerobaculum [−0.09 (−0.16; −0.02), 0.009] and Catonella [−0.08 (−0.15; −0.00), 0.046], and species Lachnospiraceae [G-7] bacterium HMT 086 [−0.07 (−0.13; −0.02), 0.011] and one Lachnoanaerobaculum unclassified species [−0.10 [−0.19; −0.02), 0.022] were significantly associated with MMSE score. Additionally, Bergeyella sp. HMT 322 [0.08 (0.03; 0.14), 0.003] and Gemella unclassified [0.07 (0.009; 0.12), 0.021] displayed a significant association. The forest plots of the taxa with suggestive p-values in the random effect models are presented in Supplemental Figure S2.
Figure 3. Meta-analyses of the significant OTUs. the forest plots display the results of the final random effects models produced by meta-analyses of all significant taxa of either cohort, adjusted for age, sex, number of medications, PPD≥6 mm and presence of caries. Effect size and 95% CI are shown separately for MINOPAR, FINORAL, and their meta-analyses.
Discussion
We showed among 376 older adults, that cognitive decline is associated with substantial compositional changes of the subgingival microbiota. Although the microbial richness or evenness did not differ significantly between the MMSE categories, we found 101 different taxa that were associated with cognitive decline. When the obvious differences between the two cohorts were considered, especially the abundance of the Lachnospiraceae [XIV] family members increased with declining cognition independently of residence type, age, sex, medications, and presence of periodontitis or caries. Genus Gemella and Bergeyella sp. were depleted in participants with cognitive impairment. Thus, the number of associated taxa decreased markedly after adjusting for the presence of deepened periodontal pockets or caries: Oral health status was the strongest determinant of the subgingival microbiota composition.
The two cohorts of the study represent very different segments of older adults with respect to age, type of housing, level of education and state of cognition. FINORAL cohort comprised Finnish long-term care facility residents from the capital area with markedly impaired ability to function. They were significantly older than MINOPAR cohort comprising Swedish residents also from the greater capital area but living independently at home. We assessed MMSE scores for both cohorts: FINORAL participants categorised as having normal cognition or mild cognitive decline formed a minority (22%), while MINOPAR comprised mainly such participants (96.5%) and none of them had severe cognitive impairment. However, these differences offer a perspective to both a wide age range and variation in the level of cognition. Strengths of the study include the careful evaluations of oral health status and registering of putative confounders, such as smoking, education level, BMI, and diabetes. The differences between the two cohorts may also be regarded as a limitation. Also, other important risk factors for cognitive decline, such as nutrition or cholesterol levels were not recorded.
The most important prerequisite for comparing these cohorts was that the microbiota was analysed in the same laboratory with a similar 16S rRNA gene amplicon sequencing method. Cohorts were examined separately in all statistical analyses; data were not combined at any stage. The main objective of the study, to recognise special features of the subgingival microbiota in groups divided based on MMSE and to identify possible marker species, was carried out by conducting a meta-analysis with microbial taxa selected among those displaying significant log2 fold changes in linear models. With this approach, we were able to avoid the challenges caused by several dissimilarities of the two data sets, and to produce an insight of microbial taxa that associate with the decrease of MMSE considering confounding factors.
We observed a declining trend of alpha diversity (Shannon index) between MINOPAR MMSE categories but not in FINORAL. In multivariate models, this association was lost. However, in these models, alpha diversity was associated significantly with gender, caries, and PPD in MINOPAR, and with the number of teeth in both cohorts. MINOPAR participants had a higher number of teeth and teeth with increased PPD, while FINORAL participants had fewer teeth and a lower number of teeth with increased PPD. In MINOPAR, the strongest association of beta-diversity was with increased PPD explaining almost 4% of the variability. In FINORAL, on the other hand, beta-diversity was associated with the presence of caries which explained 1.2% of the variability. Caries was very common in FINORAL (68% of all participants) while in MINOPAR only 15% had caries teeth. The oldest old population living in long-term facilities has often lost teeth with the most severe periodontitis earlier in life but suffer often from root caries and milder forms of periodontitis [Citation37].
In a Danish study, participants with MMSE scores less than 24 had significantly more both coronal and root surface caries than those with higher score [Citation38]. Caries experience of institutionalized older adults has been associated with dementia, disability and lack of oral care [Citation39], but less is known about the role of caries in brain health. Periodontitis has been hypothesized as being one of the most common potential risk factors for the cognitive impairment and development of dementia/neurodegenerative diseases. Our earlier study suggested that marginal periodontitis is associated with both early cognitive impairment and AD [Citation26]. In a retrospective study, 10-year exposure to periodontitis increased significantly the risk of developing dementia (AD) (odds ratio, OR 1.7) [Citation40]. A recent meta-analysis concluded that periodontitis is associated with cognitive impairment [Citation41], and subjects with moderate or severe periodontitis were at 2.13 times greater risk of developing dementia compared with persons without moderate or severe periodontitis [Citation42]. One earlier study linked MMSE and oral health: in a 5-year prospective study, severe periodontitis and periodontal inflammation were associated with increased odds for MCI among older adults segmented by a chemosensory test and cognitive scores by MMSE [Citation43]. Controversies also exist, since in our large cohort study conducted in Sweden deep periodontal probing was not associated with incidence of dementia [Citation44]. Importantly, however, in a large Japanese study, having had periodontal treatment was associated with a lower risk for dementia [Citation45].
There is some evidence that in individuals with cognitive impairment or AD, the subgingival microbiota exhibits a shift typical of periodontitis [Citation30] but that the microbial signatures change to favor opportunistic species with increasing AD severity [Citation46]. Periodontal pathogens, such as Porphyromonas gingivalis and Treponema denticola, have been found in post-mortem brain tissue samples of AD patients [Citation13–15] suggesting a direct bacterial translocation into the brain, which is also supported by the animal studies [Citation47]. Among studies using targeted methods, elevated serum antibodies to periodontal disease bacteria were associated with future cognitive impairment, AD patients compared to healthy, and risk for developing incident AD [Citation16,Citation17,Citation48,Citation49].
It is possible that weakening cognition (determined by MMSE) and consequent deficient oral hygiene will result that certain bacterium achieve a dominant role in periodontal biofilm. Oral hygiene is known to be poor especially among vulnerable, care-dependent institutionalized older people [Citation50,Citation51] [Citation52], particularly among those who need assistance with oral hygiene [Citation53,Citation54], and fast deterioration of oral health because of caries or periodontal problems is possible [Citation55]. In our earlier study [Citation29] oral disease burden was associated with functional and cognitive decline according to MMSE among long-term care facility residents in line with prior studies [Citation56,Citation57].
In the present study, the relative abundance of only a few genera correlated significantly with the MMSE categories: Streptococcus and Actinomyces displayed a decreasing trend, while Tannerella, Treponema, Corynebacterium, and Saccharibacteria [G-5] presented an increasing trend with declining cognition. Relative abundances of several common oral bacterial genera were significantly different for FINORAL and MINOPAR cohorts. Higher abundancies of periodontitis-related genera, Tannerella, Treponema, and Porphyromonas in MINOPAR can be explained by the higher number of teeth with increased PPD, especially PPD≥6 mm. Fusobacterium, more abundant in MINOPAR, and Prevotella, more abundant in FINORAL, both genera also common in periodontal diseases, are not traditionally considered as pathogenic for periodontitis as bacteria in genera Tannerella, Treponema and Porphyromonas [Citation58]. Streptococcus were twice as abundant in MINOPAR as in FINORAL, though caries was more common in FINORAL. In older long-term care living populations especially root caries is common [Citation59], and it does not need streptococci to develop [Citation60]. In FINORAL higher abundance of Veillonella, common bacteria in the gastro-intestinal tract including oral mucous membrane, can be explained by the inadequate oral hygiene of long-term care residents, as previously published in the FINORAL population [Citation29,Citation61].
We found associations between 101 taxa and MMSE score in univariate analyses. When the confounders were considered and the two cohorts subjected to a meta-analysis, eight taxa retained the significance. Among them was Lachnospiraceae [XIV] family, which belongs to the phylum of Firmicutes, the class of Clostridia and includes several genera, such as Catonella, Lacnoanaerobaculum, Lachnospiraceae [G-7], and Oribacterium. The family is anaerobic and among the most abundant taxa in the human gut microbiota [Citation62]. Several studies have shown that gut Lachnospiraceae [XIV] is less abundant in Alzheimer’s disease compared to healthy subjects [Citation63,Citation64] and associated with a better performance on MMSE and other cognitive assessments [Citation42,Citation65]. The advantageous properties are believed to derive from Lachnospiraceae’s ability to ferment indigestible carbohydrate producing beneficial metabolites [Citation66]. The health-promoting metabolites, such as butyrate, are crucial for proper function of the intestinal barrier thus maintaining immune homeostasis, preventing translocation of endotoxins, and promoting anti-inflammatory properties [Citation62]. On the other hand, Lachnospiraceae may associate with inflammation, metabolic syndrome, liver diseases, and chronic kidney diseases [Citation62]. The present study, however, confirms and strengthen our earlier results that increased subgingival Lachnospiraceae is associated with low MMSE score and Alzheimer’s disease [Citation30]. The association was independent from residence type, age, sex, medications, and presence of periodontitis or caries. Nevertheless, the relative abundance of subgingival Lachnospiraceae was less than 1% while it may be predominant in the gut with a 40% abundance [Citation64]. The result suggests that species highly common in the gut appear in the oral cavity in dementia. Lachnoaerobaculum has been detected in the oral biofilm among all adults despite the caries status [Citation67]) but it has also been associated with smoking [Citation68]. Thus, whether an altered microbiota in the oral cavity is a cause or consequence and how it relates to the Lachnospiraceae taxa in the gut remains to be investigated.
After carefully considering demographic factors and oral health status, we did not observe significant differences in the diversity of the microbiota between the MMSE categories. However, we show that cognitive decline is associated with obvious changes in the composition of the subgingival microbiota and most of them associate with poor oral health. Considering the putative contribution of oral health to dementia, good oral health-care practices require special deliberations at all ages and at all stages of cognition.
Supplemental Material
Download Zip (7.4 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20002297.2023.2178765
Additional information
Funding
References
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–12.
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12(9):1005–1015.
- Alonso R, Pisa D, Fernández-Fernández AM, et al. Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer’s disease. Front Aging Neurosci. 2018 May 24;10:159. DOI:10.3389/fnagi.2018.00159.
- Bulgart HR, Neczypor EW, Wold LE, et al. Microbial involvement in Alzheimer disease development and progression. Mol Neurodegener. 2020 Jul 24;15(1):42. DOI:10.1186/s13024-020-00378-4.
- Emery DC, Shoemark DK, Batstone TE, et al. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front Aging Neurosci. 2017 Jun 20;9:195. DOI:10.3389/fnagi.2017.00195.
- Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer’s Disease. J Alzheimers Dis. 2016;51(4):979–984.
- Narengaowa KW, Lan F, Awan, U. F. et al. The oral-gut-brain AXIS: the influence of microbes in Alzheimer’s disease. Front Cell Neurosci. 2021 Apr 14;15:633735. DOI:10.3389/fncel.2021.633735.
- Pritchard AB, Crean S, Olsen I, et al. Microbiomes and their role in Alzheimer’s disease. Front Aging Neurosci. 2017;9:336.
- Yadav P, Lee YH, Panday H, et al. Implications of microorganisms in Alzheimer’s disease. Curr Issues Mol Biol. 2022 Sep 30;44(10):4584–4615. DOI:10.3390/cimb44100314.
- Nascimento PC, Castro MML, Magno MB, et al. Association between periodontitis and cognitive impairment in adults: a systematic review. Front Neurol. 2019;10:323.
- Orr ME, Reveles KR, Yeh CK, et al. Can oral health and oral-derived biospecimens predict progression of dementia? Oral Dis. 2020;26(2):249–258.
- Ilievski V, Zuchowska PK, Green SJ, et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE. 2018;13(10):e0204941. DOI:10.1371/journal.pone.0204941
- Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333.
- Poole S, Singhrao SK, Kesavalu L, et al. Determining the presence of perodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 2013;36(4):655–677.
- Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 2002 Apr;17(2):113–118. DOI:10.1046/j.0902-0055.2001.00100.x.
- Sparks Stein P, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012;8(3):196–203.
- Noble JM, Scarmeas N, Celenti RS, et al. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS ONE. 2014;9(12):e114959. DOI:10.1371/journal.pone.0114959
- Noh H, Jeon J, Seo H. Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochem Int. 2014;69:35–40.
- Kang J, Wu B, Bunce D, et al. Bidirectional relations between cognitive function and oral health in ageing persons: a longitudinal cohort study. Age Ageing. 2020;49(5):793–799.
- Nangle MR, Manchery N. Can chronic oral inflammation and masticatory dysfunction contribute to cognitive impairment? Curr Opin Psychiatry. 2020;33(2):156–162.
- Alvarenga MOP, Frazão DR, de Matos IG, et al. Is there any association between neurodegenerative diseases and periodontitis? A systematic review. Front Aging Neurosci. 2021;13:651437.
- Arevalo-Rodriguez I, Smailagic N, Roqué-Figuls M, et al. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2021;7(7). DOI:10.1002/14651858.CD010783.pub3
- Nicholson JS, Landry KS. Oral dysbiosis and neurodegenerative diseases: correlations and potential causations. Microorganisms. 2022;10(7):1326.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. DOI:10.1016/0022-3956(75)90026-6.
- Creavin ST, Wisniewski S, Noel-Storr AH, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. 2016;2016(1). DOI:10.1002/14651858.CD011145.pub2
- Holmer J, Eriksdotter M, Schultzberg M, et al. Association between periodontitis and risk of Alzheimer′s disease, mild cognitive impairment and subjective cognitive decline: a case–control study. J Clin Periodontol. 2018;45(11):1287–1298.
- Salminen KS, Suominen MH, Kautiainen H, et al. Energy intake and severity of dementia are both associated with HRQOL among older long-term care residents. Nutrients. 2019;11(19):2261.
- Hiltunen K, Saarela RKT, Kautiainen H, et al. Relationship between fried’s frailty phenotype and oral frailty in long-term care residents. Age Ageing. 2021;50(6):2133–2139.
- Julkunen L, Hiltunen K, Kautiainen H, et al. Oral disease burden of dentate older adults living in long-term care facilities: fINORAL study. BMC Oral Health. 2021;21(1):624. DOI:10.1186/s12903-021-01984-4
- Holmer J, Aho V, Eriksdotter M, et al. Subgingival microbiota in a population with and without cognitive dysfunction. J Oral Microbiol. 2021;13(1):1854552.
- Pereira PAB, Aho VTE, Paulin L, et al. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:61–67.
- Aho VTE, Pereira PAB, Voutilainen S, et al. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707.
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMB net journal. 2011;17(1):10.
- Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541.
- Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Opens external link in new windowNucl. Acids Res. 2013;41(D1):D590–596.
- Chen T, Yu WH, Izard J, et al. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford). 2010;2010:baq013.
- Renvert S, Persson GR. Treatment of periodontal disease in older adults. Periodontol 2000. 2016 Oct;72(1):108–119. DOI:10.1111/prd.12130.
- Ellefsen BS, Holm-Pedersen P, Morse D, et al. Caries prevalence in older persons with and without dementia. J Am Geriatr Soc. 2008;56(1):59–67. DOI:10.1111/j.1532-5415.2007.01495.x
- Philip P, Rogers C, Kruger E, et al. Caries experience of institutionalized elderly and its association with dementia and functional status. Int J Dent Hyg. 2012;10(2):122–127.
- Chen CK, Wu YT, Chang YC, et al. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res Ther. 2017;9(1):56.
- Asher S, Stephen R, Mäntylä P, et al. Periodontal health, cognitive decline, and dementia: a systematic review and meta-analysis of longitudinal studies. J Am Geriatr Soc. 2022;70(9):2695–2709.
- Guo H, Chang S, Pi X, et al. The effect of periodontitis on dementia and cognitive impairment: a meta-analysis. Int J Environ Res Public Health. 2021;18(13):6823.
- Iwasaki M, Kimura Y, Ogawa H, et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: a 5-year cohort study. J Periodontal Res. 2019;54(3):233–240.
- Holmer J, Eriksdotter M, Häbel H, et al. Periodontal conditions and incident dementia: a nationwide Swedish cohort study. J Periodontol. 2022;93(9):1378–1386. Sep.
- Saito M, Shimazaki Y, Nonoyama T, et al. Utilization of dental care and the incidence of dementia: a longitudinal study of an older Japanese cohort. Dement Geriatr Cognit Disord. 2022;51(4):1–8. DOI:10.1159/000526683
- Bathini P, Foucras S, Dupanloup I, et al. Classifying dementia progression using microbial profiling of saliva. Alzheimers Dement (Amst). 2020;12(1):e12000.
- Jungbauer G, Stähli A, Zhu X, et al. Periodontal microorganisms and Alzheimer disease - a causative relationship? Periodontol 2000. 2022;89(1):59–82.
- Beydoun MA, Beydoun HA, Hossain S, et al. Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer’s disease dementia in a large national survey. J Alzheimers Dis. 2020;75(1):157–172. DOI:10.3233/JAD-200064 PMID: 32280099.
- Kamer AR, Craig RG, Pirraglia E, et al. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216(1–2):92–97.
- De Visschere L, Janssens B, De Reu G, et al. An oral health survey of vulnerable older people in Belgium. Clin Oral Investig. 2016 Nov;20(8):1903–1912. DOI:10.1007/s00784-015-1652-8.
- Janssens B, Vanobbergen J, Petrovic M, et al. The oral health condition and treatment needs assessment of nursing home residents in Flanders (Belgium). Community Dent Health. 2017 Sep;34(3):143–151. DOI:10.1922/CDH_4086Janssens09.
- Yoon MN, Ickert C, Slaughter SE, et al. Oral health status of long-term care residents in Canada: results of a national cross-sectional study. Gerodontology. 2018;35(4):359–364. Dec.
- Niesten D, Witter DJ, Bronkhorst EM, et al. Oral health care behavior and frailty-related factors in a care-dependent older population. J Dent. 2017 Jun;61:39–47. DOI:10.1016/j.jdent.2017.04.002.
- Philip P, Rogers C, Kruger E, et al. Oral hygiene care status of elderly with dementia and in residential aged care facilities. Gerodontology. 2012;29(2):e306–11. Jun.
- Boehm TK, Scannapieco FA. The epidemiology, consequences and management of periodontal disease in older adults. J Am Dent Assoc. 2007 Sep;138(26S–33S):S26–33.
- Lee KH, Jung ES, Choi YY. Association of oral health and activities of daily living with cognitive impairment. Gerodontology. 2020 Mar;37(1):38–45.
- Nadim R, Tang J, Dilmohamed A, et al. Influence of periodontal disease on risk of dementia: a systematic literature review and a meta-analysis. Eur J Epidemiol. 2020 Sep;35(9):821–833. DOI:10.1007/s10654-020-00648-x.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998 Feb;25(2):134–144. DOI:10.1111/j.1600-051x.1998.tb02419.x.
- Chan AKY, Tamrakar M, Jiang CM, et al. A systematic review on caries status of older adults. Int J Environ Res Public Health. 2021 Oct 12;18(20):10662. DOI:10.3390/ijerph182010662.
- Takahashi N, Nyvad B. Ecological hypothesis of dentin and root caries. Caries Res. 2016;50(4):422–431.
- Saarela RKT, Hiltunen K, Kautiainen H, et al. Oral hygiene and health-related quality of life in institutionalized older people. Eur Geriatr Med. 2022;13(1):213–220. DOI:10.1007/s41999-021-00547-8
- Vacca M, Celano G, Calabrese FM, et al. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8(4):573. DOI:10.3390/microorganisms8040573
- Murray ER, Kemp M, Nguyen TT. The microbiota-gut-brain axis in Alzheimer’s disease: a review of taxonomic alterations and potential avenues for interventions. Arch Clin Neuropsychol. 2022 Feb 22;37(3):595–607. DOI:10.1093/arclin/acac008.
- Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537.
- Hung CC, Chang CC, Huang CW, et al. Gut microbiota in patients with Alzheimer’s disease spectrum: a systematic review and meta-analysis. Aging (Albany NY). 2022 Jan 14;14(1):477–496. DOI:10.18632/aging.203826.
- Leung C, Rivera L, Furness JB, et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–425.
- Johansson I, Witkowska E, Kaveh B, et al. The microbiome in populations with a low and high prevalence of caries. J Dent Res. 2016;95(1):80–86.
- Duan X, Wu T, Xu X, et al. Smoking may lead to marginal bone loss around non-submerged implants during bone healing by altering salivary microbiome: a prospective study. J Periodontol. 2017 Dec;88(12):1297–1308. DOI:10.1902/jop.2017.160808.
