ABSTRACT
Objective
To explore the mechanisms underlying the virulence changes in early childhood caries (ECC) caused by Candida albicans (C. albicans) and Streptococcus mutans (S. mutans), with a focus on carbohydrate metabolism and environmental acidification.
Methods
A review of literature was conducted to understand the symbiotic relationship between C. albicans and S. mutans, and their role in the pathogenesis of ECC. The review also examined how their interactions influence carbohydrate metabolism and environmental acidification in the oral cavity.
Results
C. albicans and S. mutans play crucial roles in the onset and progression of ECC. C. albicans promotes the adhesion and accumulation of S. mutans, while S. mutans creates an environment favorable for the growth of C. albicans. Their interactions, especially through carbohydrate metabolism, strengthen their pathogenic potential. The review highlights the importance of understanding these mechanisms for the development of effective management and treatment protocols for ECC.
Conclusion
The symbiotic relationship between C. albicans and S. mutans, and their interactions through carbohydrate metabolism and environmental acidification, are key factors in the pathogenesis of ECC. A comprehensive understanding of these mechanisms is crucial for developing effective strategies to manage and treat ECC.
Introduction
Early childhood caries (ECC) is a prevalent chronic disease among young children and represents a significant public health challenge across the globe. It affects approximately half of all preschool-aged children, displaying an epidemic spread with notable geographical disparities, particularly among impoverished communities [Citation1,Citation2]. ECC can lead to severe destruction of the dental crown in primary teeth, resulting in increased pain and infection, ultimately compromising the child’s quality of life [Citation3].
Dental caries is recognized as a multifactorial disease, driven by the fermentation of dietary carbohydrates, acid production through bacterial metabolism, and biofilm-mediated dysbiosis that culminates in the demineralization of tooth tissues [Citation4–6]. Recent evidence indicates that the role of Streptococcus mutans (S. mutans) in ECC is paralleled by the involvement of Candida albicans (C. albicans), both of which have been frequently detected in the oral saliva and dental plaque of ECC-affected patients [Citation7]. A study examining saliva and dental plaque from 30 preschool children across two different locations revealed that those with ECC exhibited higher quantities of C. albicans and S. mutans compared to their healthy counterparts, as determined by log DNA copy numbers and their proportion relative to total oral bacteria [Citation8]. The tooth surface is increasingly acknowledged as a conducive environment for the colonization by C. albicans and the formation of pathogenic dental biofilms [Citation9].
Filamentation is considered a key virulence attribute of C. albicans, wherein its ability to transition from yeast to hyphal is deemed critical for pathogenicity [Citation10,Citation11]. However, recent research by Xiang et al. [Citation12] suggests that filamentation may not be as essential for C. albicans’ pathogenicity within the oral niche as previously thought. Isolates from children with severe ECC (S-ECC) displayed broad phenotypic variations but consistently exhibited traits conducive to cariogenesis, such as high proteinase activity, acidogenicity, and acid tolerance. Remarkably, these isolates enhanced sucrose metabolism and biofilm acidogenicity, creating a highly acidic environment (pH < 5.5) and forming robust biofilms with S. mutans, irrespective of their filamentous state. These findings suggest that the morphology of C. albicans does not solely determine its adaptive strategies on the tooth surface. Clinical studies have corroborated the frequent detection of C. albicans in dental plaque from children with S-ECC, implicating its association with increased caries severity [Citation13–15]. The virulence of C. albicans within the oral cavity is significantly influenced by its interactions with commensal bacteria [Citation16].
S. mutans, an acidogenic bacterium capable of biofilm formation, is a well-established pathogen in the context of ECC. It thrives in the acidic microenvironments created within dental biofilms, which confers a selective advantage over other microbial community members [Citation17]. Notable research has demonstrated that C. albicans engages in biochemical, metabolic, and physical interactions with S. mutans, leading to the establishment of highly cariogenic interkingdom biofilms [Citation18–20]. Given that the presence of C. albicans can alter the oral microbiome composition even in early biofilm development stages, it is essential to consider its potential role within healthy oral ecosystems and its impact on bacterial populations [Citation21].
This review diverges from previous discussions centered on Candida-Streptococcus biofilm interactions. Instead, we concentrate on the aspects of carbohydrate metabolism and environmental acidification, exploring the cooperative mechanisms employed by C. albicans and S. mutans that modulate their growth and virulence within the oral cavity. We aim to underscore the implications of these interactions on the oral health of children.
Alteration of oral microbiome composition in ECC through interactions between C. albicans and S. mutans, and the influence of external factors
In the oral cavity, environmental factors such as diet and oral hygiene play a pivotal role in the progression of ECC. S. mutans is a primary cariogenic bacterium that secretes glucosyltransferases (Gtfs), enzymes that catalyze the conversion of sucrose into extracellular glucans. These glucans form the major components of exopolysaccharides (EPSs), which facilitate S. mutans adhesion and promote microbial cohesion within the oral biofilm. Notably, glucosyltransferases B (GtfB), a key exoenzyme, binds to the mannan layer of the Candida albicans cell wall, enhancing extracellular matrix formation and fostering coexistence within biofilms [Citation22]. The C. albicans Checkpoint Kinase 1 (CHK1) gene as an essential component of two-component signal transduction system (TCS) plays a significant role in modulating the pathogenicity of C. albicans and its response to the host environment [Citation23]. The CHK1 gene in C. albicans is involved in various functions, including enhancing its virulence, upregulating the quorum sensing and enabling responses to stress [Citation24]. Recent studies have highlighted the importance of CHK1 gene during oral mucosal infections, its plays a crucial role in mediating cross-kingdom interactions, which can potentially increase the cariogenic potential of these microbiomes within the oral cavity [Citation23,Citation25,Citation26].
The expression of Gtfs is regulated by the VicK gene, part of the VicRKX three-component system in S. mutans. Research conducted by Yelan Deng and colleagues [Citation27] demonstrated that mutations in the VicK gene influenced EPS production and biofilm formation. This finding aligns with Yaqi Liu’s observations that the C. albicans CHK1 gene affects the VicRK pathway in S. mutans, enhancing the expression of GtfB, GtfC, and GtfD genes, and subsequently promoting biofilm and EPS production [Citation26]. The VicRKX system comprises VicK, a Histidine protein kinase; VicR, a global response regulator; and VicX, a putative hydrolase. Beyond its capacity to produce EPS via Gtf upregulation, S. mutans collaborates with C. albicans in the oral environment, contributing to the initiation and progression of ECC.
Recent RNA-Seq study reveals that C. albicans can upregulate genes associated with carbohydrate transport and metabolism when co-cultured with S. mutans [Citation19]. Furthermore, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway impact analysis indicated that both pyruvate and galactose metabolism pathways were enhanced in S. mutans during co-culture [Citation19]. These findings suggest that C. albicans significantly influences the carbohydrate utilization patterns of S. mutans. Subsequent research has identified that S. mutans genes related to the phosphotransferase system (PTS), ABC sugar transporter system, and carbohydrate metabolism, along with glycogen biosynthesis, are also upregulated in the presence of C. albicans [Citation18]. Proteomic analysis of dual-species biofilms has shown increased activity in carbohydrate metabolism, glucan biosynthesis and transferase activity, and peptidoglycan and cell wall biosynthesis in S. mutans [Citation18]. These studies collectively highlight the possibility that C. albicans contributes to the acidification of mixed-species biofilms, in part through a GtfB-mediated mechanism, thereby enhancing the acidogenic and aciduric capabilities of S. mutans.
Gtfs and their role in ECC pathogenesis
Gtfs, including GtfB, GtfC, and GtfD, are critical enzymes in the pathogenesis of S. mutans-mediated ECC. These enzymes enable S. mutans to enhance biofilm formation and colonization by accumulating extracellular polysaccharides (EPSs) [Citation28]. Although the Gtfs share structural similarities, each possesses unique functions. GtfB, for instance, primarily synthesizes insoluble glucans that facilitate microbial attachment and alter biofilm structure, playing an essential role in the interaction with other oral microbiota [Citation29] ().
Figure 1. Alteration of the Virulence of C. albicans effecting with S. mutans in the development of ECC. S. mutans prompt both the growth and pathogenesis of C. albicans in the oral via secreted and cell surface molecules. S. mutans secrete Glucosyltransferase that can attach firmly to the C. albicans cell wall that are critical for pathogenesis and virulence. EPSs present on the cell surface of S. mutans mediate the sugar metabolism transforming sucrose to glucan to sustain the virulence of S. mutans in the oral, which enhances their capacity to cariogenic. (Created with BioRender.com).
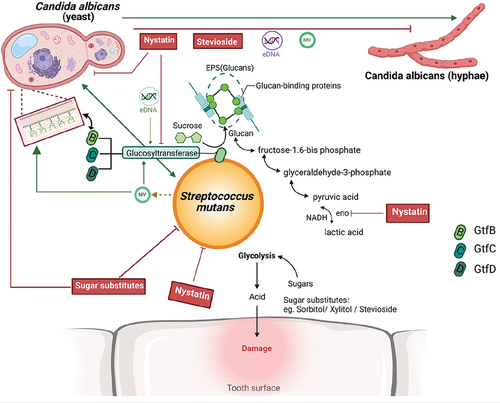
In a dual-species biofilm system, S. mutans influences a wide array of genes and proteins involved in carbohydrate metabolism, sugar transport systems, glycolysis, pyruvate degradation, and the production of ethanol and acetate, as well as the tricarboxylic acid cycle and electron transport chain [Citation18,Citation30]. Notably, while C. albicans predominantly utilizes glucose and cannot efficiently metabolize sucrose, S. mutans can decompose ingested sucrose into glucans via the Gtf system. This activity supports the growth of C. albicans and enhances acid production in the oral cavity [Citation18,Citation28,Citation31,Citation32]. Furthermore, the exoenzyme GtfB from S. mutans binds to the mannan layer of the C. albicans cell wall, promoting the in situ production of extracellular α-glucans. This interaction likely affects C. albicans colonization, and the resultant EPSs provide additional bacterial binding sites for S. mutans [Citation22,Citation33,Citation34]. The structure of the C. albicans cell wall is thus crucial not only for its growth and survival but also for mediating interactions with the extracellular environment and may play a pivotal role in ECC development.
Cell wall compositions and their impact on ECC
The cell wall of C. albicans comprises an outer layer of mannan fibrils and an inner core of cross-linked chitin and β-glucan, covalently connected to mannoproteins modified by N-linked and/or O-linked mannosylation and phosphomannosylation. These modifications regulate the dynamic nature of the fungal wall and contribute to the complexity of mechanisms controlling its structure and viscoelastic properties [Citation35,Citation36]. The composition and architecture of the C. albicans cell wall are also pivotal in modulating the host immune response. In mixed-species biofilms, S. mutans can enhance C. albicans genes involved in hyphal formation and cell wall properties, resulting in the upregulation of genes related to filamentous growth and cell wall components such as mannans and glucans, thereby reinforcing Candida biology and virulence [Citation18].
The architecture of the C. albicans cell wall is influenced by the available carbon source. Research by Elizabeth R. Ballou and colleagues [Citation37] demonstrated that L-lactate, produced by host cells or bacteria within the microbiota, can trigger β-glucan masking in C. albicans. The level of β-glucan exposure is a critical pathogen-associated molecular pattern (PAMP), and their findings indicate that changes in carbon source can regulate the visibility of C. albicans to the immune system and influence its virulence. Moreover, the expression of enzymes that link mannans to β-glucan in the C. albicans cell wall is affected by different carbon sources. It has been suggested that the outer wall mannans function as a protective shield over the β-glucan layer [Citation24]. These alterations in β-glucan exposure appear to be dependent on the carbon source rather than pH levels. Specifically, C. albicans can mask β-glucan in its cell wall when utilizing lactic acid – a byproduct of S. mutans metabolism – as an energy source, which is highly acidogenic in the oral cavity. Collectively, these findings suggest that in the oral environment, the presence of S. mutans alters the carbon sources and thus reducing the exposure of β-glucan on the C. albicans cell wall, facilitating the binding of GtfB to mannans ().
Table 1. The impact of various external factors in the dual-species interaction.
Role of membrane vesicles (MVs) in ECC pathogenesis
MVs of S. mutans are bilayer membranous structures formed when a section of the cytoplasmic membrane protrudes and buds off, encapsulating an array of components such as proteins, nucleic acids, lipids, and metabolites. These MVs are essential for processes like cell wall synthesis, cell-to-cell communication, bacterial adhesion, and biofilm formation [Citation47–49]. Notably, S. mutans MVs are implicated in caries development, aiding in both the formation of its own biofilms and the colonization of host sites, and also promoting the biofilm formation of C. albicans on tooth surfaces [Citation38,Citation50,Citation51]. Specifically, S. mutans MVs have been shown to enhance demineralization of bovine dentin by C. albicans biofilms and to augment the expression of proteins and metabolites in C. albicans related to carbohydrate metabolism [Citation52]. Furthermore, Gtfs harbored within S. mutans MVs can increase EPS production, thereby boosting C. albicans biofilm formation [Citation38,Citation48]. Even under different pH conditions, S. mutans continues to release MVs containing proteins associated with cariogenesis, contributing to the impairment of host cell function. Yina Cao’s bicinchoninic acid (BCA) assay results revealed that S. mutans discharges more MVs under acidic conditions than neutral ones, suggesting that MVs production amplifies the virulence of S. mutans and enhances adherence to tooth surfaces, ultimately leading to caries development [Citation48]. Additionally, MVs in S. mutans have been identified as vehicles for eDNA release. Investigation into a putative glycosyltransferase of S. mutans, SMU_833, which is thought to modulate biofilm matrix dynamics, revealed that an increase in eDNA was accompanied by elevated production of MVs. This suggests that SMU_833 may influence biofilm matrix composition through MV-mediated modulation of eDNA release [Citation32].
eDNA in ECC development
eDNA is a critical component of the dental plaque biofilm and the biofilm extracellular matrix of S. mutans. It serves multiple functions, including maintaining biofilm structural integrity, initiating adhesion to dental surfaces, acting as a nutrient source, and facilitating horizontal gene transfer [Citation53]. In the development of caries, the release of eDNA via MVs and lysis has been proposed as a potential virulence factor for S. mutans. Notably, eDNA and glucans have been observed to colocalize within biofilms [Citation50], with glucans providing a supportive matrix for eDNA, particularly in environments rich in sucrose [Citation54]. The presence of carbohydrates may trigger the activation of catabolite control protein A (CcpA), which regulates the two-component system (TCS) LytST, leading to degradation of the bacterial cell wall and subsequent eDNA release [Citation53]. Under acidic conditions, eDNA contributes to the formation of insoluble glucan-independent biofilms by S. mutans [Citation55].
Recent study highlights the essential role of eDNA in maintaining the structural integrity of dual-species biofilms formed by S. mutans and C. albicans. Interestingly, the removal of eDNA significantly disrupts the formation and structure of these dual-species biofilms without affecting the growth of either organism [Citation39]. eDNA is particularly influential during the initial attachment and developmental stages of biofilm formation, but less so in mature biofilms. These findings underscore the potential of targeting eDNA to mitigate dental plaque formation on tooth surfaces and promote oral health.
The critical role of saliva in regulating C. albicans and S. mutans interactions
In the oral cavity, various factors could shape the microbiota, including internal conditions and exogenous dietary intake. These factors significantly influence the interactions between the cariogenic bacterium S. mutans and the opportunistic fungal pathogen C. albicans. Understanding the impact of these factors on such microbial niches may offer new insights into preserving the dynamic balance of the oral microbiome.
Saliva as a represent of internal factor plays a multifaceted role in oral health by mediating pH buffering, lubrication, tooth mineralization, and enhancing host defenses [Citation56]. Among its components, mucin O-glycans, salivary glucose, and amylase are particularly noteworthy. Amylase, a major salivary constituent, is thought to contribute to the reduction of plaque acids produced by S. mutans that can dissolve dental enamel [Citation57]. Mucins, which are large gel-forming polymers, constitute a significant part of the salivary mucus barrier, with MUC5B and MUC7 being the predominant mucins in the human oral cavity [Citation58,Citation59]. [Citation60] Notably, mucin O-glycans have been shown to inhibit hyphal formation in C. albicans through the Nrg1 pathway and regulate microbial community behaviors [Citation58].
Recent research by Caroline A. Werlang et al. [Citation59], utilizing an ex vivo saliva model, identified that mucin glycans alone are sufficient to suppress both genetic transformation and biofilm formation in S. mutans. This finding suggests that mucin glycans can inhibit quorum-sensing-regulated phenotypes, thereby providing evidence for their significant role in modulating the oral microbiota and presenting them as potential therapeutic agents for maintaining oral health [Citation61].
Mechanistic studies focusing on the impact of other microorganisms on the dual-species system comprising C. albicans and S. mutans could establish a more mature and stable framework for future research. Such studies would enhance our understanding of the interaction mechanisms between C. albicans and S. mutans in the oral cavity, especially for potential experiments involving targeted pathway analyses ().
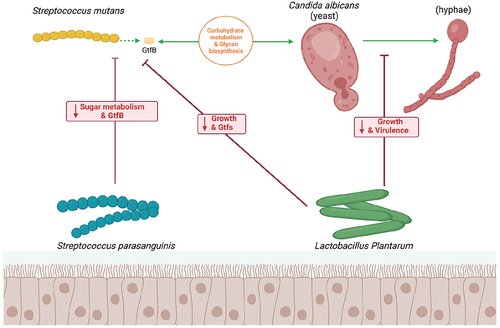
Figure 2. Model of the interplay between C. albicans, S. mutans, and Streptococcus parasanguinis/Lactobacillus plantarum in the human oral. S. mutans use GtfB to attach firmly to C. albicans to prompt each other’s growth which could regulate the carbohydrate metabolism and glycan biosynthesis. L. plantarum reduce the growth of both C. albicans and S. mutans; however, L. plantarum has also been shown to suppress the C. albicans virulence through the hyphal morphogenesis an·d inhibit the expression of GtfB of S. mutans. Like L. plantarum, S. parasanguinis also disturb the sugar metabolism and reduce the expression of GtfB of S. mutans. (Created with BioRender.com).
Insights from interactions with other oral environmental factors
The oral cavity harbors a diverse microbial community comprising fungi and bacteria, which contributes to the increased complexity and severity of oral diseases. Among the bacterial species, Streptococci are predominant in the oral cavity and saliva, encompassing groups such as mitis, sanguinis, anginosus, salivarius, downei, and mutans groups [Citation62]. Notably, common colonizers in the oral cavity include Streptococcus parasanguinis (S. parasanguinis), Streptococcus oralis (S. oralis), Streptococcus gordonii (S. gordonii), Streptococcus mitis (S. mitis), and Streptococcus sanguinis (S. sanguinis) [Citation63]. Due to S. mutans and C. albicans association with the development of caries, mutans Streptococci – Candida interactions have raised attention. Mutans Streptococci including S. mutans and Streptococcus sobrinus (S. sobrinus) is among the most abundant genera present in caries incidence and prevalence, some studies have indicated that children harboring both S. mutans and S. sobrinus exhibit an elevated risk of caries development [Citation64,Citation65]. The association of S. mutans and C. albicans with caries development has drawn significant attention to Streptococci-Candida interactions. A recent study highlighted the essential cooperative strategy where the co-adherence of C. albicans to Streptococci is crucial for its robust colonization within the oral cavity [Citation66,Citation67].
S. parasanguinis
S. parasanguinis, a member of the mitis group streptococci like S. mutans, is commonly found on the tongue dorsum and within the oral cavity [Citation63,Citation68]. Unlike S. mutans, S. parasanguinis can antagonize oral pathogens by producing hydrogen peroxide (H2O2), which also exhibits antimicrobial activity against S. mutans [Citation69,Citation70]. Recent study demonstrates that S. parasanguinis significantly disrupts the biofilm synergy of C. albicans and S. mutans, even in the absence of H2O2 [Citation40]. S. parasanguinis specifically hinders biofilm development by altering the sugar metabolism of S. mutans and inhibiting the activity of GtfB, a critical enzyme for S. mutans to bind to C. albicans mannan [Citation40]. These findings suggest that S. parasanguinis could be exploited to disrupt the synergistic interaction between C. albicans and S. mutans in ECC, underscoring the pivotal roles of sugar metabolism and Gtf enzymes of cariogenic ability in the dual-species biofilm system.
Lactobacillus plantarum (L. plantarum)
L. plantarum has been shown to inhibit the growth and biofilm formation of the C. albicans-S. mutans system, similar to the effects observed with S. parasanguinis [Citation41,Citation42,Citation71]. Studies have indicated that L. plantarum can modulate the gene expression of S. mutans and C. albicans involved in metabolic pathways. Notably, the L. plantarum strain 108 reduces the attachment and biofilm formation of S. mutans in both initial colonization and preformed biofilms by downregulating the expression of the Gtf genes (gtfB, gtfC, and gtfD) and the C. albicans genes HWP1, ALS1, and ALS3 [Citation42] Additionally, strains L. plantarum ATCC 8014 and ATCC 14917 have been shown to downregulate genes involved in exopolysaccharide (EPS) formation, carbohydrate metabolism, and glycan biosynthesis and metabolism in the mixed-species biofilms [Citation41]. Intriguingly, the inhibitory effect of L. plantarum was found to be more pronounced at higher sucrose concentrations (1%) compared to lower ones (0.1%) [Citation41]. Using rat models, Qiuxiang Zhang’s laboratory has reported a significant antagonistic relationship between L. plantarum CCFM8724 and the mixed-species biofilms, with a potential for oral cavity colonization in both treatment and prevention contexts [Citation43]. Collectively, these findings highlight the significant role of L. plantarum in modulating sugar metabolism and virulence gene expression, particularly affecting the levels of S. mutans and C. albicans.
The influence of external factors on oral microbiomes
Antifungal drugs
Antifungal drugs are well-known for targeting essential cell wall components such as β-glucans, mannans, and chitin, which play a crucial role in the interaction with GtfB of S. mutans. Nystatin, an antifungal agent with both fungicidal and fungistatic properties, is commonly used topically in dentistry [Citation72]. This study shows that nystatin can alter the formation and characteristics of C. albicans-S. mutans dual-species biofilms in vitro, consistently reducing biofilm volume and microcolony size on substrate layers [Citation45]. Notably, nystatin-treated biofilms exhibit distinctive halo-shaped microcolonies, and there is a downregulation in the core EPS coverage and expression of the gtfD and atpD genes of S. mutans [Citation45]. Furthermore, investigations into the use of nystatin oral rinse have indicated its impact on oral carriage of C. albicans and S. mutans, suggesting that oral antifungal treatments may effectively influence S. mutans salivary carriage [Citation73]. These findings point to antifungal treatments as a growing potential strategy for managing cariogenic microorganisms in oral environment. The influence of dietary factors will be discussed below.
Sugar substitutes
Sugar substitutes, representing exogenous dietary factors, play a significant role in shaping the oral microbiome and the development of ECC. The study revealed that different sugars could induce the alterations of the components and functions of microbiome biofilms [Citation74]. The consumption of sugars is especially critical given its impact on the mixed-species biofilm system in the oral cavity [Citation75–77]. Sucrose, a fermentable sugar, serves as a substrate for acid and exopolysaccharide production by microorganisms and is widely recognized as the most cariogenic carbohydrate related to dental caries [Citation78–80]. Sugar substitutes can indeed serve as an alternative to sucrose helping to reduce the risk of caries. In the recent study, Galacto-oligosaccharide (GOS), a low-calorie sweetener, has been found to suppress the hyphal formation of C. albicans and the acid resistance of the C. albicans-S. mutans interaction [Citation81]. The finding suggests that sugar substitutes may have potential in inhibiting the growth and virulence of C. albicans and its cooperation with S. mutans, which could contribute to the prevention of caries.
In the quest for sugar substitutes that could prevent for caries, one research has indicated that stevioside significantly inhibits growth and biofilm formation in mixed-species cultures, suggesting its potential as an alternative to sucrose [Citation44]. Interestingly, the addition of 1% sucrose to these cultures counteracted the inhibitory effects of stevioside, highlighting the importance of reducing sucrose intake for dental caries management [Citation44,Citation82]. Among various commercially available sugar substitutes, stevioside, along with other levorotatory carbohydrates such as xylitol and sorbitol, has demonstrated similar inhibitory effects on the mixed-species biofilm [Citation83]. These sugar substitutes appear to be effective carbon sources in disrupting the synergy between C. albicans and S. mutans. The impact of stevioside and other sugar substitutes on sugar metabolism pathways and transcriptomic profiles is complex, pointing to multifaceted mechanisms underlying their effects on oral health [Citation46].
Fluoride (F) as a caries protective agent
F is recognized for its role as a protective agent against caries, due to its ability to stabilize the dynamics of remineralization and demineralization in the oral environment [Citation84,Citation85]. This study indicates that children with access to fluoridated water and supplements exhibit a reduced risk of ECC [Citation86]. The incorporation of F into oral care regimens may enhance enamel resistance to infection by diminishing the attachment of cariogenic bacteria and glycogen accumulation, thus offering a safe protective measure for children [Citation87,Citation88].
Research has demonstrated that F significantly suppresses the expression of genes involved in the transport and modification of mono- and oligosaccharides, as well as enzymes associated with glucose metabolism [Citation89]. Although the impact of F on enolase gene expression was not found to be significant in one study, it is hypothesized that F’s mediation of sugar metabolism, resulting in reduced acid production, may be the primary mechanism of action. Notably, the efficacy of F treatments in inhibiting the growth of S. mutans biofilms is closely linked to the stage of biofilm formation and the F concentration, with treatments being more effective during the early stages of biofilm development than in mature biofilms [Citation90]. These findings suggest that F could potentially serve as an inhibitor of dual-species biofilms formed by S. mutans and C. albicans.
Supporting this notion, Thayse Yumi Hosida et al. [Citation91] proposed that in mixed-species biofilms, F could mitigate the drop in pH and thereby reduce cariogenicity. Specifically, sodium F combined with hexametaphosphate (HMP) led to higher pH levels both before and after sucrose exposure [Citation61,Citation90]. Additionally, F has been shown to interfere with the acidogenicity of S. mutans by modulating gene expression related to glycosyltransferases and glycolytic pathways, leading to an increase in pH [Citation90]. Another study reported that dual-species biofilms treated with calcium glycerophosphate and 500 ppm F exhibited a significant pH increase, resulting in the highest pH values and concentrations of F and calcium within the biofilm biomass, regardless of sucrose exposure [Citation92].
Collectively, these findings highlight the significance of F in combating cariogenic infections.
Conclusion and future perspectives
The interaction between C. albicans and S. mutans is of paramount importance, as both are prevalent oral commensals that pose challenges in pediatric oral healthcare. While the cariogenic potential of S. mutans is relatively well-understood, significant knowledge gaps remain regarding the biological capabilities of mixed-species biofilms to cause ECC. Addressing these gaps is urgent, particularly as both C. albicans and S. mutans exhibit increasing virulence with more complex carbohydrate consumption. Moreover, examining physical interactions alone is insufficient, given the imminent threat of antibiotic resistance faced by both species. The distinct carbohydrate metabolism that largely regulates their interplay provides insights into their persistent attachment and virulence. Therefore, understanding the role of sugar metabolism in facilitating these processes offers opportunities for intervention before further invasion and provides broader perspectives on microbial interactions within various niches.
To date, numerous approaches have been developed to target the cross-kingdom biofilm interaction in oral disease. Gradually, the interaction between S. mutans and C. albicans has emerged as a significantly potential treatment target for ECC. Among the cooperative strategies reviewed here, targeting Gtfs may offer the best choice to inhibit the production of EPSs and impede the co-adhesion between the two species. When dealing with children with poor compliance, rather than strictly restricting carbohydrate intake by eliminating sugar and other foods, a more feasible approach involves replacing sucrose with sugar substitutes and applying F under careful evaluation to delay the development of ECC and potentially achieve preventive effects.
Authors’ contributions
Pingping Jin: Conceptualization, Investigation, Data curation, Writing – original draft, Visualization. Lu Wang: Conceptualization, Investigation, Data curation, Writing – original draft, Visualization. Daozhen Chen: Data curation, Writing – review & editing. Yu Chen: Conceptualization, Writing – review & editing, Supervision, Project administration.
Acknowledgments
We are very grateful to the BioRender website for the draft drawing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
I have shared my data in my review.
Additional information
Funding
References
- Uribe SE, Innes N, Maldupa I. The global prevalence of early childhood caries: a systematic review with meta-analysis using the WHO diagnostic criteria. Int J Paed Dentistry. 2021;31(6):817–11. doi: 10.1111/ipd.12783
- Zou J, Du Q, Ge L, et al. Expert consensus on early childhood caries management. Int J Oral Sci. 2022;14(1):35. doi: 10.1038/s41368-022-00186-0
- Duque C, Chrisostomo DA, Souza ACA, et al. Understanding the predictive potential of the oral microbiome in the development and progression of early childhood caries. Curr Pediatr Rev. 2023;19(2):121–138. doi: 10.2174/1573396318666220811124848
- O’Connell LM, Santos R, Springer G, et al. Site-specific profiling of the dental mycobiome reveals strong taxonomic shifts during progression of early-childhood caries. Appl Environ Microbiol. 2020;86(7):e02825–19. doi: 10.1128/AEM.02825-19
- Pitts NB, Zero DT, Marsh PD, et al. Dental caries. Nat Rev Dis Primers. 2017;3(1):17030.
- Ribeiro AA, Paster BJ. Dental caries and their microbiomes in children: what do we do now? J Oral Microbiol. 2023;15(1):2198433. doi: 10.1080/20002297.2023.2198433
- Cvanova M, Ruzicka F, Kukletova M, et al. Candida species and selected behavioral factors co-associated with severe early childhood caries: case-control study. Front Cell Infect Microbiol. 2022;12. doi: 10.3389/fcimb.2022.943480
- Bachtiar EW, Bachtiar BM. Relationship between Candida albicans and Streptococcus mutans in early childhood caries, evaluated by quantitative PCR. F1000Res. 2018;7:1645. doi: 10.12688/f1000research.16275.2
- Bowen WH, Burne RA, Wu H, et al. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26(3):229–242. doi: 10.1016/j.tim.2017.09.008
- AL Bataineh MT, Soares NC, Semreen MH, et al. Candida albicans PPG1, a serine/threonine phosphatase, plays a vital role in central carbon metabolisms under filament-inducing conditions: a multi-omics approach. PLOS One. 2021;16(12):e0259588.
- Khan F, Bamunuarachchi NI, Tabassum N, et al. Suppression of hyphal formation and virulence of Candida albicans by natural and synthetic compounds. Biofouling. 2021;37(6):626–655. doi: 10.1080/08927014.2021.1948538
- Xiang Z, Wakade RS, Ribeiro AA, et al. Human tooth as a fungal niche: Candida albicans traits in dental plaque isolates. MBio. 2023;14(1):e0276922. doi: 10.1128/mbio.02769-22
- Baraniya D, Chen T, Nahar A, et al. Supragingival mycobiome and inter-kingdom interactions in dental caries. J Oral Microbiol. 2020;12(1):1729305. doi: 10.1080/20002297.2020.1729305
- Xiao J, Grier A, Faustoferri RC, et al. Association between Oral Candida and bacteriome in children with severe ECC. J Dent Res. 2018;97(13):1468–1476. doi: 10.1177/0022034518790941
- Xiao J, Moon Y, Li L, et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PLOS One. 2016;11(10):e0164242. doi: 10.1371/journal.pone.0164242
- Pérez JC. The interplay between gut bacteria and the yeast Candida albicans. Gut Microbes. 2021;13(1):1979877. doi: 10.1080/19490976.2021.1979877
- Burne RA. Oral Streptococci… products of their environment. J Dent Res. 1998;77(3):445–452. doi: 10.1177/00220345980770030301
- Ellepola K, Truong T, Liu Y, et al. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans-Candida albicans mixed-species biofilms. Infect Immun. 2019;87(10):e00339–19. doi: 10.1128/IAI.00339-19
- He J, Kim D, Zhou X, et al. RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front Microbiol. 2017;8:1036. doi: 10.3389/fmicb.2017.01036
- Kim D, Sengupta A, Niepa THR, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7(1):41332.
- Janus MM, Crielaard W, Volgenant CMC, et al. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol. 2017;9(1):1270613. doi: 10.1080/20002297.2016.1270613
- Hwang G, Liu Y, Kim D, et al. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLOS Pathog. 2017;13(6):e1006407. doi: 10.1371/journal.ppat.1006407
- Zhou Y, Cheng L, Liao B, et al. Candida albicans CHK1 gene from two-component system is essential for its pathogenicity in oral candidiasis. Appl Microbiol Biotechnol. 2021;105(6):2485–2496. doi: 10.1007/s00253-021-11187-0
- Graus MS, Wester MJ, Lowman DW, et al. Mannan molecular substructures control nanoscale glucan exposure in Candida. Cell Rep. 2018;24(9):2432–2442.e5. doi: 10.1016/j.celrep.2018.07.088
- Feng Y, Bian S, Pang Z, et al. Deletion of non-histidine domains of histidine kinase CHK1 diminishes the infectivity of Candida albicans in an oral mucosal model. Front Microbiol. 2022;13:855651. doi: 10.3389/fmicb.2022.855651
- Liu Y, Wang Z, Zhou Z, et al. Candida albicans CHK1 gene regulates its cross-kingdom interactions with Streptococcus mutans to promote caries. Appl Microbiol Biotechnol. 2022;106(21):7251–7263. doi: 10.1007/s00253-022-12211-7
- Deng Y, Yang Y, Zhang B, et al. The vicK gene of Streptococcus mutans mediates its cariogenicity via exopolysaccharides metabolism. Int J Oral Sci. 2021;13(1):45. doi: 10.1038/s41368-021-00149-x
- Zhang Q, Ma Q, Wang Y, et al. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int J Oral Sci. 2021;13(1):30. doi: 10.1038/s41368-021-00137-1
- Paes Leme AF, Koo H, Bellato CM, et al. The role of sucrose in cariogenic dental biofilm formation–new insight. J Dent Res. 2006;85(10):878–887. doi: 10.1177/154405910608501002
- Xiao J, Zeng Y, Rustchenko E, et al. Dual transcriptome of Streptococcus mutans and Candida albicans interplay in biofilms. J Oral Microbiol. 2023;15(1):2144047. doi: 10.1080/20002297.2022.2144047
- Burgain A, Tebbji F, Khemiri I, et al. Metabolic reprogramming in the opportunistic yeast Candida albicans in response to hypoxia. mSphere. 2020;5(1):e00913–19.
- Ma Q, Pan Y, Chen Y, et al. Acetylation of glucosyltransferases regulates Streptococcus mutans biofilm formation and virulence. PLOS Pathog. 2021;17(12):e1010134. doi: 10.1371/journal.ppat.1010134
- Hwang G, Marsh G, Gao L, et al. Binding force dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J Dent Res. 2015;94(9):1310–1317. doi: 10.1177/0022034515592859
- Koo H, Andes DR, Krysan DJ. Candida-streptococcal interactions in biofilm-associated oral diseases. PLOS Pathog. 2018;14(12):e1007342. doi: 10.1371/journal.ppat.1007342
- Garcia-Rubio R, de Oliveira HC, Rivera J, et al. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol. 2019;10:2993. doi: 10.3389/fmicb.2019.02993
- Gow NAR, Lenardon MD. Architecture of the dynamic fungal cell wall. Nat Rev Microbiol. 2023;21(4):248–259. doi: 10.1038/s41579-022-00796-9
- Ballou ER, Avelar GM, Childers DS, et al. Lactate signalling regulates fungal β-glucan masking and immune evasion. Nat Microbiol. 2016;2(2):1–9. doi: 10.1038/nmicrobiol.2016.238
- Wu R, Tao Y, Cao Y, et al. Streptococcus mutans membrane vesicles harboring glucosyltransferases augment Candida albicans biofilm development. Front Microbiol. 2020;11:581184. doi: 10.3389/fmicb.2020.581184
- Guo H, Chen Y, Guo W, et al. Effects of extracellular DNA on dual-species biofilm formed by Streptococcus mutans and Candida albicans. Microb Pathog. 2021;154:104838. doi: 10.1016/j.micpath.2021.104838
- Huffines JT, Scoffield JA. Disruption of Streptococcus mutans and Candida albicans synergy by a commensal streptococcus. Sci Rep. 2020;10(1):19661. doi: 10.1038/s41598-020-76744-5
- Zeng Y, Fadaak A, Alomeir N, et al. Lactobacillus plantarum disrupts S. mutans–C. albicans cross-kingdom biofilms. Front Cell Infect Microbiol. 2022;12. doi: 10.3389/fcimb.2022.872012
- Srivastava N, Ellepola K, Venkiteswaran N, et al. Lactobacillus plantarum 108 inhibits Streptococcus mutans and Candida albicans mixed-species biofilm formation. Antibiotics. 2020;9(8):478. doi: 10.3390/antibiotics9080478
- Inhibitory Effect of Lactobacillus plantarum CCFM8724 towards Streptococcus mutans- and Candida albicans-Induced Caries in Rats - PubMed. [cited 2023 Jun 13]. Available from: https://pubmed.ncbi.nlm.nih.gov/33414892/
- Guo M, Yang K, Zhou Z, et al. Inhibitory effects of stevioside on Streptococcus mutans and Candida albicans dual-species biofilm. Front Microbiol. 2023;14:1128668. doi: 10.3389/fmicb.2023.1128668
- Alomeir N, Zeng Y, Fadaak A, et al. Effect of Nystatin on Candida albicans - Streptococcus mutans duo-species biofilms. Arch Oral Biol. 2023;145:105582. doi: 10.1016/j.archoralbio.2022.105582
- Chan A, Ellepola K, Truong T, et al. Inhibitory effects of xylitol and sorbitol on Streptococcus mutans and Candida albicans biofilms are repressed by the presence of sucrose. Arch Oral Biol. 2020;119:104886. doi: 10.1016/j.archoralbio.2020.104886
- Cao Y, Lin H. Characterization and function of membrane vesicles in gram-positive bacteria. Appl Microbiol Biotechnol. 2021;105(5):1795–1801. doi: 10.1007/s00253-021-11140-1
- Cao Y, Zhou Y, Chen D, et al. Proteomic and metabolic characterization of membrane vesicles derived from Streptococcus mutans at different pH values. Appl Microbiol Biotechnol. 2020;104(22):9733–9748. doi: 10.1007/s00253-020-10563-6
- Morales-Aparicio JC, Lara Vasquez P, Mishra S, et al. The impacts of sortase a and the 4’-phosphopantetheinyl transferase homolog sfp on Streptococcus mutans extracellular membrane vesicle biogenesis. Front Microbiol. 2020;11:570219. doi: 10.3389/fmicb.2020.570219
- Rainey K, Michalek SM, Wen ZT, et al. Glycosyltransferase-mediated biofilm matrix dynamics and virulence of Streptococcus mutans. Appl environ microbiol. 2019;85(5):e02247–18. doi: 10.1128/AEM.02247-18
- Senpuku H, Nakamura T, Iwabuchi Y, et al. Effects of complex DNA and MVs with GTF extracted from Streptococcus mutans on the oral biofilm. Molecules. 2019;24(17):3131. doi: 10.3390/molecules24173131
- Wu R, Cui G, Cao Y, et al. Streptococcus mutans membrane vesicles enhance Candida albicans pathogenicity and carbohydrate metabolism. Front Cell Infect Microbiol. 2022;12:940602. doi: 10.3389/fcimb.2022.940602
- Serrage HJ, Jepson MA, Rostami N, et al. Understanding the matrix: the role of extracellular DNA in oral biofilms. Front Oral Health. 2021;2. doi: 10.3389/froh.2021.640129
- Li Y, Du Y, Ye J, et al. Effect of extracellular DNA on the formation of Streptococcus mutans biofilm under sucrose environment. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016;51(2):81–86. doi: 10.3760/cma.j.issn.1002-0098.2016.02.004
- Kawarai T, Narisawa N, Suzuki Y, et al. Streptococcus mutans biofilm formation is dependent on extracellular DNA in primary low pH conditions. J Oral Biosci. 2016;58(2):55–61. doi: 10.1016/j.job.2015.12.004
- Uchida H, Ovitt CE. Novel impacts of saliva with regard to oral health. J Prosthet Dent. 2022;127(3):383–391. doi: 10.1016/j.prosdent.2021.05.009
- Culp DJ, Robinson B, Cash MN. Murine salivary amylase protects against Streptococcus mutans-induced caries. Front physiol. 2021;12:699104. doi: 10.3389/fphys.2021.699104
- Takagi J, Aoki K, Turner BS, et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat Chem Biol. 2022;18(7):762–773. doi: 10.1038/s41589-022-01035-1
- Werlang CA, Chen WG, Aoki K, et al. Mucin O-glycans suppress quorum-sensing pathways and genetic transformation in Streptococcus mutans. Nat Microbiol. 2021;6(5):574–583. doi: 10.1038/s41564-021-00876-1
- Wu CM, Wheeler KM, Cárcamo-Oyarce G, et al. Mucin glycans drive oral microbial community composition and function. NPJ Biofilms Microbiomes. 2023;9(1):11. doi: 10.1038/s41522-023-00378-4
- Pandit S, Cai J-N, Jung J-E, et al. Effect of 1-minute fluoride treatment on potential virulence and viability of a cariogenic biofilm. Caries Res. 2015;49(4):449–457. doi: 10.1159/000434731
- Richards VP, Palmer SR, Pavinski Bitar PD, et al. Phylogenomics and the dynamic genome evolution of the genus Streptococcus. Genome Biol Evol. 2014;6(4):741–753. doi: 10.1093/gbe/evu048
- Abranches J, Zeng L, Kajfasz JK, et al. Biology of oral Streptococci. Microbiol Spectr. 2018;6(5). doi: 10.1128/microbiolspec.GPP3-0042-2018
- Fragkou S, Balasouli C, Tsuzukibashi O, et al. Streptococcus mutans, Streptococcus sobrinus and Candida albicans in oral samples from caries-free and caries-active children. Eur Arch Paediatr Dent. 2016;17(5):367–375. doi: 10.1007/s40368-016-0239-7
- Zhou Q, Qin X, Qin M, et al. Genotypic diversity of Streptococcus mutans and Streptococcus sobrinus in 3–4-year-old children with severe caries or without caries. Int J Paediatr Dent. 2011;21(6):422–431. doi: 10.1111/j.1365-263X.2011.01145.x
- Liu T, Liu J, Liu J, et al. Interspecies interactions between Streptococcus mutans and Streptococcus agalactiae in vitro. Front Cell Infect Microbiol. 2020;10. doi: 10.3389/fcimb.2020.00344
- Lueyar TK, Karygianni L, Attin T, et al. Dynamic interactions between Candida albicans and different streptococcal species in a multispecies oral biofilm. Microbiologyopen. 2023;12(5):e1381. doi: 10.1002/mbo3.1381
- Bernardi S, Karygianni L, Filippi A, et al. Combining culture and culture-independent methods reveals new microbial composition of halitosis patients’ tongue biofilm. Microbiologyopen. 2020;9(2):e958. doi: 10.1002/mbo3.958
- Redanz S, Treerat P, Mu R, et al. Pyruvate secretion by oral streptococci modulates hydrogen peroxide dependent antagonism. Isme J. 2020;14(5):1074–1088. doi: 10.1038/s41396-020-0592-8
- Scoffield J, Michalek S, Harber G, et al. Dietary nitrite drives disease outcomes in oral polymicrobial infections. J Dent Res. 2019;98(9):1020–1026. doi: 10.1177/0022034519855348
- Zeng Y, Fadaak A, Alomeir N, et al. Effect of probiotic lactobacillus plantarum on Streptococcus mutans and Candida albicans clinical isolates from children with early childhood caries. Int J Mol Sci. 2023;24(3):2991. doi: 10.3390/ijms24032991
- Talapko J, Juzbašić M, Matijević T, et al. Candida albicans-the virulence factors and clinical manifestations of infection. J Fungi. 2021;7(2):79. doi: 10.3390/jof7020079
- Aljaffary M, Jang H, Alomeir N, et al. Effects of Nystatin oral rinse on oral Candida species and Streptococcus mutans among healthy adults. Clin Oral Investig. 2023;27(7):3557–3568. doi: 10.1007/s00784-023-04969-5
- Liu Y, Daniel SG, Kim H-E, et al. Addition of cariogenic pathogens to complex oral microflora drives significant changes in biofilm compositions and functionalities. Microbiome. 2023;11(1):123. doi: 10.1186/s40168-023-01561-7
- Adler CJ, Cao K-A, Hughes T, et al. How does the early life environment influence the oral microbiome and determine oral health outcomes in childhood? BioEssays. 2021;43(9):2000314. doi: 10.1002/bies.202000314
- Quivey RG, O’Connor TG, Gill SR, et al. Prediction of early childhood caries onset and oral microbiota. Mol Oral Microbiol. 2021;36(5):255–257. doi: 10.1111/omi.12349
- Samaddar A, Shrikrishna SB, Moza A, et al. Association of parental food choice motives, attitudes, and sugar exposure in the diet with early childhood caries: Case-control study. J Indian Soc Pedod Prev Dent. 2021;39(2):171–177. doi: 10.4103/jisppd.jisppd_104_21
- Du Q, Fu M, Zhou Y, et al. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: an in vitro study. Sci Rep. 2020;10(1):2961. doi: 10.1038/s41598-020-59733-6
- Hajishengallis E, Parsaei Y, Klein MI, et al. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol Oral Microbiol. 2017;32(1):24–34. doi: 10.1111/omi.12152
- Paulino TP, Cardoso JM, Bruschi-Thedei GCM, et al. Fermentable and non-fermentable sugars: a simple experiment of anaerobic metabolism. Biochem Mol Biol Educ. 2003;31(3):180–184. doi: 10.1002/bmb.2003.494031030211
- Huang X, Bao J, Zeng Y, et al. Anti-cariogenic properties of lactobacillus plantarum in the utilization of galacto-oligosaccharide. Nutrients. 2023;15(9):2017. doi: 10.3390/nu15092017
- Guan C, Che F, Zhou H, et al. Effect of rubusoside, a natural sucrose substitute, on Streptococcus mutans biofilm cariogenic potential and virulence gene expression in vitro. Appl Environ Microbiol. 2020;86(16):e01012–20. doi: 10.1128/AEM.01012-20
- Brambilla E, Ionescu AC, Cazzaniga G, et al. Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation. J Basic Microbiol. 2016;56(5):480–492. doi: 10.1002/jobm.201500329
- Bijle MN, Abdalla MM, Hung IFN, et al. The effect of synbiotic-fluoride therapy on multi-species biofilm. J Dent. 2023;133:104523. doi: 10.1016/j.jdent.2023.104523
- Bijle MN, Ashraf U, Abdalla MM, et al. The effect of arginine-fluoride varnish on biochemical composition of multi-species biofilm. J Dent. 2021;108:103631. doi: 10.1016/j.jdent.2021.103631
- Peng S, McGrath C. What can we do to prevent small children from suffering from tooth decay? Evid Based Dent. 2020;21(3):90–91. doi: 10.1038/s41432-020-0111-9
- Do Amaral GCLS, Hassan MA, Saraiva L, et al. The effect of a multicomponent oral care regimen on gingival inflammation: A randomized controlled clinical trial. Journal Of Periodontology; N/A(n/A). doi: 10.1002/JPER.23-0361
- Chen KJ, Gao SS, Duangthip D, et al. Randomized clinical trial on sodium fluoride with tricalcium phosphate. J Dent Res. 2021;100(1):66–73. doi: 10.1177/0022034520952031
- López-López A, Mira A. Shifts in composition and activity of oral biofilms after fluoride exposure. Microb Ecol. 2020;80(3):729–738. doi: 10.1007/s00248-020-01531-8
- Pandit S, Jung J-E, Choi H-M, et al. Effect of brief periodic fluoride treatments on the virulence and composition of a cariogenic biofilm. Biofouling. 2018;34(1):53–61. doi: 10.1080/08927014.2017.1404583
- Hosida TY, Pessan JP, Cavazana TP, et al. Effects of sodium hexametaphosphate and fluoride on the ph and inorganic components of Streptococcus mutans and Candida albicans biofilm after sucrose exposure. Antibiotics. 2022;11(8):1044. doi: 10.3390/antibiotics11081044
- Cavazana TP, Hosida TY, Sampaio C, et al. Calcium glycerophosphate and fluoride affect the pH and inorganic composition of dual-species biofilms of Streptococcus mutans and Candida albicans. J Dent. 2021;115:103844. doi: 10.1016/j.jdent.2021.103844
