Abstract
Background: In this study, markers of coagulation and fibrinolysis were assessed during early and delayed microsurgical reconstruction in patients with traumatic defects of their lower legs to analyse whether an imbalance of the hemostasis after trauma might predispose the development of vascular complications.
Methods: The prospective study included 70 patients. In 35 patients, surgery was performed within 72 hours after injury. In 35 other patients, delayed free flap transfer was performed between 14–21 days after trauma. In each group, reconstruction was performed with a fasciocutaneous anterior-lateral thigh flap (ALT, n = 18) or a myocutaneous flap (latissimus dorsi flap; n = 17). Blood samples were collected preoperatively, intraoperatively, and 3, 6, 12, 24, 36, 48, 72, 96 and 120 hours after the operation. Analysed parameters included markers of coagulation such as prothrombin fragment 1 + 2 (F1 + 2), thrombin-antithrombin III-complex (TAT), and antithrombin, as well as fibrinolysis markers such as plasminogenactivator inhibitor-I (PAI-1), tissue-plasminogenactivator (t-PA), and plasminogen.
Results: Preoperatively, levels of F1 + 2, TAT, and PAI-1 were significantly higher in patients with delayed reconstruction (p < .05). Patients with later vascular complications in this group (n = 5) presented a significant higher concentration of TAT, F1 + 2, and PAI-1 (p < .05). Twelve and 24 hours after free flap surgery, patients with vascular complications presented significant elevated levels of these markers (p < .05).
Conclusions: Patients with delayed free flap surgery after lower leg trauma present a hypercoagulable state in their blood due to activation of the coagulation system and hypofibrinolysis. Early reconstruction might minimise the risk of flap failure caused by hypercoagulability.
Introduction
Most of the factors contributing to microvascular thrombosis and failure of free flaps focus on technical aspects of the anastomosis [Citation1,Citation2]. Some authors have also pointed to the importance of systemic factors [Citation3,Citation4]. The relationship between free flap failure in the lower extremities and periods of delay from the time of injury have been reported as a relevant systemic factor [Citation2,Citation5,Citation6]. Godina [Citation6] has reported the highest rate of complications in those patients undergoing surgery between 72 hours and 3 months post-injury. Posttraumatic thrombocytosis is believed to be a key factor associated with microvascular thrombosis [Citation7]. Only several studies have been conducted to examine factors of blood coagulation and fibrinolysis during microsurgery [Citation3,Citation4,Citation8–11].
Multiple enzymatic cleavage reactions, as part of the coagulation cascade, result in the production of thrombin and fibrin, which ultimately cause adherence (‘sticking’) of platelets. Several biomarkers of coagulation activation and thrombin generation exist. The conversion of prothrombin to thrombin by prothrombinase complex results in the production of a degradation product with a half-life of <90 minutes; prothrombin fragment 1 + 2 (F1 + 2) [Citation12]. Thrombin released through this enzymatic process is inactivated by antithrombin to form a thrombin–antithrombin III-complex (TAT) [Citation13]. In the consecutive process of fibrinolysis, the essential generation of plasmin takes place [Citation14]. Patients suffering from major trauma often turn into a hypercoagulable state, which then frequently leads to pathological thrombosis [Citation15]. Abnormal coagulation parameters can be found in 25% of trauma patients with major injuries [Citation16]. Previous studies in trauma patients were able to highlight a significant and persistent increase in markers of thrombin generation and action, such as F1 + 2 and TAT [Citation17].
Tissue trauma and systemic hypoperfusion appear to be the primary factors responsible for the development of acute traumatic coagulopathy immediately after injury [Citation18]. As a result of overt activation of the protein C pathway, the acute traumatic coagulopathy is characterised by coagulopathy in conjunction with hyperfibrinolysis. This coagulopathy can then be exacerbated by subsequent physiologic and physical derangements such as consumption of coagulation factors, haemodilution, hypothermia, acidemia, and inflammation, all factors being associated with ongoing haemorrhage and inadequate resuscitation or transfusion therapies [Citation19]. Later on, problems related to hypofibrinolysis can occur after different kinds of trauma due to release of plasminogenactivator inhibitor (PAI)-1 from the endothelium [Citation20].
In the setting of free tissue transfer, multiple studies state an overall success rate from 90%–99% [Citation1]. As mentioned before, trauma patients are frequently in a hypercoagulable state before surgery, which will increase the incidence of thromboembolic events and might encompass problems during or after microsurgical reconstruction [Citation21,Citation22].
In this study, we have assessed markers of coagulation and fibrinolysis at different time points in patients with previous trauma and early and delayed microsurgical reconstruction in the lower extremities. The aim was to analyse whether an activated coagulation system after trauma might predispose a patient to develop thrombosis or coincide with flap failure in the context of free flap surgery.
Patients and methods
Patients
Our prospective study included 70 patients with microsurgical reconstruction of the lower extremity due to complicated fractures of the lower leg. Patients were included in a consecutive fashion over a period of ∼18 months. Thirty-five patients (19 male, 16 female, average age = 42 ± 11 years) underwent free flap reconstruction within 72 hours after trauma (early reconstruction group). Eighteen of these patients received a fasciocutaneous flap (anterior-lateral thigh flap; ALT), 17 a myocutaneous flap (latissimus dorsi flap; LD). Thirty-five patients (21 male, 14 female, mean age = 39 ± 9 years) had a delayed free flap transfer between 14–21 days after trauma (mean = 17 ± 2 days). Seventeen of these patients had a microsurgical reconstruction with an ALT flap, 18 with a latissimus dorsi flap. The defect size ranged from ∼10 × 15 cm to 25 × 30 cm in both groups and did not differ significantly from each other. Blood samples were collected with the written permission of the informed patients and with the approval of the local Ethical Committee. None of the patients had taken aspirin or non-steroidal anti-inflammatory drugs or a history of thromboembolic events. Two patients of the early reconstruction group had a family history of thromboembolic events. Pre- and postoperatively, all patients received thrombosis prophylaxis with low-molecular-weight heparin-dalteparin (Fragmin, Pharmacia & Upjohn) at 5000 IU subcutaneously.
Analysed haemostatic markers
Plasma samples were collected serially at 11 timepoints: baseline (24 hours before surgery), after opening the microanastomoses (intraoperatively), and 3, 6, 9, 12, 24, 48, 72, 96, and 120 hours after surgery. Blood was collected into citrate tubes, centrifuged at 3500 rpm for 10 minutes, and plasma was separated, divided into aliquots, and frozen at −80 °C for subsequent analysis. The analysed parameters included markers of coagulation and fibrinolysis. Analysed markers for activation were: prothrombin fragment 1 + 2 (F1 + 2) by ELISA (Enzygnost® F1 + 2, Behring Diagnostics Inc., Westwood, MA), thrombin-antithrombin complex (TAT) by ELISA (Enzygnost® TAT, Behring Diagnostics Inc.), and antithrombin using semi quantitative ELISA assay (Asserachrom antithrombin®, Diagnostica Stago, Asnieres, France). Markers of fibrinolysis included: plasminogen activator inhibitor-1 (PAI-1) by ELISA (Asserachrom PAI-1®, Diagnostica Stago), tissue-type plasminogen activator by ELISA (Asserachrom t-PA®, Diagnostica Stago), plasminogen by ELISA (Actichrome® Plasminogen, American Diagnostica, Stamford, CT).
Statistical analysis
Statistical analysis to compare data from the two study groups was performed using the unpaired Mann–Whitney U-test. Friedman’s analysis was used to detect changes with time within each group. p < .05 was considered to be statistically significant.
Results
There was no significant difference in the operating time between both groups (4.7 ± 1.3 vs 4.9 ± 1.5 hours). In the group with early reconstruction of defects of the lower leg, two patients underwent reoperation due to venous thrombosis of the free flap. In the delayed reconstructive group, four patients had a venous thrombosis and one an arterial problem between 12–24 hours after surgery and underwent reoperation. Two flaps were lost in this group ().
Table 1. Overview of complications: early vs delayed group.
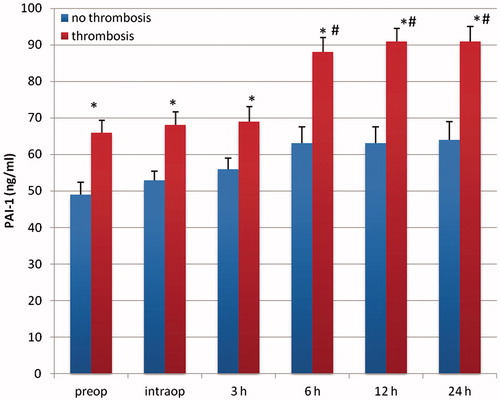
Patients with delayed microsurgery after trauma presented a hypercoagulable state with clotting activation and inhibition of fibrinolysis. Preoperatively, levels of F1 + 2 and TAT were significantly higher in patients with delayed reconstruction, indicating an activation of the clotting system (p < .05; and ). Patients in the delayed reconstructive group with later vascular complications presented a significantly higher concentration of TAT and F1 + 2 (p < .05; and ). Twelve and 24 hours after free flap surgery, patients with vascular complications presented a significant elevated level of both markers in comparison with the preoperative value (p < .05; and ). PAI-1 was significantly higher in patients with delayed reconstruction (p < .05; ). Between 12–36 hours after operation, this factor showed a significant increase in patients with delayed reconstruction in comparison to the preoperative value (p < .05). Patients with later vascular complications in this group had a significantly higher PAI-1 concentration (p < .05) than those with no complications, starting preoperatively (). They also presented a significantly higher concentration between 6–24 hours after operation in comparison to the preoperative value (p < .05). Plasminogen, antithrombin, and t-PA presented no significant difference between both patient groups.
Figure 1. F1 + 2 (nM) in patients with early and delayed microsurgical reconstruction of a traumatic defect of their lower leg. *p < .05 vs early reconstruction.
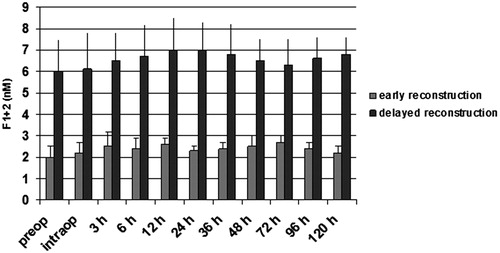
Figure 2. TAT (ng/ml) in patients with early and delayed microsurgical reconstruction of a traumatic defect of their lower leg. * p < .05 vs early reconstruction.
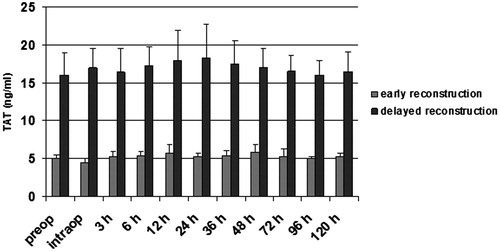
Figure 3. F1 + 2 (nM) in patients with vascular and without vascular complications after delayed microsurgical reconstruction of a traumatic leg defect. *p < .05 vs no complications. #p < .05 vs preop.
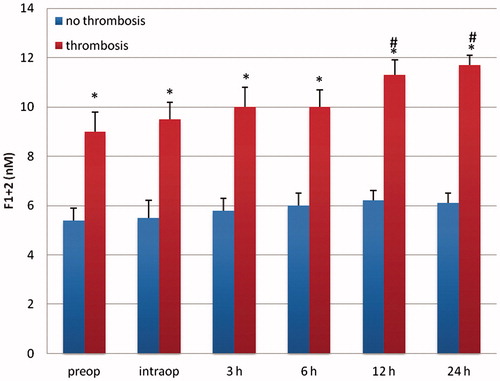
Figure 4. TAT (ng/ml) in patients with vascular and without vascular complications after delayed microsurgical reconstruction of a traumatic leg defect. *p < .05 vs no complications. #p < .05 vs preop.
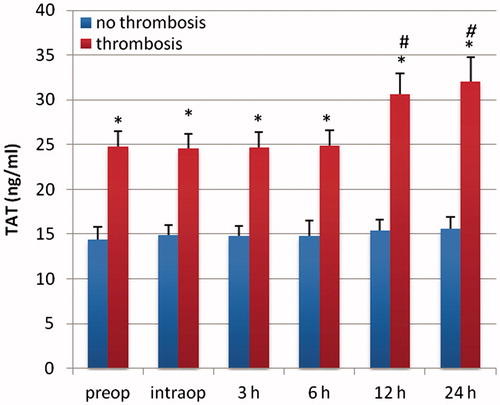
Figure 5. PAI-1 (ng/ml) in patients with early and delayed microsurgical reconstruction of a traumatic defect of their lower leg. *p < .05 vs early reconstruction. #p < .05 vs preop.
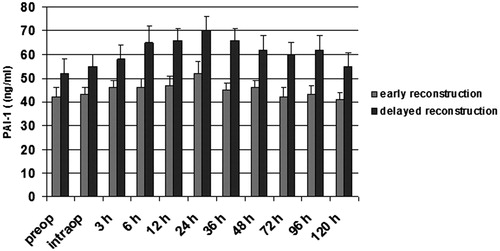
Figure 6. PAI-1 (ng/ml) in patients with vascular and without vascular complications after delayed microsurgical reconstruction of a traumatic leg defect. *p < .05 vs no complications. #p < .05 vs preop.
No significant differences in the concentration of the analysed parameters of the haemostasis and fibrinolysis could be observed when comparing patients that were reconstructed with a fasciocutaneous (ALT) or myocutaneous flap (LD).
Discussion
One reason for free flap failure can be a hypercoagulable state in the patient’s blood [Citation3,Citation4]. Thrombembolic events based on a hypercoagulable predisposition (genetic and/or acquired causes) are quite prevalent in the general population. In the field of genetically determined hypercoagulable predispositions, mutations of factor V (factor V Leiden gene R506Q mutation) and factor II (prothrombin G20210A mutation) are undoubtedly the most prevalent in clinical settings. Furthermore, elevated homocystein levels as a consequence of mutations of the methylene tetrahydrofolate reductase gene (C677T and A1298C polymorphisms) as well as modulation of the endogenous fibrinolytic activity based on mutations of the plasminogen activator inhibitor-type 1 gene (4 G/5 G alleles) are two other clinical relevant genetically hypercoagulable states [Citation3]. Davison et al. [Citation3] presented four patients with major free flap microvascular complications who were later diagnosed with multiple risk factors for hypercoagulability and biochemical abnormalities. Also patients with malignancy or autoimmune disorders like antiphospholipid antibody syndrome can produce a hypercoagulable state with problems for microsurgical reconstruction.
The influence of a disturbed balance between clotting system and fibrinolysis on the complication rate of microsurgical reconstructions after trauma is still unknown. Severe trauma significantly predisposes the patient for venous thromboembolic events and syndromes of ‘micro thrombosis’ such as disseminated intravascular coagulation (DIC) and systemic inflammatory response syndrome (SIRS) based on elevated levels of procoagulatory factors [Citation23]. A plethora of studies have demonstrated a pronounced imbalance between pro- and anticoagulatory factors in the setting of trauma, e.g. upregulation of tissue factor (TF) and markers of thrombin generation [Citation19,Citation24–26] and simultaneous reduction of natural anticoagulants/fibrinolytic factors such as antithrombin (AT), protein C (PC), and protein S (PS) [Citation25,Citation26]. However, to which extent the posttraumatic hypofibrinolytic state contributes to this hypercoagulability with all its deleterious clinical consequences remains unclear [Citation18,Citation27].
A prothrombotic shift of the coagulation after trauma has been demonstrated by several authors. Selby et al. [Citation15] measured several markers of in vivo coagulation and fibrinolysis and their regulation serially for 2 weeks after multi-system trauma in a prospective cohort of 135 patients who received no anticoagulant prophylaxis. Thrombin generation had increased within 24 hours of injury, indicated by a marked increase in the patients’ baseline levels of F1 + 2 compared to normal controls. Another major increase occurred by day 5. The levels decreased to a level of 2.0 nM, which was still elevated in comparison to normal controls, within 12–14 days. PAI-1 levels were high within 24 hours after trauma, but decreased by 48 hours after injury, and decreased further until the end of the study. D-Dimer was markedly elevated in almost all patients at baseline, and remained elevated at all subsequent time points for the entire 2-week study period.
Meissner et al. [Citation24] analysed markers of the clotting system in 101 trauma patients, and found significantly elevated F1 + 2 levels on admission. The levels remained elevated throughout the first month of follow-up, indicating an activation of the clotting system. All patients also presented significantly elevated D-Dimer levels.
Beside trauma, also after major surgery, an activation of the clotting system can be observed [Citation14,Citation21,Citation22]. Several factors can alter postoperative coagulation changes. These include the type of operation performed and the type of anaesthesia [Citation14,Citation21,Citation22].
Only a few studies have addressed hypercoagulable states as a risk factor for the thrombosis in the context of microsurgery [Citation9–11,Citation28]. Olsson et al. [Citation9] analysed coagulation and fibrinolysis activities in relation to trauma, surgery, and thrombosed microanastomoses during free-flap surgery in eight patients with lower extremity defects due to recent trauma or chronic ulcers. One of their patients had an intraoperative thrombosis. Three patients required reoperations on the same day due to postoperative thromboses. At the end of primary surgery, distinct thrombin generation was seen in three patients with excessive bleeding and later reoperations by high levels of TAT and F1 + 2. D-dimer was higher in patients with recent trauma. In two of these patients with high D-dimer concentration, reoperation was performed the same day. These patients also presented increased PAI-1 levels with hypofibrinolysis. TAT and F1 + 2 were associated with the threat of flap failure. Komuro et al. [Citation8] investigated blood coagulation activity in nine patients who underwent microsurgical reconstruction. Parameters included prothrombin time, activated partial thromboplastin time, fibrinopeptide A, F1 + 2, and TAT. In one patient with free flap failure, FPA, F1 + 2, and TAT values significantly increased. The authors postulated that these markers might be important in indicating a hypercoagulable state.
Other groups investigated the impact of timing of microvascular reconstruction on flap survival as well [Citation29–32]. Kolbenschlag et al. [Citation29] described a significant reduction in flap loss in the early reconstruction group (within the first 7 days) vs reconstruction after day 7, and advocate some form of platelet inhibition in addition. Karanas et al. [Citation30], on the other hand, found no increased level of complications in delayed reconstruction when certain ramification are considered, such as transfer to a microsurgical facility, radical debridement, and vessel anastomoses outside the zone of injury.
We have investigated blood coagulation activity in patients who underwent early and delayed microsurgical reconstruction of the lower leg to determine whether these changes could be responsible for failures in delayed reconstructive surgery. Our results indicate that, after trauma of the lower extremity, the balance of endothelial properties can be tipped to favour clot formation through co-ordinated induction of procoagulant and suppression of anticoagulant mechanisms.
Free tissue transfer is always prone to bleeding from the operation field. This circumstance can induce coagulation and fibrinolysis not only at the wound sites, but also systemically [Citation9]. A thrombembolic event at the site of the microsurgical anastomosis could further stimulate the elevation of thrombin and plasmin levels, at least locally. Our patients with high levels of PAI-1 suggest a reduced activity of the fibrinolytic system, which appears to be another negative factor to stimulate further deposition of fibrin after trauma in vessels of the lower extremities. Activators and inhibitors of fibrinolysis are produced and kept in endothelial cells. An excess of fibrin deposition in the microcirculation under physiological conditions is prevented by a relative overabundance of t-PA over PAI-1, thus predominantly stimulating fibrinolytic processes. Trauma increases the synthesis or release of PAI-1, which leads to a temporary hypofibrinolysis and a thrombotic diathesis in the patients’ blood.
We are well aware of the circumstance that two different types of flaps were used in this study regarding their vascular anatomy. While the LD is based on an axial blood supply, the ALT-Flap can be based on variable amounts of septo- or musculocutaneous perforators of different quality. The authors believe that the anatomical differences are of minor relevance, and are convinced that both flaps can be used equally in lower leg reconstruction.
We were able to demonstrate an activation of the coagulation and inhibition of the fibrinolytic system during microsurgical free tissue transfers for the treatment of defects of the lower extremities. The disturbed balance between coagulation and fibrinolysis leads to a hypercoagulability, as seen in our patients by increased levels of TAT and F1 + 2. Our data indicated accelerated conversion of pro-thrombin to thrombin (high F1 + 2) and an increase in circulating thrombin (high TAT), whereas fibrinolysis was disturbed by the increase of PAI-1. Interestingly, there was no significant difference in clotting activation between fasciocutaneous and muscle flaps that have a larger wound surface area. All of our patients received low-molecular-weight heparin to reduce the risk of thrombotic events during or after surgery. Anticoagulant therapy has proved effective for preventing both venous and arterial thromboembolism. Multiple well-designed studies have confirmed that thromboprophylaxis decreases the risk of deep vein thrombosis and pulmonary embolism [Citation33].
Many anticoagulatory regimens have been described in scientific literature in order to prevent or at least reduce the incidence of deleterious postoperative thrombembolic complications in microsurgical tissue transfer [Citation31]. These include employment of systemic medications such as Heparin (intravenously and PTT-controlled or as subcutaneous injections), HES, Albumine, and techniques of haemodilution using different concentrations of saline infusions. Especially the effect of haemodilution has been reported contradictory with groups describing a significant increase in flap loss [Citation34–39]. As one of the more recent approaches, Sildenafil (Viagra®) has been shown to postoperatively increase the bloodflow in free LD transfers in one experimental animal study [Citation40].
Finally, flap loss is attributed to a multitude of colliding factors at the same time and eventually on the background of insufficient microsurgery or surgical planning in some instances. Finding the best postoperative individualised anticoagulatory treatment scheme without increasing the risk for postoperative bleeding and subsequent constricting haematoma formation while prevailing sufficient anticoagulatory power remains a matter of ongoing research. Based on common clinical experience and based on studies [Citation41], low-dose Heparine reduces the postoperative thrombotic risk to some extent. To assess this effect of postoperative low-dose heparine in detail, we would have had to carry out further extensive diagnostic testings, such as e.g. Thrombelastography (TEG). The shortcoming of these results could hypothetically cause a bias in the delayed group, even more so within a hypercoaguable state. Omitting postoperative low-dose Heparine in all patients would have adequately addressed that bias, but would have been considered unethical in terms of prevention of postoperative thrombotic complications, and could have hypothetically impacted flap survival rates.
According to the results of our study demonstrating a significant increase of prothrombotic factors such as F1 + 2, PAI-1, and TAT preoperatively, as well as postoperatively in the delayed reconstruction group with a concomitant increase in flap failure rate, we favour early microsurgical reconstruction after lower leg trauma to minimise the risk of flap failure due to prothrombotic diasthesis. One the other hand, several factors often preclude early reconstruction. In these patients, markers of blood coagulation and fibrinolysis can be used preoperatively to target antithrombotic control and to minimise the risk of flap failure due to a hypercoagulable state in the patient’s blood. Based on our findings in the delayed reconstructive group, we therefore suggest to consider pre- and postoperative screening of F1 + 2, PAI-1, and TAT, especially when microsurgical reconstruction is carried out in a delayed fashion. Further studies have to evaluate the best therapeutic interventions in these settings, such as awaiting normalisation of these parameters with the disadvantage of further delay of the reconstruction or adjusting the postoperative anticoagulatory regimen accordingly.
Future work of our department will predominantly focus on platelet function assessment and subsequent therapeutic interventions in the perioperative course of microsurgical lower leg reconstruction.
Disclosure statement
The authors have no financial interests in this research project or in any of the techniques or equipment used in this study. This study was self-funded by the Department of Plastic and Reconstructive Surgery of the Erasmus University Hospital, Rotterdam, and the Department of Plastic and Reconstructive Surgery of the University Hospital Aachen.
References
- Bui DT, Cordeiro PG, Hu QY, et al. Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg 2007;119:2092–100.
- Lineaweaver WC, Buncke HJ. Complications of free flap transfers. Hand Clin 1986;2:347–51.
- Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg 2009;124:490–5.
- Höijer P, Olsson E. Elevated coagulation factor VIII, postoperative thrombosis and flap failure in late breast reconstruction with a free TRAM flap: a case report. J Plast Reconstr Aesthet Surg 2006;59:102–4.
- Byrd HS, Spicer TE, Cierney G. III. Management of open tibial fractures. Plast Reconstr Surg 1985;76:719–30.
- Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg 1986;78:285–92.
- Miniello S, Testini M, Balzanelli MG, Cristallo G. Coagulation disorders following severe trauma: surgeon’s role in prevention. Ann Ital Chir 2004;75:293–7.
- Komuro Y, Sekiguchi J, Nomura S, et al. Blood coagulation activity during microsurgery. Ann Plast Surg 1998;40:53–8.
- Olsson E, Svartling N, Asko-Seljavaara S, Lassila R. Activation of coagulation and fibrinolysis in microsurgical reconstructions in the lower extremities. Br J Plast Surg 2001;54:597–603.
- Olsson E, Svartling N, Asko-Seljavaara S, Lassila R. Activation of coagulation and fibrinolysis during reconstructive microsurgery in patients with cancer. Microsurgery 2001;21:208–13.
- Olsson E, Höijer P. Activated protein C resistance due to factor V Leiden, elevated coagulation factor VIII and postoperative deep vein thrombosis in late breast reconstruction with a free TRAM flap: a report of two cases. Br J Plast Surg 2005;58:720–3.
- Haeberli A. Prothrombin fragment; F1 + 2. In: Jespersen J, Bertina RM, Haverkater F, editors. Laboratory techniques in thrombosis – a manual. Dordrecht-Boston-London: Kluwer; 1999. pp 217–222.
- Lau HK, Rosenberg RD. The isolation and characterization of a specific antibody population directed against the thrombin antithrombin complex. J Biol Chem 1980;255:5885–93.
- Schietroma M, Carlei F, Mownah, et al. Changes in the blood coagulation, fibrinolysis, and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc 2004;18:1090–6.
- Selby R, Geerts W, Ofosu F, et al. Hypercoagulability after trauma: hemostatic changes and relationship to venous thromboembolism. Thromb Res 2009;124:281–7.
- Ganter MT, Pittet JF. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol 2010;24:15–25.
- Gando S, Kameue T, Nanzaki S, et al. Participation of tissue factor and thrombin in posttraumatic systemic inflammatory syndrome. Crit Care Med 1997;25:1820–6.
- Sorensen JV. Levels of fibrinolytic activators and inhibitors in plasma after severe trauma. Blood Coagul Fibrinolysis 1994;5:43–9.
- Dries DJ. Activation of the clotting system and complement after trauma. New Horizons 1996;4:276–88.
- Ulrich D, Pallua N, Lichtenegger F, et al. Influence of low frequency electric fields on anti- and procoagulability of the vascular endothelium: new insights into high-voltage electrical injury. Thromb Haemost 2004;91:1000–8.
- Bennett B, Towler HMA. Haemostatic response to trauma. Br Med Bull 1985;41:274–80.
- Falanga A, Ofosu FA, Cortelazzo S, et al. Preliminary study to identify cancer patients at high risk of venous thrombosis following major surgery. Br J Haematol 1993;85:745–50.
- Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med 1994;331:1601–6.
- Meissner MH, Chandler WL, Elliott JS. Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability? J Trauma 2003;54:224–31.
- Engelman DT, Gabram SGA, Allen L, et al. Hypercoagulability following multiple trauma. World J Surg 1996;20:5–10.
- Owings JT, Bagley M, Gosselin R, et al. Effect of critical injury on plasma antithrombin activity: low antithrombin levels are associated with thromboembolic complications. J Trauma 1996;41:396–406.
- Chen JP, Rowe DW, Enderson BL. Contrasting post-traumatic serial changes for D-dimer and PAI-1 in critically injured patients. Thromb Res 1999;94:175–85.
- Ayala C, Blackwell KE. Protein C deficiency in microvascular head and neck reconstruction. Laryngoscope 1999;109:259–65.
- Kolbenschlag J, Klinkenberg M, Hellmich S, et al. Impact of timing of admission and microvascular reconstruction on free flap success rates in traumatic upper extremity defects. J Reconstr Microsurg 2015;31:414–19.
- Karanas YL1, Nigriny J, Chang J. The timing of microsurgical reconstruction in lower extremity trauma. Microsurgery 2008;28:632–4.
- Brinkman JN1, Derks LH, Klimek M, Mureau MA. Perioperative fluid management and use of vasoactive and antithrombotic agents in free flap surgery: a literature review and clinical recommendations. J Reconstr Microsurg 2013;29:357–66.
- Kolbenschlag J, Daigeler A, Lauer S, et al. Can rotational thromboelastometry predict thrombotic complications in reconstructive microsurgery? Microsurgery 2014;34:253–60.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:338S–400S.
- Tocantins LM, Carroll RT, Holburn RH. The clot accelerating effect of dilution on blood and plasma. Relation to the mechanism of coagulation of normal and hemophilic blood. Blood 1951;6:720–39.
- Ruttmann TG, James MF, Viljoen JF. Haemodilution induces a hypercoagulable state. Br J Anaesth 1996;76:412–14.
- Egli GA, Zollinger A, Seifert B, et al. Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br J Anaesth 1997;78:684–9.
- Amoroso M, Özkan Ö, Başsorgun CI, et al. The effect of normovolemic and hypervolemic hemodilution on a microsurgical model: Experimental study in rats. Plast Reconstr Surg 2015;136:512–19.
- Namdar T, Bartscher T, Stollwerck PL, et al. Complete free flap loss due to extensive hemodilution. Microsurgery 2010; 30:214–17.
- Hill JB, Patel A, Del Corral GA, et al. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann Plast Surg 2012;69:364–7.
- Gravvanis A, Papalois A, Delikonstantinou I, et al. Changes in arterial blood flow of free flaps after the administration of sildenafil in swine. Microsurgery 2011;31:465–71.
- Handoll HH1, Farrar MJ, McBirnie J, et al. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev 2002;(4):CD000305.
