ABSTRACT
Background
This study aimed to investigate the effect of honokiol combined with resveratrol on bacteria responsible for oral malodor and their biofilm.
Method
This study investigated drug’s MIC, FICI and dynamic bactericidal susceptibility activities against Pg and Fn. The effects of drugs on biofilm metabolic activity, biofilm total amount, and biofilm microstructure were determined by CCK-8 experiment, semi-quantitative adhesion experiment and SEM, respectively. The effects of drugs on biofilm genes, extracellular polysaccharides, proteins and DNA content were determined by qRT-PCR, phenol-sulfuric acid method, BCA method and Nano Drop one C, respectively.
Results
The combination had synergistic antibacterial effect on Pg and Fn. 1/2×MIC and 1×MIC combination inhibit the whole process of Pg and Fn growth. The results showed that the combination effectively reduce biofilm metabolic activity and total amount, and destroy biofilm microstructure. The results showed that the combination downregulate the gene expression both Pg and Fn, reduce extracellular polysaccharides and DNA of Pg, and reduce extracellular proteins and DNA of Fn.
Conclusion
This study showed that the combination had a synergistic antibacterial effect on Pg and Fn, reduced the biofilm extracellular matrix, inhibited biofilm formation, and downregulated the expression of genes related to biofilm formation.
Introduction
Oral malodor or halitosis refers to odors, mainly composed of volatile sulfur-containing compounds (VSCs), emanating from the mouth or other air-filled cavities (such as the nose, sinuses, pharynx). When the oral microbiome is broken, the proportion of bacteria responsible for oral malodor such as Pg and Fn increases significantly [Citation1]. On the one hand, these bacteria produce substances such as VSCs, amines, and organic acids by breaking down sulfur-containing substrates in food or saliva, which can lead to halitosis [Citation2,Citation3]. On the other hand, the above-mentioned bacteria destroy the oral microbiome through coaggregation, biofilm, quorum sensing, etc.
Pg produces many virulence factors, such as fimbriae, gingipains, hemagglutinin, etc., which can destroy the host defense mechanism, help itself or other oral microorganisms colonize the lesion, and play an important role in the development of oral diseases [Citation4,Citation5]. Fimbriae of Pg promote biofilm formation, bacterial motility, bacterial adhesion and invasion to host cells. Upregulation of fimA gene of Pg promotes the formation of FimA protein, thereby accelerating the adhesion of bacterial to the host surface. On the other hand, it also promotes the formation of the initial biofilm [Citation6]. Kgp-encoded lysine gingipains and hagA-encoded hemagglutinin A participate in the co-aggregation of Pg with other bacteria, interfering with the host’s immune response, thereby helping to evade the host’s defense mechanism [Citation5,Citation7].
Fn is an adherent bacterium, and its mechanism of inducing halitosiss mainly include colonization and transmission, and induction of host response [Citation8]. Adhesin is one of the important virulence factors of Fn, which can mediate the co-aggregation between Fn and colonizing bacteria in plaque (such as co-adhesion between planktonic bacteria), thereby stabilizing the development of dental plaque [Citation9]. Current studies believe that Fn is essential for the formation and maturation of dental plaque biofilms, while many virulence factors of Fn directly disrupt oral microbiome [Citation10]. In addition, specific adhesins encoded by fap2 and fadA contribute to Fn attache to and invade host cells, and subgingival biofilms expressing the fadA gene also exhibit greater pathogenicity [Citation11,Citation12]. Studies have shown that reducing the expression of F. nucleatum’s fadA and its main outer membrane protein, fomA protein, can inhibit the invasion of Fn into human oral epithelial cells [Citation13].
The biofilm of oral pathogenic bacteria greatly improves its drug resistance. Biofilm is a group of organized bacteria that attach to biological or non-living surfaces and are embedded in a protective extracellular polymeric substance (EPS) matrix to form a robust three-dimensional structure. Drugs can easily exert antimicrobial effects on planktonic bacteria, but biofilms prevent drugs from penetrating deep into bacterial cells, so mature biofilms can increase drug resistance [Citation14,Citation15]. Therefore, the study of biofilm inhibitors of oral pathogenic bacteria to avoid bacterial resistance to antimicrobials is a topic of widespread concern among researchers.
The quorum-sensing (QS) system is an intercellular bacteria communication mechanism. AI-2 (autoinducer-2) is the signaling molecule for the LuxS/AI-2 QS system of Pg and Fn. Pg and Fn use the luxS gene to regulate and synthesize their signaling molecule AI-2, which is considered to be the ‘common language’ of oral biofilm interspecific communication, and AI-2 plays a decisive role in promoting biofilm formation and maturation, and AI-2 improves the efficiency of signal transduction through co-adhesion [Citation16]. Studies have proven that the luxS gene not only regulates the signaling molecule AI-2 but also controls the expression of more than 400 genes related to bacterial surface adhesion, motility, and toxin production processes [Citation17]. Meanwhile, most bacteria with biofilm can sense bacterial density through quorum sensing (QS) system to regulate the expression of genes related to their growth and metabolism, so as to control the unified action of the entire bacterial population, and ensure the normal secretion of bacterial metabolites and the stability of the biofilm microenvironment, providing guarantee for the biofilm formation and the normal growth and reproduction of bacteria, while planktonic bacteria do not have the above functions [Citation18,Citation19].
Targeted inhibition of bacterial QS systems is also one of the effective strategies for the treatment of biofilm infections [Citation20]. A large number of studies have shown that QS is related to the regulation of oral bacterial population density, the formation of biofilms, interspecific communication of bacteria, the emergence of drug resistance, and the expression of bacterial virulence factors. Therefore, the activation of the QS system and the formation of biofilms will increase the difficulty of treating bacterial diseases [Citation21]. Therefore, it is important to treat bacterial diseases by inhibiting biofilms and QS systems to reduce bacterial virulence without causing drug resistance [Citation22–24].
The main cause of halitosis is the imbalance of oral microbiome, that is, the bacteria responsible for oral malodor and their biofilms increase significantly, and produce a larger number of VSCs. At present, mechanical cleaning or antibiotics are commonly used to solve the problem of oral malodor, but mechanical cleaning only plays a supplementary role, and antibiotics easily lead to drug resistance in the process of killing bacteria. A significant incidence of antibiotic resistant isolates was reported for Pg and Fn, and clindamycin-resistant and amoxicillin-resistant Pg increased significantly during 1999–2000 [Citation25,Citation26]. Chlorhexidine mouthrinse had beneficial effects in reducing oral malodor [Citation27]. Meanwhile, long-term use of chlorhexidine can cause adverse effects such as extrinsic tooth staining, calculus build up, transient taste disturbance and effects on the oral mucosa. But chlorhexidine is still the most commonly prescribed mouthrinse for oral malodor control due to its excellent results [Citation28]. For the present situation, it is necessary to search for natural sources of antibacterial agents that can inhibit bacteria responsible for oral malodor and their biofilms better than chlorhexidine and have fewer side effects.
The natural source of honokiol and resveratrol has an inhibitory effect on oral pathogens. Magnolia refers to the dried root and stem bark of Magnolia officinalis rehd.et wils. Or Magnolia offinalis rehd.et wils.var.biloba rehd.et wils. [Citation29]. Honokiol is an active ingredient with a high content of Magnolia and an important quality indicator for it [Citation30]. Honokiol demonstrated a strong germ-kill effect against bacteria responsible for halitosis, and Streptococcus mutans involved in dental caries formation [Citation31]. And resveratrol is a novel therapeutic agent for periodontal disease with its ability to be antibacterial, protect the keratinocytes barrier and attenuate the inflammatory response of monocytes [Citation32]. Resveratrol prevents the formation of Pg biofilms, a key pathogen of periodontal disease, and attenuates the virulence of Pg by reducing the expression of virulence factor genes such as fimbriae (types II and IV) and proteases kgp (Lys-gingipain) and rgpA (arg-gingipains A) [Citation33].
Meanwhile, the previous experiments of our research group showed that honokiol and resveratrol had good antibacterial effects on Pg and Fn, but compared with chlorhexidine, the effect was still a certain gap. It is of great interest to find a natural antibacterial agent that has the similar antibacterial effect as chlorhexidine, but without the side effects of chlorhexidine. On the other hand, there are no research reports related to the effect of honokiol combined with resveratrol on bacteria (Pg, Fn) responsible for oral malodor and their biofilms. Therefore, chlorhexidine was set as the positive control, we studied the antibacterial activity of honokiol combined with resveratrol on Pg and Fn, its scavenging effect on the biofilm, its effect on the genes of biofilm formation, and its effect on the formation of biofilm matrix.
Materials and methods
Antimicrobial agents and chemicals
Honokiol (C18H18O2) was purchased from Shanghai Macklin Biochemical Technology Co., (Ltd≥purity ≥98%, lot number C10026295). Resveratrol (C14H12O3) was purchased from Chengdu Must Bio-technology Co., Ltd (purity 98.71%, lot number MUST-19101105).Chlorhexidine (C22H30Cl2N10) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd (purity 98%, lot number C12196688). Honokiol and resveratrol were dissolved in dimethyl sulfoxide and prepared as a stock solution at a concentration of 256 mg/mL. Chlorhexidine was prepared as a positive control drug stock solution at a concentration of 128 mg/mL.
Bacterial strain and growth conditions
Porphyromonas gingivalis (P. gingivalis, Pg, standard strain, ATCC 33,277) and Fusobacterium nucleatum (F. nucleatum, Fn, standard strain, ATCC 25,586) were purchased from Beijing Microbiological Culture Collection Center. The quality control strain Bacteroides fragilis (ATCC 25,285) was purchased from Guangdong Microbial Culture Collection Center.
The bacteria used for the experiment were inoculated on brain heart infusion agar (BHI) medium (Qingdao Haibo Biotechnology Co., Ltd, China), cultured in a sealed anaerobic bags (Mitsubishi Gas Chemical Company, Inc, Japan), and kept in a constant temperature incubator at 37°C for 48–72 hours. During low-temperature storage, all isolates were stored in sterile purified water containing 20% glycerol at − 80°C. BHI broth and BHI agar were prepared and sterilized according to the manufacturer’s recommendations before use.
Antibacterial susceptibility testing of antimicrobial agents on tested strains
Determination of the minimal inhibitory concentration (MIC)
The determination of MIC was carried out according to the method described by the M11-A7 scheme of Clinical and Laboratory Standards Institute (CLSI). In short, BHI broth was used to dilute the bacterial solution to 1 × 106 CFU/mL.
Honokiol, resveratrol and chlorhexidine were diluted in a 96-well microtiter plate to a final concentration of 2560–5 μg/mL, 2560–5 μg/mL and 1280–0.039 μg/mL, respectively. The 96-well plate was incubated at 37°C anaerobically for 24–48 h, and the lowest concentration of the agent that inhibits the growth of P. gingivalis or F. nucleatum is considered the MIC. All MICs were confirmed by repeated tests.
Quality control. According to the M11-A7 scheme formulated by CLSI, the test process and environment need to be tested for quality control during the drug susceptibility test. Bacteroides fragilis (ATCC 25,285) was used as the quality control strain, and Metronidazole as the quality control drug. The MIC of the quality control strain is in the range of 0.25 to 2 µg/mL under the conditions of parallel operation, the determination result is considered valid and reliable.
Determination of fractional inhibitory concentration index (FICI)
Based on references and slightly modified [Citation34], the micro-checkerboard dilution method was used to determine the FICI of honokiol and resveratrol to explore the interaction between them. According to MICs of honokiol and resveratrol, in the checkerboard format, each row of 96-well microtiter plate contains a decreased concentration of honokiol (in twofold decrements; 1MIC–1/16MIC), and each column contains a decreased concentration of resveratrol (in twofold decrements; 1MIC–1/16MIC). The 96-well plate was incubated at 37°C anaerobically for 24–48 h. The lowest concentration of the agent that inhibits the growth of tested strain is considered the MIC of combination. All MICs were confirmed by repeated tests.
The calculation method of FICI is as follows: FICI = (MIC of drug A in combined use/MIC of drug A used alone) + (MIC of drug B in combined use/MIC of drug B used alone). FICI was used as the basis for the judgment of combined drug susceptibility test. FICI ≤ 0.5 denoted synergistic effect; 0.5 < FICI ≤ 1 denoted additive effect; 1 < FICI ≤ 2 denoted irrelevant effect; FICI > 2 denoted antagonistic effect.
Time–kill curve assay
Honokiol and resveratrol were, respectively, diluted into three concentrations of MIC, 1/2MIC and 1/4MIC, and the concentrations were combined with each other to determine the time kill curve. A growth group, a honokiol group, a resveratrol group, a chlorhexidine group, a combination of honokiol and resveratrol group were set up, respectively. Drugs with different concentrations or drugs with different combined concentrations were added to the BHI medium with a microbial concentration of 1 × 106 CFU/mL, and cultured in a constant temperature shaking box at 37°C anaerobically. The optical density at 630 nm (OD630 nm) was measured by iMark microplate reader (BIO-RAD, USA) at 0, 4, 8, 12, 24, 36, 48, 60, 72 h, respectively. The time–kill curve was drawn by taking culture time of the tested strain as the abscissa and the OD630 nm value as the ordinate.
Antibacterial susceptibility testing of antimicrobial agents on biofilm
Determination of minimal biofilm inhibitory concentration (MBIC)
200 μL BHI medium containing 1 × 106 CFU/mL microbial concentration was inoculated on sterile 96 micro-well plates, and incubated at 37°C anaerobically for 48–72 h until biofilm formation. Then BHI medium and planktonic bacteria were discarded, and the plate was rinsed 3 times with sterile PBS. The antimicrobial agent was diluted with BHI medium to obtain honokiol (5120 ~ 5 μg/mL), resveratrol (5120 ~ 5 μg/mL) and chlorhexidine (2560 ~ 2.5 μg/mL). A 200 μL sample of each dilution was taken and added to 96-well plate, growth control wells (containing BHI medium and bacterial solution) and negative control wells (containing BHI medium) were also set, and incubated at 37°C anaerobically for 24 h. The lowest concentration of the agent that inhibits the growth of P. gingivalis or F. nucleatum is considered the MBIC [Citation35].
Determination of the MBIC of honokiol combined with resveratrol on the tested strain
Based on references and slightly modified [Citation36], the micro-checkerboard dilution method was used to determine the MBIC of honokiol combined with resveratrol on the tested strain. 200 μL BHI medium containing 1 × 106 CFU/mL microbial concentration was inoculated on sterile 96 micro-well plates, and incubated at 37°C anaerobically for 72 h until biofilm formation. Then BHI medium and planktonic bacteria were discarded, and the plate was rinsed 3 times with sterile PBS.
In the checkerboard format, each row of 96-well microtiter plate contains an decreased concentration of honokiol (in twofold decrements; 1 MIC-1/16 MIC), and each column contains an decreased concentration of resveratrol (in twofold decrements; 1 MIC-1/16 MIC). The 96-well plate was incubated at 37°C anaerobically for 24 h. The lowest concentration of the agent that inhibits the growth of tested strain is considered the MBIC of combination. All MBICs were confirmed by repeated tests.
Determination of the eradication effect of antimicrobial agents on biofilm
The optimal concentration combination of antimicrobial agents was selected according to the above-mentioned MBIC measurement experiment results. For the tested strain Pg, 20 μg/mL honokiol combined with 80 μg/mL resveratrol was selected, and 10 μg/mL chlorhexidine was selected. For the tested strain Fn, 16.67 μg/mL honokiol combined with 53.33 μg/mL resveratrol was selected, and 5.62 μg/mL chlorhexidine was selected.
CCK-8 experiment
The CCK-8 experiment was used to determine the effect of drugs on the metabolic activity of tested strain in the biofilm
WST-8 in CCK-8 can be reduced to the water-soluble product Formazan by the dehydrogenase in the mitochondria, and the amount of Formazan is proportional to the metabolic activity of cells. 200 μL BHI medium containing 1 × 106 CFU/mL microbial concentration was inoculated on 96 micro-well plates, and incubated at 37°C. Anaerobic culture of Pg for 36, 60, and 84 h. Anaerobic culture of Fn for 24, 42, and 60 h. After anaerobic culture, the medium and planktonic bacteria in each well were discarded, wash with PBS 3 times, add 200 μL antibacterial agents and continue to culture for 24 h, then the plate was rinsed with sterile PBS 3 times, dried naturally and 100 μL BHI medium and 10 μL CCK-8 reagent (Dalian Meilun Biotechnology Co., LTD, China) were added to each well. After incubating at 37°C anaerobically for 1 h in the dark, the bacterial growth (OD450 nm) was measured with a iMark microplate reader (BIO-RAD, USA), and the influence of drugs on growth kinetic curve of tested strain biofilm was drawn.
Semi-quantitative adhesion experiment
Semi-quantitative adhesion test was used to determine the effect of drugs on the total amount of biofilm
Samples were treated in the same way as CCK-8 experiment above. After washing with PBS, the biofilm was fixed with methanol for 30 min, the methanol was then discarded, 200 μL of 0.1% crystal violet staining solution was added to each well, and the unadhered dye was washed off after staining for 15 min. Finally, 95% ethanol was added to dissolve the dye bound to the biofilm, it was gently shaken to make it fully dissolved for 10 min, the bacterial growth (OD570 nm) was measured with a iMark microplate reader (BIO-RAD, USA).
Effect of antimicrobial agents on the biofilm microstructure
Based on references and slightly modified [Citation37], SEM (JSM-7610F Plus, JEOL, Japan) was used to observe the effect of the drug on the biofilm microstructure. A sterile round slide was placed on a 24-microwell plate, 1 mL of inoculum (1 × 106 CFU/mL) was added to each well. Anaerobic culture of Pg for 36, 60, and 84 h. Anaerobic culture of Fn for 24, 42, and 60 h.
The BHI medium and planktonic bacteria in each well were then discarded and after rinsing 3 times, 1 mL of 2.5% glutaraldehyde fixative solution for electron microscopy was added for fixation overnight at 4°C. After aspirating and discarding the fixative, ethanol of different concentration gradients (30, 50, 70, 80, 90, and 95%) was used to perform gradient elution on each well. Each well was eluted twice by each concentration gradient, for 15 min each time, and finally it was dehydrated with 100% ethanol twice, for 30 min each time. After dehydration, 100% tert-butanol was added to replace ethanol twice, each for 30 min. After the dehydrated samples were freeze-vacuum dried, the samples to be tested were glued to the stage with conductive adhesive, and the samples were sprayed with gold for 90 s using a vacuum ion sputtering instrument, and then placed in a SEM for observation and the images were taken.
Gene of biofilm formation expression analysis
The tested strain biofilm with or without drug was established according to the previous method. The primers used here are listed in . And Trizol reagent was used to extract RNA according to the manufacturer’s protocol. A 1 μL aliquot of 4 RNA samples was randomly taken, and the 5S rRNA, 18S rRNA and 28S rRNA bands of total RNA were observed with a gel imaging system. If the three bands are complete, the total RNA extraction is relatively intact. Then the Prime Script TMRT reagent Kit was used for the reverse transcription reaction. Finally, the SYBR® Green Pro Taq HS premixed qPCR reagent Kit was used to prepare the reaction system for qRT-PCR detection. The results of qRT-PCR data were analyzed using the relative expression level 2−△△CT method with 16s rRNA as the internal reference gene.
Table 1. Primer sequence of target genes.
Determination of effect of antimicrobial agents on biofilm matrix
Preparation of biofilm EPSs
The tested strain biofilm with or without drug was established according to the previous method, and growth control group was set up. The formed biofilm was resuspended with sterile PBS to a bacterial suspension with OD600 nm = 0.40 ± 0.01. The bacterial suspension was bathed in water at 80°C for 30 min, centrifuged at 5 000 r/min for 15 min, and the supernatant was collected and filtered with a 0.22 μm filter to obtain biofilm extracellular polymers (EPSs).
Determination of extracellular polysaccharides in biofilm
Phenol-sulfuric acid method was used to determine the polysaccharides in biofilm. The glucose standard curve was drawn by preparing a series of glucose solution with gradient concentrations, concentrated sulfuric acid and 5% phenol solution, respectively, and then measuring the OD490 nm, using the OD value as the vertical coordinate and the glucose concentration (ug/mL) as the horizontal coordinate, drawing the standard curve and plotting the linear regression equation. The biofilm EPSs was taken and reacted with concentrated sulfuric acid/phenol reagent to determine OD490 nm, and the corresponding polysaccharide content was calculated according to its OD value of the glucose standard curve.
Determination of extracellular protein in biofilm
The biofilm extracellular protein content was determined by BAC method. The biofilm EPSs was measured using the BCA kit (Jiancheng Biotech, Nanjing, China), and at 595 nm was measured by iMark microplate reader (BIO-RAD, USA). The biofilm extracellular protein of the sample was calculated according to the OD value.
Determination of eDNA in biofilm
Nano Drop one C was used to determine the eDNA in biofilm. The tested strain biofilm with or without drug was established according to the previous method, and growth control group was set up. The formed biofilm was resuspended with TEN buffer to a bacterial suspension with OD600 nm = 0.40 ± 0.01. The bacterial suspension was extracted with a bacterial genomic DNA extraction kit (Solarbio, Beijing, China) to obtain the eDNA-containing solutions. The eDNA content of the eDNA-containing solutions were determined with Nano Drop one C.
Statistical analysis
Each analysis was repeated at least three times, and the software GraphPad Prism 8.0.1 was used for statistical analysis of the data. Ordinary one-way ANOVA was used for comparison between the two groups. A value of p < 0.05 was considered to indicate if the difference was statistically significant.
Results
The result of antibacterial susceptibility testing of antimicrobial agents on tested strains
The MIC results
shows that honokiol and resveratrol have significant antibacterial effects on both Pg and Fn. The MIC values of honokiol against Pg and Fn are 30.00 ± 11.55 μg/mL and 22.50 ± 12.58 μg/mL, respectively. The MIC values of resveratrol against Pg and Fn are 160.00 ± 0.00 μg/mL. The MIC values of chlorhexidine against Pg and Fn are 1.88 ± 0.63 μg/mL and 0.94 ± 0.31 μg/mL, respectively.
Table 2. The MIC results of drugs against tested strains (n = 3).
Honokiol combined with resveratrol will be selected as the antimicrobial agents for the follow-up experiment.
The FICI results
shows that FICI values of honokiol combined with resveratrol against Pg and Fn are 0.45 ± 0.07 μg/mL and 0.38 ± 0.00 μg/mL. FICI ≤ 0.5 denoted synergistic effect. For Pg and Fn, both honokiol and resveratrol MICcombination values are equal to a quarter of their MICsingle values.
Table 3. The FICI results of drugs against tested strains (n = 3).
Time–kill curve assay
The effects of honokiol combined with resveratrol on the growth of Pg.
(A) and (B) shows that time–kill curve of Pg. The growth control group showed rapid growth from 6 to 48 h, indicating that this period was the logarithmic growth phase of Pg. After 48 h, the growth slowed down and entered a stable growth period.
Figure 1. Effect of honokiol combined with resveratrol on the growth curve of Pg and Fn (n=5).
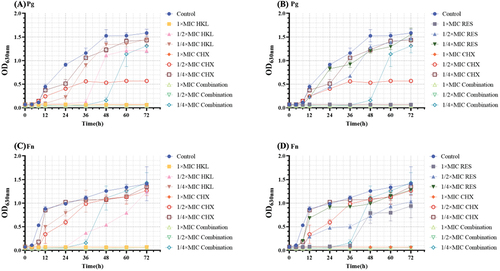
With the increase of single-agent concentration, the growth rate decreases and the killing effect becomes more and more obvious, showing concentration dependence. During the logarithmic growth phase, the growth rate and growth of Pg treated with CHX, honokiol or resveratrol were significantly lower than those in the growth control group, and the growth process of Pg could be inhibited with 1×MIC CHX, 1×MIC HKL or 1×MIC RES. 1/2×MIC CHX halves the growth of Pg. The logarithmic growth phases of 1/4×MIC HKL group and 1/2×MIC HKL group were delayed until the 24 h and 36 h, respectively.
1/2×MIC combination and 1×MIC combination had a killing effect on the whole process of Pg growth, and 1/4×MIC combination delayed Pg growth to the 36 h. Compared with the single-agent group, the effect of 1/2×MIC combination group can achieve the effect of the 1×MIC CHX group, and its effect is also significantly better than honokiol and resveratrol single-agent group with corresponding concentration.
The effects of honokiol combined with resveratrol on the growth of Fn
(C) and (D) shows that time–kill curve of Fn. The growth control group showed rapid growth from 4 to 12 h, indicating that this period was the logarithmic growth phase of Fn. At 12–48 h, the growth rate slowed down and entered the growth stability period, and the growth rate flattened at 48–72 h and entered the mature stage.
Effect of single-agent on Fn growth. 1×MIC HKL and 1×MIC CHX can inhibit the whole process of Fn growth. The logarithmic growth phases of 1/2×MIC HKL group and 1×MIC RES group were delayed until the 24 h and 36 h, respectively. 1/2×MIC RES reduces the growth of Fn.
The single-agent group was compared to the combination group. Fn growth in the 1/4×MIC Combination was delayed until 36 h. Moreover, as the concentration increased, the 1/2×MIC and 1×MIC Combination groups had a killing effect on the whole process of Fn growth. The effect of 1/2×MIC Combination was comparable to that of 1×MIC CHX, and its bactericidal effect was stronger than that of the single-drug group with the corresponding concentration.
The result of antibacterial susceptibility testing of antimicrobial agents on biofilm
Pg means Porphyromonas gingivalis; Fn means Fusobacterium nucleatum
The results are shown as . The MBICsingle values of honokiol against Pg and Fn are 60 ± 23.09 μg/mL, and that of resveratrol against Pg and Fn are 280.00 ± 80.00 μg/mL and 240.00 ± 92.37 μg/mL, and that of chlorhexidine against Pg and Fn are 10.00 ± 4.08 μg/mL and 5.62 ± 3.14 μg/mL, respectively.
Table 4. MBIC of drugs on Pg and Fn (n = 3).
The concentration of combined drug can be reduced to 1/3 ~ 1/4 that of a single drug. The MBICcombination values of honokiol combined with resveratrol against Pg are 20.00 ± 0.00 μg/mL honokiol + 80.00 ± 0.00 μg/mL resveratrol, and that against Fn are 16.67 ± 5.77 μg/mL honokiol + 53.33 ± 23.09 μg/mL resveratrol, respectively.
The result of the eradication effect of antimicrobial agents on biofilms
CCK-8 experiment
The results are shown in (A1), (B1) and (C1)
The combination of honokiol and resveratrol can effectively inhibit the bacterial metabolic activity in the Pg biofilm at all stages, which is better than honokiol or resveratrol alone, and similar to the effect of the positive drug chlorhexidine.
Figure 2. Effect of drugs on bacterial metabolic activity at each stage of Pg and Fn biofilm formation (n=3).
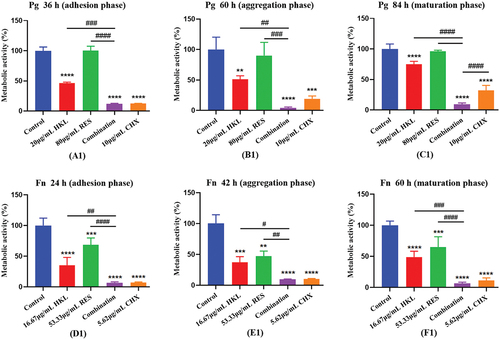
Compared with the control group, the bacteria metabolic activity of combination group in the adhesion, aggregation and mature stages decreased by 96.78%, 96.19% and 92.13%, respectively (P < 0.0001).
Compared with the HKL or RES group, the combination group had better effects in the adhesion stage, aggregation stage and mature stage (p < 0.01), and the effects were 12.79 times, 13.01 times and 9.44 times that of HKL group, and 29.04 times, 22.87 times and 11.92 times that of RES group.
There was no significant difference in the effect of the combination group compared with the CHX group (p > 0.05).
The results are shown in (D1), (E1) and (F1)
The combination of honokiol and resveratrol can effectively inhibit the bacterial metabolic activity in the Fn biofilm at all stages, which is better than honokiol or resveratrol alone, and similar to the effect of the positive drug chlorhexidine.
Compared with the control group, the bacteria metabolic activity of combination group in the adhesion, aggregation and mature stages decreased by 96.73%, 81.28% and 82.44%, respectively (P < 0.0001).
Compared with HKL or RES group, the combination group had better effects in the adhesion stage and mature stage (p < 0.01), and the effects were 11.99 times and 2.93 times that of HKL group, and 10.48 times and 3.67 times that of RES group. Compared with HKL or RES group, the combination group had better effects in the aggregation stage (p < 0.05), and the effects were 3.17 times and 3.61 times that of HKL and RES group, respectively.
There was no significant difference in the effect of the combination group compared with the CHX group (p > 0.05).
Semi-quantitative adhesion experiment
The results are shown as (A2), (B2), (C2)
The combination of honokiol and resveratrol can effectively reduce the total amount of biofilm formation at each stage of Pg, which is better than that of honokiol and resveratrol alone, and similar to the effect of positive drug chlorhexidine.
Figure 3. Effect of drugs on the Pg and Fn biomass at each stage of biofilm formation (n=3).
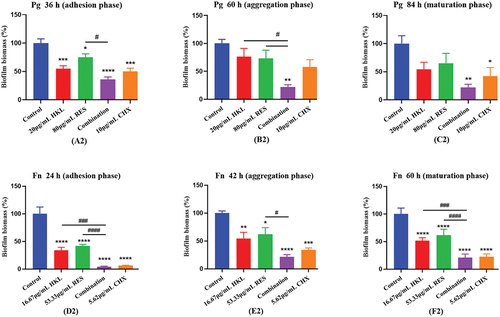
Compared with the control group, the total amount of Pg biofilm of combination group in the adhesion, aggregation and mature stages decreased by 66.05%, 78.01% and 81.42%, respectively.
The combination group was compared with HKL or RES group. During the adhesion phase, the effect of the combination group was better than that of RES group (p < 0.05), and the effect was 2.09 times that of RES group. During the aggregation phase, the effect of the combination group was better than that of the HKL or RES group (p < 0.05), and the effects were 3.48 times and 3.33 times that of HKL and RES group, respectively. During the maturation phase, the effects of the combination group were 3.54 times and 2.44 times higher than those of HKL and RES group, respectively.
There was no significant difference in the effect of the combination group compared with the CHX group (p > 0.05).
The results are shown as (D2), (E2), (F2)
The combination of honokiol and resveratrol can effectively reduce the total amount of biofilm formation at each stage of Fn, which is better than that of honokiol and resveratrol alone, and similar to the effect of positive drug chlorhexidine.
Compared with the control group, the total amount of Fn biofilm of combination group in the adhesion, aggregation and mature stages decreased by 93.34%, 91.44% and 94.13%, respectively (P < 0.0001).
The combination group was compared with HKL or RES group. The effect of the combination group was better than that of the HKL or RES group (P < 0.001), and the effects were 5.05 times and 8.78 times that of HKL group in adhesion and maturation phase, and the effects were 10.35 times and 12.16 times that of RES group in adhesion and maturation phase, respectively. During the aggregation phase, The effect of the combination group was better than that of RES group (P < 0.05), and the effects were 5.69 times that of RES group.
There was no significant difference in the effect of the combination group compared with the CHX group (p > 0.05).
Effect of antimicrobial agents on the biofilm microstructure
The results are shown as
Figure 4. Observe the effect of drugs on the Pg biofilm morphological structure at different stages by SEM (×10000).
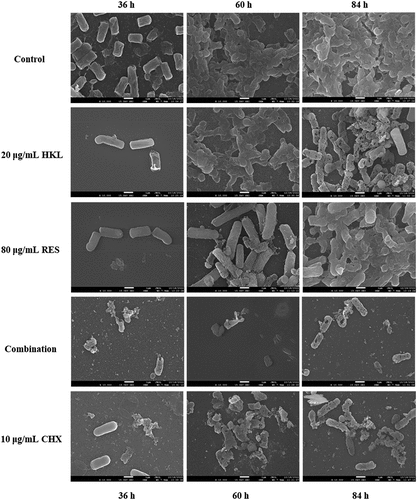
During the adhesion phase (36 h), compared with the control group, the bacteria number of group treated with the drug was reduced, and their structure changed to varying degrees. The bacteria number of HKL and RES groups decreased, and their structure was slightly deformed, but cell division and differentiation could still proceed normally. The bacteria number of combination group was drastically reduced, and almost no formed bacteria remained. The bacteria number of the CHX group decreased, and some bacteria were severely deformed.
During the aggregation phase (60 h), the bacteria of control group gradually accumulate into clumps, and they are adhered by the extracellular matrix. The bacteria number of group treated with the drug was relatively reduced, and their bacterial bodies were deformed and ruptured. Honokiol and resveratrol caused a decrease in bacterial numbers, but there are still partially aggregated bacterial clumps. The number of bacteria in combination group was greatly reduced, and the bacterial clumps disappeared, and the bacterial morphology appeared obvious deformation and rupture, and there were traces of bacterial content flow. Chlorhexidine leads to a significant reduction in the number of bacteria and visible rupture on the surface of the organism.
During the maturation phase (84 h), the extracellular matrix of the bacteria in the control group is adhered to form a dense, three-dimensional biofilm. Under the influence of drugs, the number and bacteria morphology are reduced and deformed to varying degrees, respectively. Honokiol and resveratrol cause slight rupture and deformation of the bacterial surface, but the dense biofilm remains. The number of bacteria in the combined group was greatly reduced and the bacterial clumps were broken, and the three-dimensional biofilm was severely damaged, leaving traces of a large number of bacterial deaths. The number of bacteria in the CHX group was greatly reduced, the biofilm was destroyed, only a small number of bacterial clumps remained, and the bacteria morphology was significantly broken and deformed.
(2) The results are shown in .
Figure 5. Observe the effect of drugs on the Fn biofilm morphological structure at different stages by SEM (×20000).
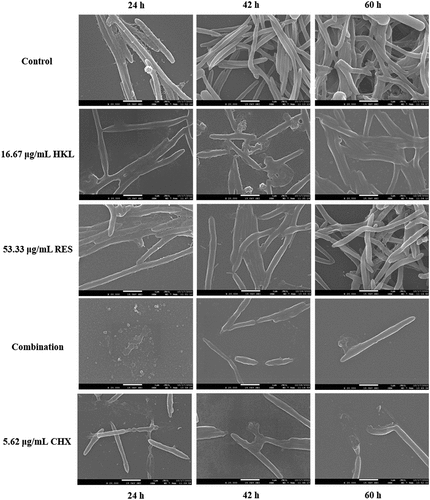
During the adhesion phase (24 h), the bacteria in the control group were rod-shaped; however, the bacteria number of group treated with drug was relatively reduced. Honokiol and resveratrol reduce the bacteria number, but do not affect their morphological structure, and bacteria can still carry out cell division and differentiation normally. The number of bacteria in combination group was greatly reduced, and there were no formed bacteria, only traces of bacterial body rupture remained. The bacteria number of CHX group decreased, and bacteria rupture severely.
During the aggregation phase (42 h), the bacteria of control group aggregate into clumps by extracellular matrix adhesion. The bacteria number of group treated with drug was relatively reduced, bacteria show varying degrees of deformation and rupture. Bacteria in HKL and RES groups decreased, but there were still partially aggregated bacterial clumps. The bacterial clumps in combination group disappeared, leaving only a single bacterium that was significantly deformed and ruptured. The bacteria in the CHX group were greatly reduced, and the bacteria ruptured and shrunk without forming.
During the maturation phase (60 h), the bacteria in the control group formed a three-dimensional dense biofilm with a certain thickness.
Under the influence of drugs, the number and bacteria morphology are reduced and deformed to varying degrees, respectively. The bacteria in the HKL and RES groups became shorter and decreased in number, but the dense biofilm remained. The bacteria in the combination group were greatly reduced and the bacteria were ruptured, the biofilm thinned and its three-dimensional structure was severely damaged. The bacteria in the CHX group were greatly reduced, the bacteria changed from slender to short, and the biofilm was destroyed.
Gene of biofilm formation expression
The results are shown as .
Figure 6. Expression of Pg biofilm formation related genes after drug intervention (n=3).
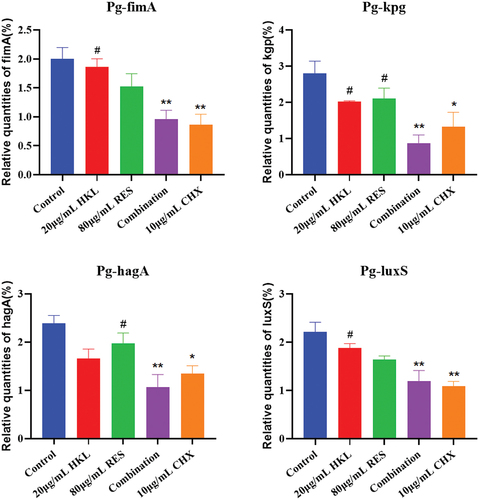
Compared with the growth control group, the relative expression of fimA genes in the HKL, RES, Combination and CHX group decreased by 6.67%, 18.39%, 51.83% and 56.67%, and kgp genes decreased by 27.50%, 25.00%, 68.69% and 52.62%, and hagA gene decreased by 30.68%, 17.57%, 55.37% and 43.65%, and luxS gene decreased by 14.78%, 25.64%, 45.85% and 50.68%, respectively.
Compared with the growth control group, the expression of fimA, kgp, hagA and luxS gene in the Combination group decreased significantly (p < 0.01).
Compared with HKL group, the expression of fimA, kgp and luxS gene in the Combination group decreased significantly (P < 0.05). Compared with RES group, the expression of hagA gene in the Combination group decreased significantly (P<0.05). There was no significant difference between the Combination group and the CHX group (p > 0.05).
The results are shown as .
Figure 7. Expression of Fn biofilm formation related genes after drug intervention (n=3).
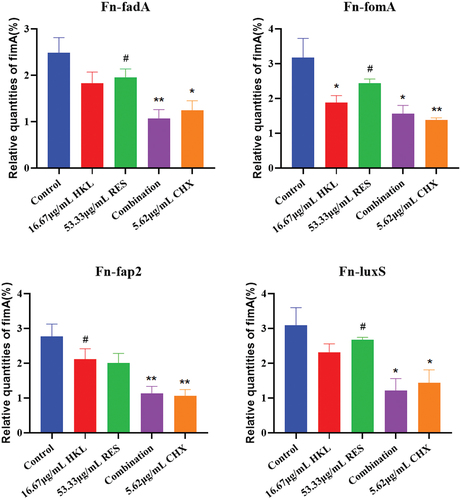
Compared with the growth control group, the relative expression of fadA genes in the HKL, RES, Combination and CHX group decreased by 26.21%, 21.24%, 56.85% and 49.87%, and fomA genes decreased by 40.69%, 23.13%, 50.58% and 56.47%, and fap2 gene decreased by 23.35%, 27.32%, 58.97% and 61.49%, and luxS gene decreased by 24.92%, 13.27%, 60.73% and 53.61%, respectively.
Compared with the growth control group, the expression of fadA and fap2 gene in the Combination group decreased significantly (p < 0.01), and that of fomA and luxS gene in the Combination group decreased significantly (p < 0.05)
Compared with HKL group, the expression of fap2 gene in the Combination group decreased significantly (P<0.05). Compared with RES group, the expression of fadA, fomA and luxS gene in the Combination group decreased significantly (P < 0.05). There was no significant difference between the Combination group and the CHX group (p > 0.05).
Effect of antimicrobial agents on biofilm matrix
Effect of antimicrobial agents on biofilm extracellular polysaccharides
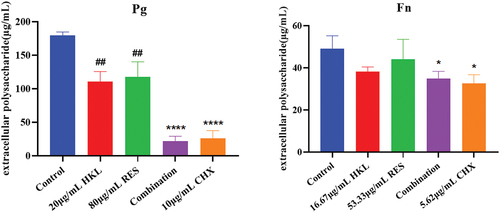
The results are shown in . Compared with the growth control group, biofilm extracellular polysaccharides of Pg in the HKL, RES, Combination and CHX group decreased by 38.37%, 34.33%, 74.71% and 85.55%, respectively. There were significant differences compared the Combination group and the CHX group with the growth control group (P < 0.01). The combination group had a better effect on the biofilm extracellular polysaccharides of Pg than HKL or RES group (p < 0.01).
The results are shown in . Compared with the growth control group, biofilm extracellular polysaccharides of Fn in the HKL, RES, Combination and CHX group decreased by 22.09%, 10.40%, 33.46% and 44.98%, respectively. There were significant differences compared the Combination group and the CHX group with the growth control group (P < 0.05).
Figure 8. Effect of drugs on biofilm extracellular polysaccharide of Pg and Fn (n = 3).
Effect of antimicrobial agents on biofilm extracellular protein
The results are shown in . Compared with the growth control group, biofilm extracellular proteins of Pg in the HKL, RES, Combination and CHX group decreased by 12.38%, 23.80%, 23.19% and 29.93%, respectively. There were significant differences compared the RES, Combination and CHX group with the growth control group (P < 0.05).
The results are shown in . Compared with the growth control group, biofilm extracellular proteins of Fn in the HKL, RES, Combination and CHX group decreased by 3.56%, 3.56%, 78.29% and 29.89%, respectively. There was a significant difference compared the Combination group with the growth control group (P < 0.001).
Figure 9. Effect of drugs on biofilm extracellular proteins of Pg and Fn (n=3).
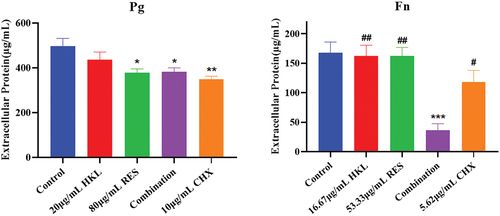
The combination group had a better effect on the biofilm extracellular protein of Fn than the CHX group (p < 0.05) and was more effective than HKL or RES group (p < 0.01).
Effect of antimicrobial agents on biofilm extracellular DNA
The results are shown in . Compared with the growth control group, biofilm extracellular DNA of Pg in the HKL, RES, Combination and CHX group decreased by 9.97%, 15.43%, 50.13% and 50.85%, respectively. There were significant differences compared the Combination and CHX group with the growth control group (P < 0.001). The effect of biofilm extracellular DNA of Pg in Combination group was similar to that of CHX group (p > 0.05), and the effect was more significant than that of HKL group (p < 0.01) and RES group (p < 0.05).
The results are shown in . Compared with the growth control group, biofilm extracellular DNA of Fn in the HKL, RES, Combination and CHX group decreased by 48.40%, 5.10%, 64.60% and 72.41%, respectively. There were significant differences compared the HKL (P < 0.0001), RES (P < 0.01), Combination (P<0.0001) and CHX group (P<0.0001) with the growth control group.
Figure 10. Effect of drugs on biofilm extracellular DNA of Pg and Fn (n=3).
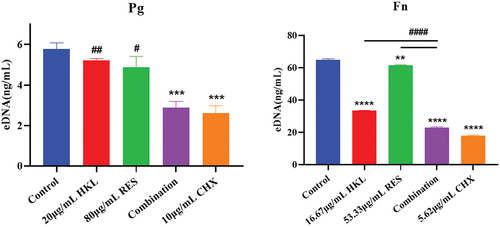
The combination group had a better effect on the reduction of Fn biofilm extracellular DNA than that of HKL or RES group (p < 0.0001), and the effect was similar to that of the CHX group (p > 0.05).
Discussion
The resistance of oral-derived halitosis pathogens and their biofilms to antibiotics continues to increase, seriously hindering the treatment of halitosis. Specifically, Fn and Pg proliferate, decomposing food scraps to produce volatile sulfur-containing compounds (VSCs) that cause halitosis. On the other hand, Fn and Pg easily adhere and form drug-resistant biofilms, which increases the difficulty of halitosis treatment. Meanwhile, long-term use of chlorhexidine can cause adverse effects although chlorhexidine mouthrinse has excellent effect for oral malodor control. Therefore, it is necessary to find natural compounds that can inhibit bacteria responsible for oral malodor, inhibitory effect is similar to that of chlorhexidine, have fewer side effects, and do not cause pathogenic bacteria to resist drugs.
The combination of natural antibacterial agents to achieve synergistic antibacterial effect is a new method to solve the problem of drug-resistant bacteria and biofilm infection. Studies have shown that honokiol has antibacterial activity against Pg and Fn, and has inhibitory effect on Streptococcus mutans and its biofilms [Citation31,Citation38,Citation39]. Resveratrol demonstrated ant-bacterial and anti-biofilm activity against Pg. Resveratrol attenuated the virulence of Pg by reducing the expression of virulence factor genes such as fimbriae (type Il and IV) and gingipain (kgp and rgpA) [Citation33]. In addition, resveratrol has an inhibitory effect on Fn and its biofilms [Citation40]. Another application direction of natural antimicrobials is to combine antibiotics or other natural antimicrobials to reduce the amount of antibiotics, reduce drug-resistant bacteria, and achieve synergistic antibacterial effects. Resveratrol combined with aminoglycoside antibiotics can produce synergistic antibacterial activity against Staphylococcus aureus [Citation41]. Curcumin combined with gentamicin has synergistic antibacterial effect, inhibits Pseudomonas aeruginosa biofilm formation and down-regulates QS related genes [Citation42]. Sapindoside A combined with sapindoside B produced synergistic anti-biofilm activity against Cutibacterium acnes [Citation43]. Therefore, this study focused on honokiol combined with resveratrol to inhibit Pg and Fn, inhibit its biofilm formation, downregulate its biofilm formation-related genes, and reduce its biofilm matrix.
Our study showed that the FICI of the combination drug against Pg and Fn was 0.45 ± 0.07 and 0.38 ± 0.00, respectively. That is, the combination drug has a synergistic antibacterial effect on both Pg and Fn. Some studies show that resveratrol has a MIC value of 100 μg/mL for F. nucleatus (ATCC10953), and resveratrol at concentrations below 25 μg/mL could not inhibit the growth of F. nucleatus (ATCC10953) [Citation40]. Our study showed that the MIC of resveratrol against planktonic Fn decreased from 160.00 μg/mL to 26.67 μg/mL after combination, which increased the antimicrobial activity of resveratrol against planktonic Fn. Obviously, the MIC values of resveratrol against Fn obtained in our study is different from the previous studies reported in the literature. The variation in MIC values in comparison to previous studies may be due to resveratrol purity and source of the strain.
Oral malodor not only brings social annoyance but also accompanies oral diseases such as periodontitis and gingivitis, so maintaining oral health and reducing oral malodor is an urgent need for patients with halitosis [Citation44]. The main oral care products that are commonly used to treat oral malodor are antibacterial agents such as chlorhexidine or triclosan, and antibiotics such as metronidazole, clindamycin and amoxicillin that are used clinically [Citation45]. The above antibacterial agents and antibiotics work quickly in the short term, but they cannot completely cure oral malodor. Long-term use them will destroy the oral microecological balance and produces bacterial resistance, which makes oral malodorh treatment more difficult. The advantage of our study is that chlorhexidine is used as a positive control, natural antibacterial agents honokiol is combined with resveratrol, and the antibacterial effect of combined antibacterial agents and chlorhexidine is compared, which provides a reference for solving the problem of halitosis. However, this study only demonstrated the effect of honokiol combined with resveratrol on bacteria and their biofilms in vitro. But, the oral environment is complex, and there are many influencing factors for biofilm formation. Therefore, the effect of the combination drug on the biofilms of Pg and Fn in vivo should be further studied.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mysak J, Podzimek S, Sommerova P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014:476068. doi: 10.1155/2014/476068
- Awano S, Gohara K, Kurihara E, et al. The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis. Int Dent J. 2002;52(3):212–17. doi: 10.1002/j.1875-595X.2002.tb00927.x
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and Malodor-Etiology and Management. Int J Mol Sci. 2020;21(8):2886. doi: 10.3390/ijms21082886
- Plančak D, Musić L, Puhar I. Quorum sensing of periodontal pathogens. Acta Stomatol Croat. 2015;49(3):234–241. doi: 10.15644/asc49/3/6
- Lunar Silva I, Cascales E. Molecular strategies underlying porphyromonas gingivalis virulence. J Mol Biol. 2021;433(7):166836. doi: 10.1016/j.jmb.2021.166836
- Kuboniwa M, Amano A, Hashino E, et al. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by porphyromonas gingivalis. BMC Microbiol. 2009;9(1):105. doi: 10.1186/1471-2180-9-105
- Ito R, Ishihara K, Shoji M, et al. Hemagglutinin/Adhesin domains of porphyromonas gingivalis play key roles in coaggregation with treponema denticola. FEMS Immunol Med Microbiol. 2010;60(3):251–260. doi: 10.1111/j.1574-695X.2010.00737.x
- Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013
- Yang R, Liu T, Pang C, et al. The regulatory effect of coaggregation between fusobacterium nucleatum and streptococcus gordonii on the synergistic virulence to human gingival epithelial cells. Front Cell Infect Microbiol. 2022;12:879423. doi: 10.3389/fcimb.2022.879423
- Zhao T, Chen J, Liu S, et al. Transcriptome analysis of Fusobacterium nucleatum reveals differential gene expression patterns in the biofilm versus planktonic cells. Biochem Biophys Res Commun. 2022;593:151–157. doi: 10.1016/j.bbrc.2021.11.075
- Liu P, Liu Y, Wang J, et al. Detection of fusobacterium nucleatum and fadA adhesin gene in patients with orthodontic gingivitis and non-orthodontic periodontal inflammation. PLoS One. 2014;9(1):e85280. doi: 10.1371/journal.pone.0085280
- Coppenhagen-Glazer S, Sol A, Abed J, et al. Fap2 of fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect Immun. 2015;83(3):1104–1113. doi: 10.1128/IAI.02838-14
- Zhang Z, Liu S, Zhang S, et al. Porphyromonas gingivalis outer membrane vesicles inhibit the invasion of fusobacterium nucleatum into oral epithelial cells by downregulating FadA and FomA. J Periodontol. 2022;93(4):515–525. doi: 10.1002/JPER.21-0144
- Van Acker H, Van Dijck P, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22(6):326–333. doi: 10.1016/j.tim.2014.02.001
- Kurtzman GM, Horowitz RA, Johnson R, et al. The systemic oral health connection: biofilms. Medicine (Baltimore). 2022;101(46):e30517. doi: 10.1097/MD.0000000000030517
- Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. 2017;44(18):S12–S22. doi: 10.1111/jcpe.12679
- Hardie KR, Cooksley C, Green AD, et al. Autoinducer 2 activity in Escherichia coli culture supernatants can be actively reduced despite maintenance of an active synthase, LuxS. Microbiology (Reading). 2003;149(Pt 3):715–728. doi: 10.1099/mic.0.25853-0
- Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2(5):435–444. doi: 10.4161/viru.2.5.16140
- Lazar V. Quorum sensing in biofilms–how to destroy the bacterial citadels or their cohesion/power. Anaerobe. 2011;17(6):280–285. doi: 10.1016/j.anaerobe.2011.03.023
- Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2006;254(1):1–11. doi: 10.1111/j.1574-6968.2005.00001.x
- Neelakantan P, Romero M, Vera J, et al. Biofilms in endodontics-current status and future directions. Int J Mol Sci. 2017;18(8):1748. doi: 10.3390/ijms18081748
- Jiang Q, Chen J, Yang C, et al. Quorum sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed Res Int. 2019;2019:2015978. doi: 10.1155/2019/2015978
- Polizzi A, Donzella M, Nicolosi G, et al. Drugs for the quorum sensing inhibition of oral biofilm: New frontiers and insights in the treatment of periodontitis. Pharmaceutics. 2022;14(12):2740. doi: 10.3390/pharmaceutics14122740
- Mohamad F, Alzahrani RR, Alsaadi A, et al. An explorative review on advanced approaches to overcome bacterial resistance by curbing bacterial biofilm formation. Infect Drug Resist. 2023;16:19–49. doi: 10.2147/IDR.S380883
- Ng E, Tay J, Boey SK, et al. Antibiotic resistance in the microbiota of periodontitis patients: an update of current findings. Crit Rev Microbiol. 2023;50(3):1–12. doi: 10.1080/1040841X.2023.2197481
- Rams TE, Sautter JD, van Winkelhoff AJ. Emergence of antibiotic-resistant porphyromonas gingivalis in United States periodontitis patients. Antibiotics. 2023;12(11):1584. doi: 10.3390/antibiotics12111584
- Blom T, Slot DE, Quirynen M, et al. The effect of mouthrinses on oral malodor: a systematic review. Int J Dent Hyg. 2012;10(3):209–222. doi: 10.1111/j.1601-5037.2012.00546.x
- James P, Worthington HV, Parnell C, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3(3): CD008676. doi: 10.1002/14651858.CD008676.pub2
- Luo H, Wu H, Yu X, et al. A review of the phytochemistry and pharmacological activities of magnoliae officinalis cortex. J Ethnopharmacol. 2019;236:412–442. doi: 10.1016/j.jep.2019.02.041
- Sarrica A, Kirika N, Romeo M, et al. Safety and toxicology of magnolol and honokiol. Planta Med. 2018;84(16):1151–1164. doi: 10.1055/a-0642-1966
- Greenberg M, Urnezis P, Tian M. Compressed mints and chewing gum containing magnolia bark extract are effective against bacteria responsible for oral malodor. J Agric Food Chem. 2007;55(23):9465–9469. doi: 10.1021/jf072122h
- Ben Lagha A, Andrian E, Grenier D. Resveratrol attenuates the pathogenic and inflammatory properties of porphyromonas gingivalis. Mol Oral Microbiol. 2019;34(3):118–130. doi: 10.1111/omi.12260
- Kugaji MS, Kumbar VM, Peram MR, et al. Effect of resveratrol on biofilm formation and virulence factor gene expression of porphyromonas gingivalis in periodontal disease. APMIS. 2019;127(4):187–195. doi: 10.1111/apm.12930
- Nair S, Desai S, Poonacha N, et al. Antibiofilm activity and synergistic inhibition of staphylococcus aureus biofilms by bactericidal protein P128 in combination with antibiotics. Antimicrob Agents Chemother. 2016;60(12):7280–7289. doi: 10.1128/AAC.01118-16
- Wang L, Di Luca M, Tkhilaishvili T, et al. Synergistic activity of fosfomycin, ciprofloxacin, and gentamicin against escherichia coli and pseudomonas aeruginosa biofilms. Front Microbiol. 2019;10:2522. doi: 10.3389/fmicb.2019.02522
- Behbehani JM, Irshad M, Shreaz S, et al. Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates. J Mycol Med. 2019;29(2):158–167. doi: 10.1016/j.mycmed.2019.01.011
- Cai Z, Mo Z, Zheng S, et al. Flavaspidic acid BB combined with mupirocin improves its anti-bacterial and anti-biofilm activities against Staphylococcus epidermidis. BMC Microbiol. 2022;22(1):179. doi: 10.1186/s12866-022-02578-y
- Sakaue Y, Domon H, Oda M, et al. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol Immunol. 2016;60(1):10–16. doi: 10.1111/1348-0421.12343
- Ren S, Yang Y, Xia M, et al. A Chinese herb preparation, honokiol, inhibits Streptococcus mutans biofilm formation. Arch Oral Biol. 2023;147:105610. doi: 10.1016/j.archoralbio.2022.105610
- He Z, Huang Z, Zhou W, et al. Anti-biofilm activities from resveratrol against fusobacterium nucleatum. Front Microbiol. 2016;7:1065. doi: 10.3389/fmicb.2016.01065
- Abedini E, Khodadadi E, Zeinalzadeh E, et al. A comprehensive study on the antimicrobial properties of resveratrol as an alternative therapy. Evid Based Complement Alternat Med. 2021;2021:8866311. doi: 10.1155/2021/8866311
- Bahari S, Zeighami H, Mirshahabi H, et al. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J Glob Antimicrob Resist. 2017;10:21–28. doi: 10.1016/j.jgar.2017.03.006
- Wei MP, Yu H, Guo YH, et al. Synergistic combination of sapindoside a and B: A novel antibiofilm agent against Cutibacterium acnes. Microbiol Res. 2022;254:126912. doi: 10.1016/j.micres.2021.126912
- Li Z, Li J, Fu R, et al. Halitosis: etiology, prevention, and the role of microbiota. Clin Oral Investig. 2023;27(11):6383–6393. doi: 10.1007/s00784-023-05292-9
- Saad S, Greenman J, Shaw H. Comparative effects of various commercially available mouthrinse formulations on oral malodor. Oral Dis. 2011;17(2):180–186. doi: 10.1111/j.1601-0825.2010.01714.x
